Published online Nov 21, 2018. doi: 10.3748/wjg.v24.i43.4920
Peer-review started: September 3, 2018
First decision: October 8, 2018
Revised: October 15, 2018
Accepted: November 8, 2018
Article in press: November 8, 2018
Published online: November 21, 2018
Processing time: 79 Days and 1.1 Hours
To describe the prevalence of posttransplant metabolic syndrome (PTMS) after donation after cardiac death (DCD) liver transplantation (LT) and the pre- and postoperative risk factors.
One hundred and forty-seven subjects who underwent DCD LT from January 2012 to February 2016 were enrolled in this study. The demographics and the clinical characteristics of pre- and post-transplantation were collected for both recipients and donors. PTMS was defined according to the 2004 Adult Treatment Panel-III criteria. All subjects were followed monthly for the initial 6 mo after discharge, and then, every 3 mo for 2 years. The subjects were followed every 6 mo or as required after 2 years post-LT.
The prevalence of PTMS after DCD donor orthotopic LT was 20/147 (13.6%). Recipient’s body mass index (P = 0.024), warm ischemia time (WIT) (P = 0.045), and posttransplant hyperuricemia (P = 0.001) were significantly associated with PTMS. The change in serum uric acid levels in PTMS patients was significantly higher than that in non-PTMS patients (P < 0.001). After the 1st mo, the level of serum uric acid of PTMS patients rose continually over a period, while it was unaltered in non-PTMS patients. After transplantation, the level of serum uric acid in PTMS patients was not associated with renal function.
PTMS could occur at early stage after DCD LT with growing morbidity with the passage of time. WIT and post-LT hyperuricemia are associated with the prevalence of PTMS. An increased serum uric acid level is highly associated with PTMS and could act as a serum marker in this disease.
Core tip: The objective of the current retrospective analysis was to describe the pre- and postoperative risk factors for prevalence of posttransplant metabolic syndrome (PTMS) after liver transplantation (LT) with donation after cardiac death (DCD). PTMS could occur at early stage after DCD LT with growing morbidity as time goes on. The warm ischemia time and posttransplant hyperuricemia were associated with the prevalence of PTMS. An increased serum uric acid level was highly relevant to PTMS and could act as a serum marker in this disease.
- Citation: Hu LS, Chai YC, Zheng J, Shi JH, Zhang C, Tian M, Lv Y, Wang B, Jia A. Warm ischemia time and elevated serum uric acid are associated with metabolic syndrome after liver transplantation with donation after cardiac death. World J Gastroenterol 2018; 24(43): 4920-4927
- URL: https://www.wjgnet.com/1007-9327/full/v24/i43/4920.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i43.4920
Liver transplantation (LT) is still the standard treatment for patients with end-stage liver disease. The increasing disparity between patients and supply of donor livers prompts the surgeons to expand the donor pool. Thus, the usage of livers from donation after cardiac death (DCD) donors has increased rapidly. In the last two decades, about 5.0% of the adult LTs were performed using grafts from DCD donors in the United States[1]. Despite the high risk of a series of acute complications correlated with the warm ischemia time (WIT)[2], several parallel studies showed that the clinical outcomes of LT using more restrictive DCD donor criteria including body mass index (BMI) < 29 kg/m2 and functional WIT < 20 min were comparable to those with standard brain-dead donors[3]. Thus, the long-term prognosis of DCD LT has gained increasing attention.
After LT, patients often develop a series of metabolic alterations such as hyperglycemia, hypertension, dyslipidemia, and obesity[4]. These metabolic derangements were defined as posttransplant metabolic syndrome (PTMS), which is correlated with cardiovascular disease, and hence, under intensive focus. Reportedly, the prevalence of PTMS is 39%-58% over a period of 1-6 years after LT in Western countries and 35.6% in Asia[5,6]. However, data on specific assessment of the morbidity of PTMS after DCD LT are still lacking.
The present retrospective analysis described the prevalence of PTMS after DCD LT and the pre- and postoperative risk factors.
This is a retrospective cohort study. One hundred and forty-seven subjects with DCD liver transplantation at the First Affiliated Hospital of Xi’an Jiao Tong University from January 2012 to February 2016 were enrolled in this study that was approved by the Ethics Committee of the Institute (No. XJTU1AF2018LSK-084).
Since the Chinese organ donation system has been developed, DCD donor is the primary legal source of the organ for transplantation. All the organ donations are confirmed by the Maastricht categories of DCD type III: Awaiting cardiac arrest controlled[7]. Mechanical support is withdrawn in the operating room in a majority of the cases. Five minutes after the declaration of death by an independent physician, donor grafts are recovered via double in situ perfusion with combined liver and kidney rapid resection technique. Organ allocation is conducted according to the waiting list from the organ transplantation division of Chinese Medical Association. All the transplants are performed using the primary orthotopic LT (OLT) as the standard technique. For immunosuppressive protocol, we used the standard three-drug regimen including steroid, tacrolimus/cyclosporine, and mycophenolate mofetil (MMF). For recipients with hepatocellular carcinoma (HCC), steroid-free protocol was applied as described by Shen et al[8].
The pre- and post-transplantation demographics and clinical characteristics were collected for both recipients and donors. PTMS was defined according to the 2004 Adult Treatment Panel-III criteria[9]. A patient would be diagnosed as PTMS if ≥ 3 of the following five conditions were fulfilled: (1) Obesity; (2) high fasting glucose level (≥ 100 mg/dL); (3) hypertriglyceridemia (≥ 150 mg/dL); (4) low high-density lipoprotein (HDL) level [< 40 mg/dL (male) or < 50 mg/dL (female)]; and (5) high blood pressure (≥ 130/85 mmHg); or pharmacological treatment for each of these conditions. The diagnosis of obesity was adjusted according to the characteristics of the Asian population and defined as a BMI ≥ 27.5 kg/m2 in accordance with the 2004 World Health Organization (WHO) guidelines[10]. Hyperuricemia was defined as a serum uric acid level > 420 μmol/L within 1 mo post-transplantation, and the complications included acute kidney injury (AKI), renal insufficiency, acute rejection, and biliary complications.
All subjects were followed monthly for the initial 6 mo after discharge, and then, every 3 mo for 2 years. The subjects were followed every 6 mo or as required after 2 years post-LT. Data of the donors and recipients were uploaded to the China Liver Transplant Registry, a database and official website for national data gathering.
Statistical analyses were performed using SPSS Statistics 22 (SPSS Inc., Chicago, IL, United States). Continuous data are presented as mean ± standard deviation and were compared using t-tests. Categorical data are presented as frequencies (percentages) and were compared using chi-squared tests or Fisher’s exact test as appropriate. Univariate and multivariate logistic regressions were conducted to explore the factors associated with PTMS. Odds ratios (OR) were presented with 95% confidence intervals (CIs). Variables with P < 0.1 in the univariate analysis were included in the multivariate logistic regression. The stepwise procedure was used to identify the variables independently associated with PTMS in the final multivariate model. Kaplan-Meier method was employed to determine the survival of patients after LT, and log-rank test was used to determine the difference in survival between PTMS and non-PTMS patients. P < 0.05 was considered statistically significant.
The mean age of the recipients was 45.6 ± 10.8 year old, and 121/147 (82.3%) recipients were male. Furthermore, 98 (66.7%) patients in the cohort underwent transplantation because of hepatitis B virus (HBV)-related liver disease, while other original diseases of the recipients included hepatitis C virus (HCV) infection (15, 10.2%), alcoholic liver disease (6, 4.1%), autoimmune hepatitis (9, 6.1%), and drug-induced liver dysfunction. Thirty-four percent of patients of the cohort received LT because of HCC.
The BMI of PTMS patients was significantly greater than that of non-PTMS patients (23.5 ± 4.3 vs 21.8 ± 2.8, P = 0.021). The proportions of obesity (20% vs 3.1%, P = 0.002) and metabolic syndrome (10% vs 3.1%, P = 0.02) at transplantation, AKI (35% vs 10.2%, P = 0.002), and hyperuricemia (55.0% vs 15.0%, P < 0.001) within one month after transplantation were significantly higher in PTMS patients as compared to non-PTMS patients. The other characteristics pre- and post-transplantation did not differ significantly between the two groups (Table 1). None of the patients presented a clinical history of gout.
| Variable | PTMS (n = 20) | Non-PTMS (n = 127) | P value |
| Demographics | |||
| Age, yr | 46.3 ± 9.0 | 45.5 ± 11.0 | 0.746 |
| Male | 17 (85.0) | 104 (81.9) | 0.735 |
| BMI, kg/m2 | 23.5 ± 4.3 | 21.8 ± 2.8 | 0.021 |
| MELD score | 18.4 ± 8.3 | 17.7 ± 8.5 | 0.768 |
| Child-Pugh score | 10.5 ± 2.2 | 10.0 ± 2.0 | 0.374 |
| Smoking | 5 (25.0) | 39 (30.7) | 0.604 |
| Alcohol | 6 (30.0) | 19 (15.0) | 0.096 |
| HBV | 13 (65.0) | 85 (66.9) | 0.865 |
| HCV | 1 (5.0) | 14 (11.0) | 0.408 |
| Pre-LT comorbidity | |||
| Obesity | 4 (20.0) | 4 (3.1) | 0.002 |
| Diabetes mellitus | 4 (20.0) | 13 (10.2) | 0.210 |
| Hypertension | 1 (5.0) | 4 (3.1) | 0.671 |
| Dyslipidemia | 2 (10.0) | 13 (10.2) | 0.974 |
| Metabolic syndrome | 3 (10.0) | 4 (3.1) | 0.020 |
| Laboratory test | |||
| Pre-LT serum uric acid, μmol/L | 265 ± 116 | 280 ± 97 | 0.545 |
| Pre-LT serum creatinine, μmol/L | 55.2 ± 16.9 | 60.0 ± 19.0 | 0.288 |
| Pre-LT eGFR, mL/min per 1.73 m2 | 158.7 ± 54.6 | 139.2 ± 43.9 | 0.076 |
| Operative characteristic | |||
| Anhepatic phase, min | 50.8 ± 9.5 | 53.5 ± 11.3 | 0.193 |
| Operation time, h | 6.7 ± 1.5 | 6.2 ± 1.1 | 0.084 |
| Length of ICU stay, d | 6.9 ± 3.0 | 6.8 ± 3.6 | 0.923 |
| Post-LT clinical characteristic | |||
| Steroid-free protocol for HCC | 3 (15.0) | 43 (33.9) | 0.091 |
| Tacrolimus use ≥ 24 mo | 12 (60.0) | 68 (53.5) | 0.590 |
| Cyclosporine use ≥ 24 mo | 8 (40.0) | 59 (46.5) | 0.590 |
| MMF use ≥ 24 mo | 13 (65.0) | 84 (66.1) | 0.920 |
| Acute graft rejection | 3 (15.0) | 12 (9.4) | 0.446 |
| Biliary complication | 3 (15.0) | 28 (22.0) | 0.472 |
| Acute kidney injury | 7 (35.0) | 13 (10.2) | 0.002 |
| Hyperuricemia | 11 (55.0) | 19 (15.0) | 0.002 |
The mean age of the donors was 41.1 ± 14.2 years, and 121/147 (82.3%) donors were male. The BMI and WIT of donors for PTMS patients were significantly greater than those of donors for non-PTMS patients (BMI: 24.0 ± 4.7 vs 22.2 ± 3.2, P = 0.029; WIT: 10.8 ± 2.7 vs 9.2 ± 2.5, P = 0.034) (Table 2).
| Variable | PTMS (n = 20) | Non-PTMS (n = 127) | P value |
| Demographics | |||
| Age, yr | 42.0 ± 13.7 | 41.0 ± 14.3 | 0.762 |
| Male, n (%) | 17 (85.0) | 104 (81.9) | 0.735 |
| BMI, kg/m2 | 24.0 ± 4.7 | 22.2 ± 3.2 | 0.029 |
| Operative characteristic | |||
| WIT, min | 10.8 ± 2.7 | 9.2 ± 2.5 | 0.034 |
| CIT, h | 5.1 ± 1.9 | 5.2 ± 1.6 | 0.864 |
The medical reasons for donors were predominantly brain trauma (61.9%) as well as cerebrovascular accident, anoxia, encephalopathy, and brain tumor.
The median follow-up period was 32.1 (range: 14-81) mo. Twenty (13.6%) among one hundred and forty-seven subjects were diagnosed with PTMS; among these, seven (4.8%) were diagnosed by the 6th mo after DCD LT. The morbidities of postoperative obesity, diabetes, hypertension, and dyslipidemia were 12.2%, 31.3%, 10.9%, and 22.4%, respectively.
The BMI of the recipients, WIT, AKI, and hyperuricemia were found to be significantly associated with PTMS according to the univariate logistic regression (Table 3). Moreover, BMI (OR = 10.9, 95%CI: 1.38-86.3, P = 0.024), WIT (OR = 1.23, 95%CI: 1.01-1.50, P = 0.045), and hyperuricemia (OR = 11.8, 95%CI: 2.85-48.8, P = 0.001) remained significant parameters according to the final multivariate logistic regression analysis (Table 4).
| Variable | OR (95%CI) | P value |
| Age | 1.26 (0.48-3.31) | 0.642 |
| Male | 1.25 (0.34-4.64) | 0.735 |
| Smoking | 1.33 (0.45-3.92) | 0.605 |
| Alcohol | 2.44 (0.83-7.13) | 0.104 |
| HBV | 0.92 (0.34-2.47) | 0.865 |
| HCV | 0.43 (2.25-13.6) | 0.408 |
| BMI | 7.69 (1.75-33.8) | 0.007 |
| Pre-LT diabetes mellitus | 2.19 (0.64-7.55) | 0.214 |
| Pre-LT hypertension | 1.62 (0.17-15.3) | 0.674 |
| Pre-LT dyslipidemia | 2.17 (0.27-17.5) | 0.469 |
| Donor age | 2.42 (0.67-8.74) | 0.178 |
| Donor BMI | 1.91 (0.37-9.90) | 0.443 |
| WIT | 1.21 (1.04-1.41) | 0.014 |
| CIT | 0.95 (0.69-1.31) | 0.741 |
| Steroid-free protocol for HCC | 0.35 (0.10-1.24) | 0.065 |
| Tacrolimus use ≥ 24 mo | 1.77 (0.39-4.01) | 0.591 |
| Cyclosporine use ≥ 24 mo | 1.30 (0.50-3.40) | 0.591 |
| MMF use ≥ 24 mo | 1.05 (0.39-2.83) | 0.920 |
| Acute graft rejection | 1.69 (0.43-6.61) | 0.450 |
| Biliary complication | 0.48 (0.17-2.28) | 0.467 |
| Acute kidney injury | 4.72 (1.60-14.0) | 0.005 |
| Hyperuricemia | 6.95 (2.54-19.0) | < 0.001 |
| Variable | OR (95%CI) | P value |
| Steroid-free protocol for HCC | 0.22 (0.41-1.16) | 0.219 |
| BMI | 10.9 (1.38-86.3) | 0.024 |
| Warm ischemia time | 1.23 (1.01-1.50) | 0.045 |
| Acute kidney injury | 3.58 (0.94-13.6) | 0.062 |
| Hyperuricemia | 11.8 (2.85-48.8) | 0.001 |
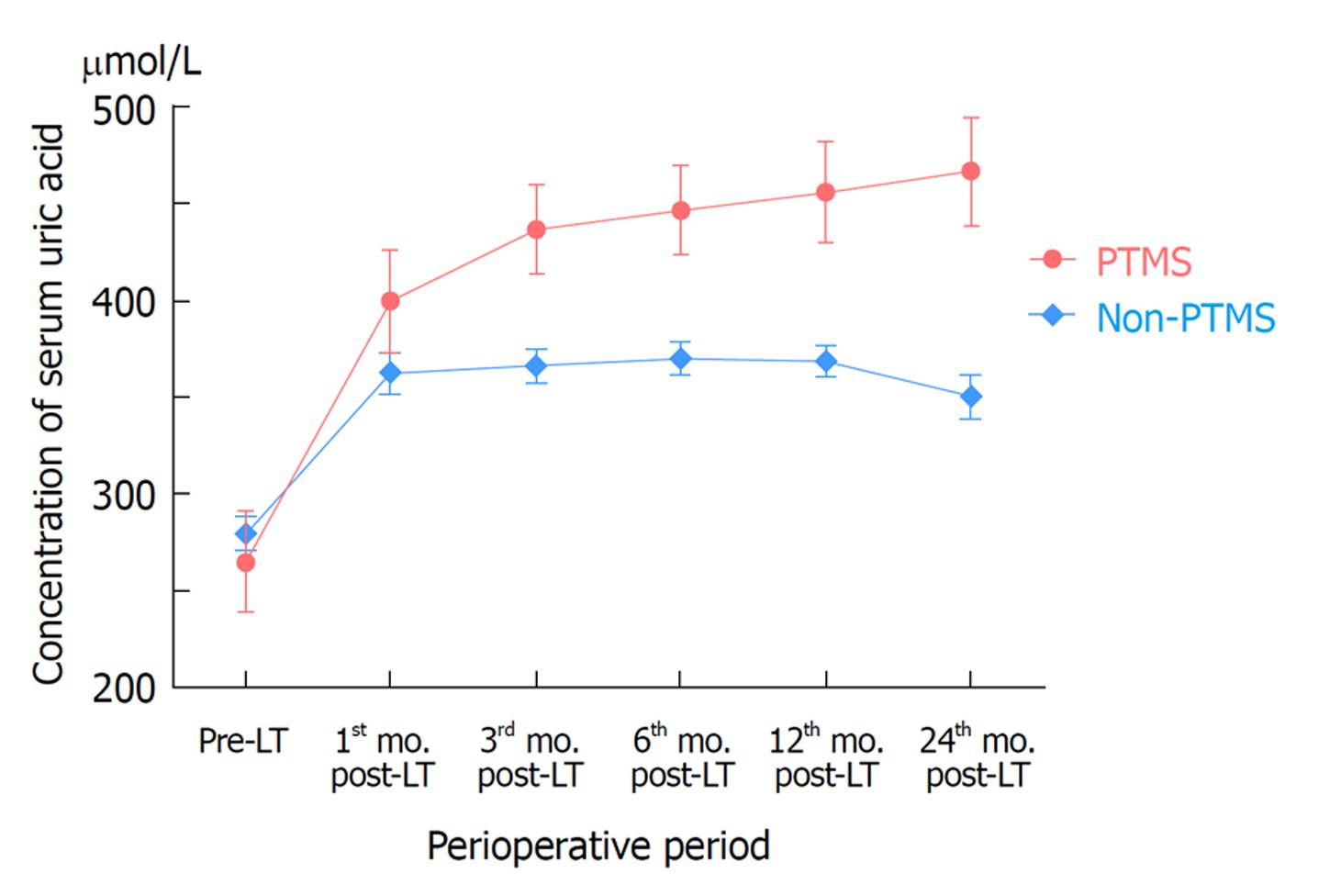
The pre-LT level of serum uric acid was compared between PTMS and non-PTMS, and no significant difference was found (255 ± 96 vs 273 ± 84, P = 0.545). The change in the serum uric acid level post-LT over a period was verified with PTMS and non-PTMS, respectively. The level of serum uric acid rose from 255 ± 96 to 400 ± 118 μmol/L in PTMS patients (P < 0.001) and from 273 ± 84 to 350 ± 103 μmol/L in non-PTMS patients (P < 0.001) during the first month after the surgery. After the 1st mo, the level of serum uric acid in PTMS patients continued to increase over time (P24 mo-1 mo < 0.001), while it remained unchanged in non-PTMS patients (P24 mo-1 mo = 0.847) (Table 5 and Figure 1).
| Time point | PTMS | Non-PTMS | ||
| Mean ± STD, μmol/L | Overall P value | Mean ± STD, μmol/L | Overall P value | |
| Pre-LT(Baseline) | 255 ± 96 | < 0.001 | 273 ± 84 | < 0.001 |
| P1st mo | 400 ± 118 | 350 ± 103 | ||
| P3rd mo | 432 ± 80 | 355 ± 81 | ||
| P6th mo | 446 ± 72 | 360 ± 78 | ||
| P12th mo | 460 ± 96 | 360 ± 83 | ||
| P24th mo | 512 ± 76 | 348 ± 90 | ||
Serum uric acid was significantly correlated with estimated glomerular filtration rate (eGFR) pre-transplantation in all patients. Subsequently, the changes in the serum uric acid were significantly associated with the corresponding changes in eGFR among non-PTMS patients, while serum uric acid in PTMS patients did not appear to be correlated with eGFR over a period (Table 6).
| PTMS | Non-PTMS | |||
| r | P value | r | P value | |
| Pre-LT | -0.74 | < 0.001 | -0.28 | 0.002 |
| P1 mo-Baseline | -0.44 | 0.052 | -0.43 | < 0.001 |
| P3 mo-Baseline | 0.076 | 0.750 | -0.22 | 0.014 |
One-year patient and graft estimated survival rates of DCD LT were 94.8% and 88.2%, respectively. The graft loss might be attributed to infection (2/16, 12.5%), biliary complications (3/16, 18.8%), vascular complications (2/16, 12.5%), primary graft failure (1/16, 6.3%), intra-abdominal hemorrhage (2/16, 12.5%), rejection (2/16, 12.5%), and tumor recurrence (4/16, 25%). No events were recorded in the recipients with PTMS.
Controlled DCD donors constitute the most potential donors in China since 2010. This is the first study assessing the prevalence of MS for DCD LT. In the current study, the prevalence of PTMS is 13.6% for the whole cohort. PTMS could occur at the early stage after DCD LT with growing morbidity with the passage of time.
The prevalence of PTMS was found to be remarkably lower than that reported in the previous studies[5,6,11,12]. Several potential reasons might be able to explain the relatively low prevalence. First, the morbidities of pre-transplant MS and its components in the current study were significantly lower than those reported previously. The prevalence of PTMS was almost triple with respect to pre-transplant MS within 2 years post-surgery. The prevalence might further rise in prolonged follow-up periods. Second, different etiologies might also cause the lower morbidity of PTMS. Unlike Europe or United States, HBV, not non-alcoholic steatohepatitis (NASH) or HCV, was the most common indication for LT observed at our center. HCV is reported to be related to diabetes mellitus and NAFLD in liver disease[13,14]. Some investigators also found a higher rate of PTMS in HCV recipients in multiple studies[4,6,15,16]. Conversely, only a few studies showed the relationship between HBV and post-LT metabolic issues. Finally, the mean age (46 years old) of this cohort was relatively young. Although we did not find age as an independent risk factor for PTMS in the current study, it has been widely accepted that older recipients have a higher prevalence of metabolic disorders[17].
In the current study for DCD patients, BMI, WIT, and hyperuricemia were found to be associated with PTMS. Ischemia-reperfusion injury resulting from prolonged WIT could lead to a series of post-OLT complications. However, whether WIT exerted any influence on PTMS has not yet been elucidated. Also, hepatic ischemia-reperfusion injury induced insulin resistance[18]. A clinical study found an early occurrence of new-onset diabetes after transplantation, which is related to the type of liver graft and warm ischemic injury[19]. Perera et al[20] reported the differences in the metabolites in the microdialysate samples of liver grafts from DCD and brain deaths. These studies indicated that WIT might contribute to hepatic metabolomic changes post-transplantation. In this study, the ineluctable WIT, rather than cold ischemia time, for DCD LT was found to be an independent risk factor of the post-transplantation metabolic syndrome; nonetheless, further experiments are essential for exploring the underlying mechanism.
Another intriguing finding of this research was derived from the analysis of serum uric acid after LT. To date, only a few studies are available on the predictors of PTMS[21]. Hyperuricemia is one of the potential metabolic complications of LT. The elevated level of serum uric acid has frequently been observed post-transplantation and reportedly, associated with ischemia-reperfusion injury, renal dysfunction, and immunosuppressive therapy[22-24]. In addition, some studies demonstrated the correlation of hyperuricemia with the development of metabolic syndrome; however, its role on PTMS has not yet been deduced[25]. Herein, we found hyperuricemia to be associated with PTMS.
Subsequently, we explored whether the elevated serum uric acid level was associated with PTMS and found rapidly increased levels of the acid in patients with PTMS in the first month post-surgery. Compared with the non-PTMS cohort, patients with metabolic syndrome exhibited a higher preoperative BMI and donor BMI. Although obesity is one of the risk factors of hyperuricemia, the mean uric acid level before surgery of the two groups was normal. Furthermore, BMI may not be the reason for the sudden increase in the level of uric acid level immediately after LT. Other differences between the two cohorts were that patients in the PTMS group suffered longer WIT and more AKI in the perioperative period than the non-PTMS group; the prolonged WIT of DCD LT led to worse ischemia-reperfusion injury and caused AKI[26]. Warm ischemia could also induce breakdown of hepatocellular ATP to purine catabolites that are oxidized, and in turn, become uric acid after reperfusion[27]. This phenomenon renders uric acid as one of the markers to predict the hepatic injury due to ischemia[28]. Renal dysfunction caused by AKI is also associated with the elevated level of uric acid[22,29]. Although not found in the current study, prolonged WIT remains a potential cause for the tendency of a rapid rise in the level of serum uric acid in the perioperative period of LT.
After a sharp increase in the first month, the level of uric acid stabilized in the non-PTMS cohort. Moreover, it continued to increase in PTMS patients and overstepped the upper limit of normal blood uric acid concentration. Intriguingly, after adjusting for renal function, the disparity in the values persisted. This indicated that the increased serum uric acid level was highly associated with PTMS. Recently, accumulating evidence suggested that uric acid, the final product of the purine degradation in human, was an independent predictor of metabolic syndrome. Choi et al[30] found a significantly high prevalence of MS in the hyperuricemia population. Li et al[31] reported that the increase in the serum uric acid level within the normal range could predict the risk of metabolic syndrome. A meta-analysis reported a linear disposition from a uric acid increase on the prevalence of MS[32]. However, any evidence supporting the relationship between hyperuricemia and PTMS was absent. Based on the current data, we hypothesized that uric acid could serve as a serum marker for the prevalence of PTMS.
Nevertheless, the present study had several limitations. First, our results were based on a single center retrospective study. Second, the follow-up period of the current study was relatively short than the previous long-term retrospective studies, thereby limiting the results of patients’ survival and complications. Third, the donor source did not allow comparison of the data from DCD with DBD LT.
In conclusion, the current study showed that PTMS could occur at the early stage after DCD LT with growing morbidity with the passage of time. For the first time, we found that prolonged warm ischemia and post-LT hyperuricemia were associated with prevalent PTMS. Also, increased serum uric acid level was highly associated with PTMS and could serve as a serum marker for monitoring such a disease.
Liver transplantation (LT) is still the standard treatment for patients with end-stage liver disease. The usage of livers from donation after cardiac death (DCD) donors has increased rapidly. Current research shows that some risks of a series of acute and chronic complications are correlated with the warm ischemia time (WIT). Thus, the long-term prognosis of DCD LT has gained increasing attention.
After LT, patients may develop a series of metabolic disorders which is called posttransplant metabolic syndrome (PTMS). However, data on specific assessment of the morbidity of PTMS after DCD LT are still lacking. Therefore, this study aimed to further explore the prevalence of PTMS after DCD LT and the pre- and postoperative risk factors, to provide evidence for clinical decision rules.
The present retrospective analysis describes the prevalence of PTMS after DCD LT and the pre- and postoperative risk factors that are relevant to the occurrence of PTMS, and provides evidence for clinical judgment.
This is a retrospective cohort study. One hundred and forty-seven subjects who underwent DCD liver transplantation from January 2012 to February 2016 were enrolled in this study. The pre- and post-transplantation demographics and clinical characteristics were collected for both recipients and donors. All subjects were followed monthly for the initial 6 mo after discharge, and then, every 3 mo for 2 years. The subjects were followed every 6 mo or as required after 2 years post-LT. All data were used to perform statistical analysis and identify the variables independently associated with PTMS in the final multivariate model.
In this retrospective cohort study, the prevalence of PTMS after DCD donor orthotopic LT was 13.6%. Recipient’s body mass index, WIT, and posttransplant hyperuricemia were significantly associated with PTMS. The change in serum uric acid level in PTMS patients was significantly higher than that in non-PTMS patients. After the 1st month, the level of serum uric acid of PTMS patients rose continually over a period, while it was unaltered in non-PTMS patients. After transplantation, the level of serum uric acid in PTMS patients was not associated with renal function.
PTMS could occur at early stage after LT DCD with growing morbidity as time goes on. For the first time, we found that prolonged WIT and the posttransplant hyperuricemia were associated with the prevalence of PTMS, and an increased serum uric acid level was highly associated with PTMS and could serve as a serum marker for monitoring such a disease.
In this study, the ineluctable WIT rather than cold ischemia time for DCD LT was found initially as an independent risk factor of PTMS. Nonetheless, further experiments are essential for exploring the underlying mechanism. Our data also indicated that the increased serum uric acid level was highly associated with PTMS. Although prolonged WIT remains a potential cause for the tendency of a rapid rise in the level of serum uric acid in the perioperative period of LT, after a sharp increase in the first month, the level of uric acid stabilized in the non-PTMS cohort. However, it continued to increase in PTMS patients and overstepped the upper limit of normal blood uric acid concentration. Intriguingly, after adjusting for renal function, the disparity in the values persisted. Recently, accumulating evidence also suggests the standpoint that uric acid is an independent predictor of metabolic syndrome. In consideration of our research results, more prospective studies are urgently required to provide evidence for clinical verification. Future research should include larger cohorts of patients from multiple centers to expand the sample size and establish a more comprehensive long-term follow-up to improve the statistical database containing more factors, including PTMS and survival rate.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kazuya S, Parakkal D, Pavel MC S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Doyle MB, Collins K, Vachharajani N, Lowell JA, Shenoy S, Nalbantoglu I, Byrnes K, Garonzik-Wang J, Wellen J, Lin Y. Outcomes Using Grafts from Donors after Cardiac Death. J Am Coll Surg. 2015;221:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | O’Neill S, Roebuck A, Khoo E, Wigmore SJ, Harrison EM. A meta-analysis and meta-regression of outcomes including biliary complications in donation after cardiac death liver transplantation. Transpl Int. 2014;27:1159-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Khorsandi SE, Giorgakis E, Vilca-Melendez H, O’Grady J, Heneghan M, Aluvihare V, Suddle A, Agarwal K, Menon K, Prachalias A. Developing a donation after cardiac death risk index for adult and pediatric liver transplantation. World J Transplant. 2017;7:203-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Satapathy SK, Charlton MR. Posttransplant metabolic syndrome: new evidence of an epidemic and recommendations for management. Liver Transpl. 2011;17:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Lunati ME, Grancini V, Agnelli F, Gatti S, Masserini B, Zimbalatti D, Pugliese G, Rossi G, Donato MF, Colombo M. Metabolic syndrome after liver transplantation: short-term prevalence and pre- and post-operative risk factors. Dig Liver Dis. 2013;45:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Tan HL, Lim KB, Iyer SG, Chang SK, Madhavan K, Kow AW. Metabolic syndrome after a liver transplantation in an Asian population. HPB (Oxford). 2015;17:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Morrissey PE, Monaco AP. Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation. 2014;97:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Liu YY, Li CP, Huai MS, Fu XM, Cui Z, Fan LL, Zhang S, Liu Y, Ma J, Li G. Comprehensive comparison of three different immunosuppressive regimens for liver transplant patients with hepatocellular carcinoma: steroid-free immunosuppression, induction immunosuppression and standard immunosuppression. PLoS One. 2015;10:e0120939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7515] [Cited by in RCA: 8452] [Article Influence: 402.5] [Reference Citation Analysis (0)] |
| 10. | WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7065] [Cited by in RCA: 8582] [Article Influence: 390.1] [Reference Citation Analysis (0)] |
| 11. | Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, Nashan B, Peltekian KM. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Bianchi G, Marchesini G, Marzocchi R, Pinna AD, Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008;14:1648-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 544] [Article Influence: 24.7] [Reference Citation Analysis (1)] |
| 14. | Gitto S, Villa E. Non-Alcoholic Fatty Liver Disease and Metabolic Syndrome after Liver Transplant. Int J Mol Sci. 2016;17:490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 16. | Pagadala M, Dasarathy S, Eghtesad B, McCullough AJ. Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15:1662-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Anastácio LR, Diniz KG, Ribeiro HS, Ferreira LG, Lima AS, Correia MI, Vilela EG. Prospective evaluation of metabolic syndrome and its components among long-term liver recipients. Liver Int. 2014;34:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Liu C, Wang X, Chen Z, Zhang L, Wu Y, Zhang Y. Hepatic ischemia-reperfusion induces insulin resistance via down-regulation during the early steps in insulin signaling in rats. Transplant Proc. 2008;40:3330-3334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Hartog H, May CJ, Corbett C, Phillips A, Tomlinson JW, Mergental H, Isaac J, Bramhall S, Mirza DF, Muiesan P. Early occurrence of new-onset diabetes after transplantation is related to type of liver graft and warm ischaemic injury. Liver Int. 2015;35:1739-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Perera MT, Higdon R, Richards DA, Silva MA, Murphy N, Kolker E, Mirza DF. Biomarker differences between cadaveric grafts used in human orthotopic liver transplantation as identified by coulometric electrochemical array detection (CEAD) metabolomics. OMICS. 2014;18:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Anastácio LR, de Oliveira MC, Diniz KG, Ferreira AM, Lima AS, Correia MI, Vilela EG. Adipokines, inflammatory mediators, and insulin-resistance parameters may not be good markers of metabolic syndrome after liver transplant. Nutrition. 2016;32:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Neal DA, Tom BD, Gimson AE, Gibbs P, Alexander GJ. Hyperuricemia, gout, and renal function after liver transplantation. Transplantation. 2001;72:1689-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Stamp L, Searle M, O’Donnell J, Chapman P. Gout in solid organ transplantation: a challenging clinical problem. Drugs. 2005;65:2593-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Layton ME, Wood JG, Yan ZY, Forster J. Ischemia/reperfusion alters uric acid and ascorbic acid levels in liver. J Surg Res. 1996;64:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005;1:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | Leithead JA, Tariciotti L, Gunson B, Holt A, Isaac J, Mirza DF, Bramhall S, Ferguson JW, Muiesan P. Donation after cardiac death liver transplant recipients have an increased frequency of acute kidney injury. Am J Transplant. 2012;12:965-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Rosser BG, Gores GJ. Liver cell necrosis: cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 263] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Fernández L, Heredia N, Grande L, Gómez G, Rimola A, Marco A, Gelpí E, Roselló-Catafau J, Peralta C. Preconditioning protects liver and lung damage in rat liver transplantation: role of xanthine/xanthine oxidase. Hepatology. 2002;36:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Kim KM, Kim SS, Yun S, Lee MS, Han DJ, Yang WS, Park JS, Park SK. Uric acid contributes to glomerular filtration rate deterioration in renal transplantation. Nephron Clin Pract. 2011;118:c136-c142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. 2007;120:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 448] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 31. | Li Q, Lu J, Cao X, Shi TT, Feng JP, Yang JK. An Increase in Normal SUA Level Within the Normal Range Predicts Risk of Metabolic Syndrome, Especially in Women: A Cross-Sectional Study. Horm Metab Res. 2017;49:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Liu Z, Que S, Zhou L, Zheng S. Dose-response Relationship of Serum Uric Acid with Metabolic Syndrome and Non-alcoholic Fatty Liver Disease Incidence: A Meta-analysis of Prospective Studies. Sci Rep. 2015;5:14325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |