Published online Nov 7, 2018. doi: 10.3748/wjg.v24.i41.4698
Peer-review started: August 1, 2018
First decision: August 24, 2018
Revised: September 29, 2018
Accepted: October 16, 2018
Article in press: October 16, 2018
Published online: November 7, 2018
Processing time: 98 Days and 14.9 Hours
To assess clinical outcomes for submucosal (T1b) oesophageal adenocarcinoma (OAC) patients managed with either surgery or endoscopic eradication therapy.
Patients found to have T1b OAC following endoscopic resection between January 2008 to February 2016 at University College London Hospital were retrospectively analysed. Patients were split into low-risk and high-risk groups according to established histopathological criteria and were then further categorised according to whether they underwent surgical resection or conservative management. Study outcomes include the presence of lymph-node metastases, disease-specific mortality and overall survival.
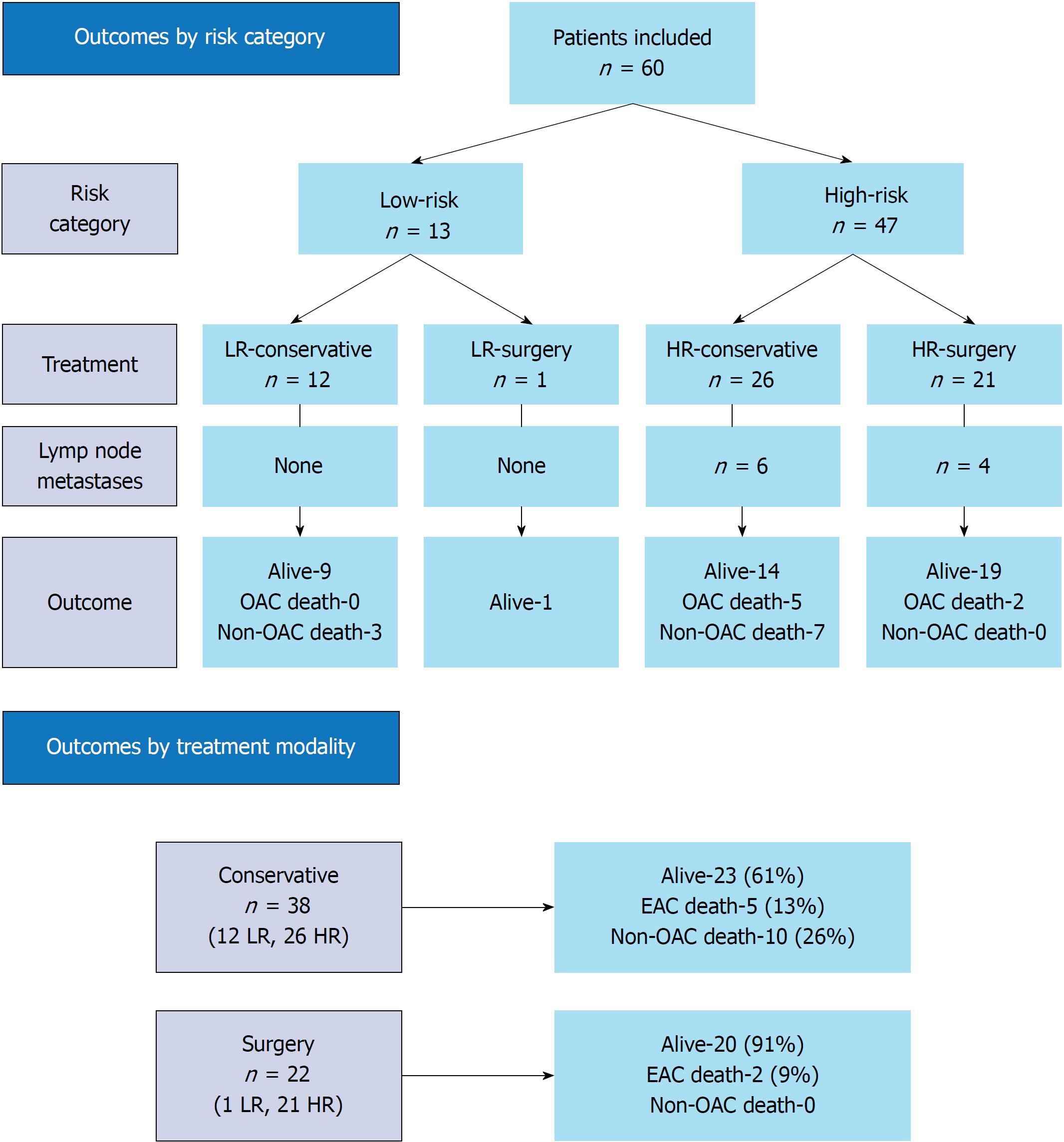
A total of 60 patients were included; 22 patients were surgically managed (1 low-risk and 21 high-risk patients) whilst 38 patients were treated conservatively (12 low-risk and 26 high-risk). Overall, lymph node metastases (LNM) were detected in 10 patients (17%); six of these patients had undergone conservative management and LNM were detected at a median of 4 mo after endoscopic mucosal resection (EMR). All LNM occurred in patients with high-risk lesions and this represented 21% of the total high-risk lesions. Importantly, there was no statistically significant difference in tumor-related deaths between those treated surgically or conservatively (P = 0.636) and disease-specific survival time was also comparable between the two treatment strategies (P = 0.376).
T1b tumours without histopathological high-risk markers of LNM can be treated endoscopically with good out-comes. In selected patients, endoscopic therapy may be appropriate for high-risk lesions.
Core tip: Our retrospective cohort data supports previously published work demonstrating that endoscopic therapy is a safe and effective option for T1b oesophageal adenocarcinoma without markers of high-risk for lymph-node metastasis. Furthermore, our work suggests that endoscopic therapy is a viable alternative to surgery in selected patients with high-risk lesions (particularly those with poor performance status) and highlights the need for further work exploring whether endoscopic therapy could be a viable option for all submucosal lesions.
- Citation: Graham D, Sever N, Magee C, Waddingham W, Banks M, Sweis R, Al-Yousuf H, Mitchison M, Alzoubaidi D, Rodriguez-Justo M, Lovat L, Novelli M, Jansen M, Haidry R. Risk of lymph node metastases in patients with T1b oesophageal adenocarcinoma: A retrospective single centre experience. World J Gastroenterol 2018; 24(41): 4698-4707
- URL: https://www.wjgnet.com/1007-9327/full/v24/i41/4698.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i41.4698
Endoscopic eradication therapy (EET) for the treatment of early neoplasia arising in Barrett’s oesophagus (BE) is widely established to avoid progression to advanced oesophageal adenocarcinoma (OAC). This approach is safe and highly effective with data demonstrating minimal risk to patients with durable long- term disease free survival. Prospective data from several high-volume studies demonstrate our ability to successfully treat BE-related neoplasia has markedly improved over the past 10 years. Following EET the complete resolution of dysplasia is above 90% and the complete resolution of intestinal metaplasia is above 80% at one year[1-4]. Endoscopic resection and subsequent field ablation with radiofrequency ablation (RFA) is the established standard of care for the majority of patients with BE-related neoplasia confined to the mucosa (dysplasia and T1a OAC)[5-7].
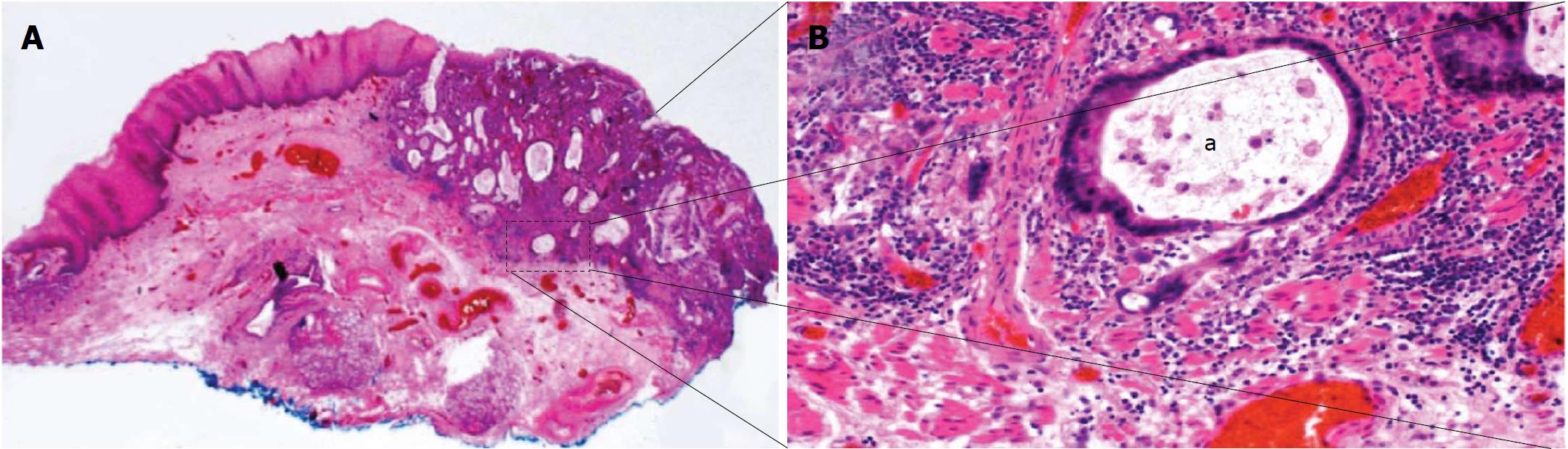
Compared to T1a OAC, the decision making and optimal management strategy for patients with BE neoplasia where lesions have submucosal invasion (T1b) is less well-defined (Figure 1). Historically, these lesions are thought to be associated with a significantly higher risk of subsequent loco-regional lymph node metastases (LNM) compared to T1a OAC and therefore surgery with oesophagectomy and nodal clearance is offered[8-13]. However, emerging data suggest that lesions confined to the uppermost layer of the submucosa (T1bSm1) with low-risk histological features (complete resection, lack of poor tumour differentiation and no lymphovascular invasion) may have a rate of LNM comparable to the 30-d mortality of surgery with oesophagectomy, leaving aside the surgery related morbidity and reduced quality of life that those who undergo this operation endure[8,10,14-19]. Given these figures EET with endoscopic follow-up may therefore be appropriate treatment for these patients with low-risk T1b OAC. Although solid clinical data are lacking, several societies now advocate this conservative management approach for low-risk T1bSm1 OAC patients[5-7]. Surgical resection remains the gold standard treatment for patients who have T1bSm1 lesions with high-risk histological features or in whom the lesion extends beyond the uppermost layer of the submucosa (Sm2/3) in view of the significantly higher rate of subsequent LNM. Emerging data suggest that in selected high-risk T1b patients, most notably those deemed unfit to undergo surgical management, conservative follow-up may be a valid alternative although again larger prospective high-quality studies are lacking[16,18,19].
The aim of this single-centre retrospective cohort study was to assess the risk of LNM in consecutive patients found to have T1b OAC following endoscopic resection and evaluate the short and long term clinical outcomes.
Consecutive patients who were found to have a T1b OAC on endoscopic resection specimen at University College London Hospital from January 2008 to February 2016 were retrospectively analysed for this study. Patients were included if they had OAC arising within BE with submucosal invasion on endoscopic resection specimen. Exclusion criteria were the presence of LNM at baseline staging prior to endoscopic mucosal resection (EMR) or distant metastases on diagnostic staging with adjunct imaging [endoscopic ultrasonography (EUS), computed tomography (CT), positron emission tomography (PET)-CT], follow-up time shorter than 24 mo, and insufficient or incomplete follow-up data. For example if patients with a high-risk visible lesion (large polypoid lesion or ulcerated lesion) would undergo EUS, PET-CT and CT scan. If any were found to have LNM then they would not undergo EMR. Patients were not excluded if they had received previous EET for dysplasia within BE [including photodynamic therapy, argon plasma coagulation (APC), radiofrequency ablation (RFA) or endoscopic resection] prior to the T1b OAC diagnosis.
All endoscopic resections were performed by endoscopic mucosal resection (EMR) with multiband mucosectomy technique (Duette, Cook Endoscopy or Captivator, Boston Scientific).
Resected specimens were reviewed by two expert gastrointestinal pathologists, who reported on each of the following tumour characteristics: Depth of submucosal invasion[15], tumour differentiation, lymphovascular invasion, and presence of tumour cells within or less than 1000 μm from the vertical resection margin. If the initial histopathology report was incomplete or made by a non-expert gastrointestinal histopathologist, the slides were reviewed by a third expert gastrointestinal pathologist for the purpose of this study before the patient was included or excluded. If a tumour characteristic was inconclusive despite re-evaluation it was reported as such and considered a high-risk feature in further assessment.
All patients were discussed at a multidisciplinary meeting (MDM) attended by gastroenterologists, upper gastrointestinal surgeons, oncologists, radiologists and histopathologists. Further treatment plans were formulated based on tumour histopathological characteristics found on EMR specimen, diagnostic staging (EUS and/or CT and/or PET-CT), and a patient’s surgical fitness, medical history and preferences. Patients, with OAC T1b, who were eligible for surgery underwent oesophagectomy with lymphadenectomy. After histopathologic review of surgically resected specimen, some patients received chemoradiotherapy (CRT) if they were upstaged or if there was evidence of LNM on pathology. Conservative treatment included additional endoscopic eradication therapy with field ablation (RFA, APC) or further EMR in an attempt to eradicate residual flat dysplasia and metaplastic BE mucosa.
After the planned EET was concluded and successful eradication of disease histologically confirmed, patients received follow-up by one or more of the following modalities: upper GI endoscopy with biopsies, EUS, CT and/or PET-CT in order to exclude local recurrence, LNM or distant metastases. Patients who had achieved complete eradication of BE-related neoplasia were followed-up endoscopically by 3, 6, 9, 12, 18 and 24 mo intervals and then annually thereafter.
Data were collected from March 2000 until July 2017 or until death of the patient. Information sources included medical history, endoscopic procedures, histopathology reports, imaging studies, outpatient clinic reports, MDM records and correspondence with referring hospitals and general practitioners.
For the purpose of our study, patients were divided into 2 tumour risk groups based on the histopathological assessment of the initial EMR specimens: Low-risk (LR group) and high-risk (HR group). The characteristics of the OAC lesions within the low-risk (LR group) were superficial submucosal invasion (Sm1, < 500 μm), well or moderate tumour differentiation (G1-2), complete resection (R0), and absence of lymphovascular invasion (LVI-). These features have previously been demonstrated to be independent risk factors carrying a favourable prognosis due to a low propensity for metastases[11,13,20]. British, European and American BE neoplasia guidelines employ similar criteria if a conservative approach over surgery is considered, especially in patients who are poor candidates for surgery[5-7].
The OAC lesions within the high-risk (HR group) consisted of tumours exhibiting one or more of the following features: Deep submucosal invasion (Sm2/3, > 500 μm); poor tumour differentiation (G3); lymphovascular invasion (LVI+); or incomplete resection (R1). Tumours were also considered high-risk if any histopathologic feature was indefinite (X), despite re-evaluation by an expert histopathologist.
When combining risk groups with treatment modalities, patients were further divided into LR - conservative treatment (LR-conservative), LR - surgical treatment (LR-surgery), HR - conservative treatment (HR-conservative), and HR - surgical treatment (HR-surgery). A study flowchart is depicted in Figure 2.
The following primary outcomes have been assessed in this study: (1) Incidence of LNM and/or distant metastases after index EMR; (2) survival of patients (time in months from the initial EMR until death or the end of the study); and (3) surgical mortality, OAC-related and non-OAC-related deaths.
Statistical analysis was performed using SPSS software (IBM, ver. 23). Parameters included Kaplan-Meier estimator compared with log-rank (Mantel-Cox) test for survival and average or median with range and/or interquartile range [IQR = first quartile (Q1) - third quartile (Q3)] for continuous variables compared with long-rank (Mantel-Cox) test or Mann-Whitney-Wilcoxon test in case of non-normal distribution. Categorical variables were expressed as percentages and compared with Pearson Chi-Square or Fischer’s exact test.
During the study period 68 potential patients were identified. Eight patients were excluded as they did not meet the inclusion criteria: 6 had short follow-up time (< 24 mo from EMR), one had insufficient follow-up data and one patient was upstaged after histopathologic review (T2 - muscularis propria invasion). A total of 60 patients were included in the study, 85% were male and the mean age at the time of the initial EMR was 70 years (range 66-75). The mean length of BE circumference (C) at baseline was 5 cm (range 0-13) and maximum (M) 7 cm (range 1-15). Sixty one percent of patients were under endoscopic surveillance for BE when the T1b OAC lesion was detected. The median period of time within the BE surveillance programme was 6 years (IQR 3-9) (Table 1). Based on the aforementioned histopathological criteria, 13/60 (22%) patients had LR tumours and 47/60 (78%) had HR tumours (Table 2).
| Patients | |
| Total, n | 60 (100%) |
| Average age at EMR (IQR) | 70 (66-75) |
| Gender | |
| Male | 51 (85%) |
| Female | 9 (15%) |
| Barrett's oesophagus | |
| Circumference (range) | 5 (0-13) |
| Maximum (range) | 7 (1-15) |
| In surveillance program | |
| Yes, median years (IQR) | 36 (61%), 6 (3-9) |
| No, new finding | 19 (32%) |
| Data N/A | 5 (7%) |
| Tumour location | |
| Oesophagus | 55 (92) |
| Cardia (Siewert 2) | 5 (8) |
| Tumour histopathology | |
| Differentiation | |
| Well (G1) | 2 (3) |
| Moderate (G2) | 28 (47) |
| Poor (G3) | 29 (49) |
| X (GX) | 1 (1) |
| Depth of submucosal invasion | |
| Sm1 (< 500 μm) | 25 (42) |
| Sm2/3 (> 500 μm) | 25 (42) |
| X | 10 (16) |
| Lymphovascular invasion | |
| Negative (LVI-) | 40 (67) |
| Positive (LVI+) | 14 (23) |
| X (LVX) | 6 (10) |
| Resection (vertical margin) | |
| Complete (R0) | 17 (28) |
| Incomplete (R1) | 43 (84) |
| Tumour risk group | |
| Low-risk (LR) | 13 (22) |
| High-risk (HR) | 47 (78) |
After initial EMR 22/60 (37%) patients underwent oesophagectomy; one patient from the LR group and 21 patients from the HR group. There were 38/60 (63%) patients treated conservatively; 12 patients from the LR group and 26 patients from the HR group (Figure 2).
There was no surgical mortality observed in our cohort. Most patients had an Ivor-Lewis procedure (18/22), 2 patients underwent oesophagogastrectomy, one patient had a three stage oesophagectomy, and one patient had a transhiatal resection. Surgery was performed at an average of 3 mo (range 1-10) after the EMR. Residual OAC after EMR was found in 14/22 (64%) surgically resected specimens and staged as T1a (4 patients), T1b (7 patients), T2 (2 patients) and T3 (1 patient). All these patients had an incompletely resected (R1) tumour on initial EMR. In 7/22 (32%) patients who underwent surgery, there was no evidence of residual tumour in the surgical resection specimen despite being classified as R1 on initial EMR. One patient with R0 EMR also had no residual cancer in surgical specimen confirming the initial histopathological assessment. One patient went on to have CRT following surgery. This patient had an R1 surgical resection of a T2 tumour with cancer cells present at less than 1 mm from the oesophageal resection margin. Residual dysplasia in the surgical margin was reported in 4/22 (18%) patients. Case specific details are summarized in Table 3.
| Case | Endoscopic resection specimen | Surgical resection specimen | Note |
| 1 | Sm1, G3, LVI-, R1 | T1a, LVI-, R0 | / |
| 2 | Sm1, G3, LVI-, R1 | T1b Sm1, G3, R0 | / |
| 3 | SmX, G2, LVI-, R1 | T1b, G3, LVI-, R0 | / |
| 4 | Sm2/3, G3, LVI-, R1 | No residual cancer | LGD in surgical margin |
| 5 | Sm1, G2, LVI-, R1 | No residual cancer | HGD in surgical margin |
| 6 | Sm1, G3, LVI-, R1 | T1b, G2, R0 | / |
| 7 | Sm2/3, G3, LVI+, R1 | T1a, G3, R0 | Positive for LNM (2/16) |
| 8 | Sm2/3, G2, LVI-, R1 | No residual cancer | LGD in surgical margin |
| 9 | Sm2/3, G3, LVI-, R1 | No residual cancer | / |
| 10 | Sm2/3, G3, LVI-, R1 | T1a, G3, LVI+, R0 | / |
| 11 | Sm2/3, G3, LVIX, R1 | T1b, G2, R0 | Positive for LNM (3/12) |
| 12 | Sm2/3, G3, LVI+, R1 | T2, G3, R1 | R1, negative for LNM (16), preop EUS T1b |
| 13 | Sm2/3, G2, LVI+, R1 | No residual cancer | / |
| 14 | Sm1, G3, LVI+, R1 | T1b, G1, LVI-, R0 | / |
| 15 | SmX, G3, LVI+, R1 | T1b, G3, R0 | Positive for LNM (1/23) |
| 16 | Sm2/3, G3, LVI+, R1 | No residual cancer | / |
| 17 | Sm1, G3, LVIX, R1 | T2, R0 | Preop EUS T1 stage |
| 18 | SmX, G3, LVI+, R1 | T3, G3, R0 | Positive for LNM (3/78), preop EUS T1b |
| 19 | Sm2/3, G3, LVI-, R1 | T1a, G3, R0 | / |
| 20 | Sm2/3, G2, LVI-, R1 | T1, R0 | / |
| 21 | SmX, G3, LVI-, R1 | No residual cancer | / |
| 22 | Sm1, G2, LVI-, R0 | No residual cancer | LR tumour, HGD in surgical margin |
A median of 20 lymph nodes (IQR 15-24, range 11-55) were retrieved per surgical patient. LNM were found in 4/22 (18%) of the surgical resection specimens. These four patients all had G3 tumours, three of which showed lymphovascular invasion and one where this could not be determined (LVIX). Two of these four cases were Sm2/3 and the other two were SmX. None of the patients with an Sm1 tumour, regardless of other histological markers of risk, was found to have LNM. In addition, none of the patients who did not have lymphovascular invasion on the EMR specimen developed LNM. When stratified by histological high-risk feature, 9/22 had LVI+ or LVIX and of these 9 patients, 4 patients were found to have LNM (44%); 16/22 patients had G3 tumours and of these, 4 had LNM (25%). In total 8/22 tumours were G3 and showed LVI+ or LVIX. Thus 4/8 (50%) of these had LNM.
Three of the 4 patients found to have LNM on their surgical resection specimen received adjuvant CRT. Two patients have died of their disease and 2 patients were alive at last follow-up, 87 and 55 mo after their initial EMR.
A total of 38/60 (63%) patients were treated with conservative endoscopic follow-up. Twelve of these patients had LR disease and 26 had HR disease. It was concluded at the MDM that 11 of 12 patients with LR tumours had been cured of OAC and endoscopic follow-up was indicated. One patient underwent radiotherapy to reduce the risk of metachronous LNM. This patient was alive and disease-free at most recent follow-up.
The main indication for non-surgical treatment of patients with HR tumours (24 of 26 patients) was poor surgical fitness due to co-morbidities and high anaesthetic risk; 2 surgically fit patients chose a conservative approach after discussing the long-term risk for cancer recurrence and LNM. Of the 26 HR tumours, 7 patients had additional RFA and/or EMR of the remaining BE segment, 14 patients received adjuvant CRT, whilst five patients had regular endoscopic follow-up alone.
In 6/38 (16%) patients treated conservatively, LNM were detected at 3, 4, 4, ,13, 17 and 38 mo after the EMR. These LNM were detected with PET CT and EUS and 4 of the 6 patients had LN sampling to confirm LNM. These were all HR lesions and represent 23% of HR tumours treated conservatively (Table 4). All 6 of these patients received CRT once the LNM were identified. 1 of these 6 also underwent an EMR for a visible oesophageal lesion. This latter patient remains alive after 26-mo follow-up, whereas the 5 others have died of their disease. Of these 6 patients, all had an R1 EMR resection specimen; 5 of 6 patients had Sm2/3 tumours; 3 of 6 patients either had LVI+ or were LVIX; and 2 of 6 patients were G3.
| Case | EMR tumour histopathology | Treatment after index EMR | Time till metastases (mo)1 | Additional treatment | End study outcome | Cause of death | Survival2 (mo) |
| 1 | Sm2/3, G3, LVIX, R1 | Surgery | 4 | Chemotherapy | Deceased | OAC | 45 |
| 2 | SmX, G3, LVI+, R1 | Surgery | 2 | None | Alive | / | 87 |
| 3 | SmX, G3, LVI+, R1 | Surgery | 4 | CRT | Deceased | OAC | 8 |
| 4 | Sm2/3, G3, LVI+, R1 | Surgery | 2 | Chemotherapy | Alive | / | 55 |
| 5 | Sm2/3, G3, LVIX, R1 | Conservative | 38 | CRT | Deceased | OAC | 40 |
| 6 | Sm2/3, G2, LVI+, R1 | Conservative | 4 | CRT | Deceased | OAC | 9 |
| 7 | Sm2/3, G2, LVI-, R1 | Conservative | 3 | CRT | Deceased | OAC | 31 |
| 8 | Sm2/3, G2, LVI+, R1 | Conservative | 4 | CRT | Deceased | OAC | 18 |
| 9 | Sm1, G2, LVI-, R1 | Conservative | 17 | EMR T1a, CRT | Alive | / | 26 |
| 10 | Sm2/3, G3, LVI-, R1 | Conservative | 13 | Radiotherapy | Deceased | OAC | 30 |
LNM were detected in 10 of 60 (17%) patients. With regard to treatment modality, LNM were detected in 4/22 (18%) patients treated surgically and 6/38 (16%) treated conservatively. All patients with LNM had a HR OAC (10 of 47, 21%). None of the 13 patients with a LR lesion developed metastatic disease during follow-up. When stratified by risk factor, 1/25 (4%) of Sm1 cancers compared with 9/35 (26%) of Sm2/3/X lesions showed LNM (P = 0.035); 3/40 (8%) with no LVI compared with 7/20 (35%) positive for LVI/X tumours showed LNM (P = 0.012); 4/30 (13%) of G1/2 tumours compared with 6/30 (20%) of G3 tumours showed LNM (P = 0.488); and 0/17 (0%) of R0 tumours compared with 10/43 (23%) for R1 tumours showed LNM (P = 0.049). An overview of all patients with LNM is provided in Table 4.
At the end of the study 43 of 60 (73%) patients were alive with a median follow-up time of 45 mo (IQR 32-72; range 24-102). The median survival of the 17 of 60 (27%) deceased patients was 24 mo (IQR 9-40; range 3-59) from EMR diagnosis of pT1b OAC. Of these 17 deceased patients, 7 (42%) died of OAC and 10 (58%) died of non-OAC-related conditions: One patient died after a cerebrovascular accident, 2 patients died of renal cancer, and 7 patients died due to complications of ischaemic heart disease.
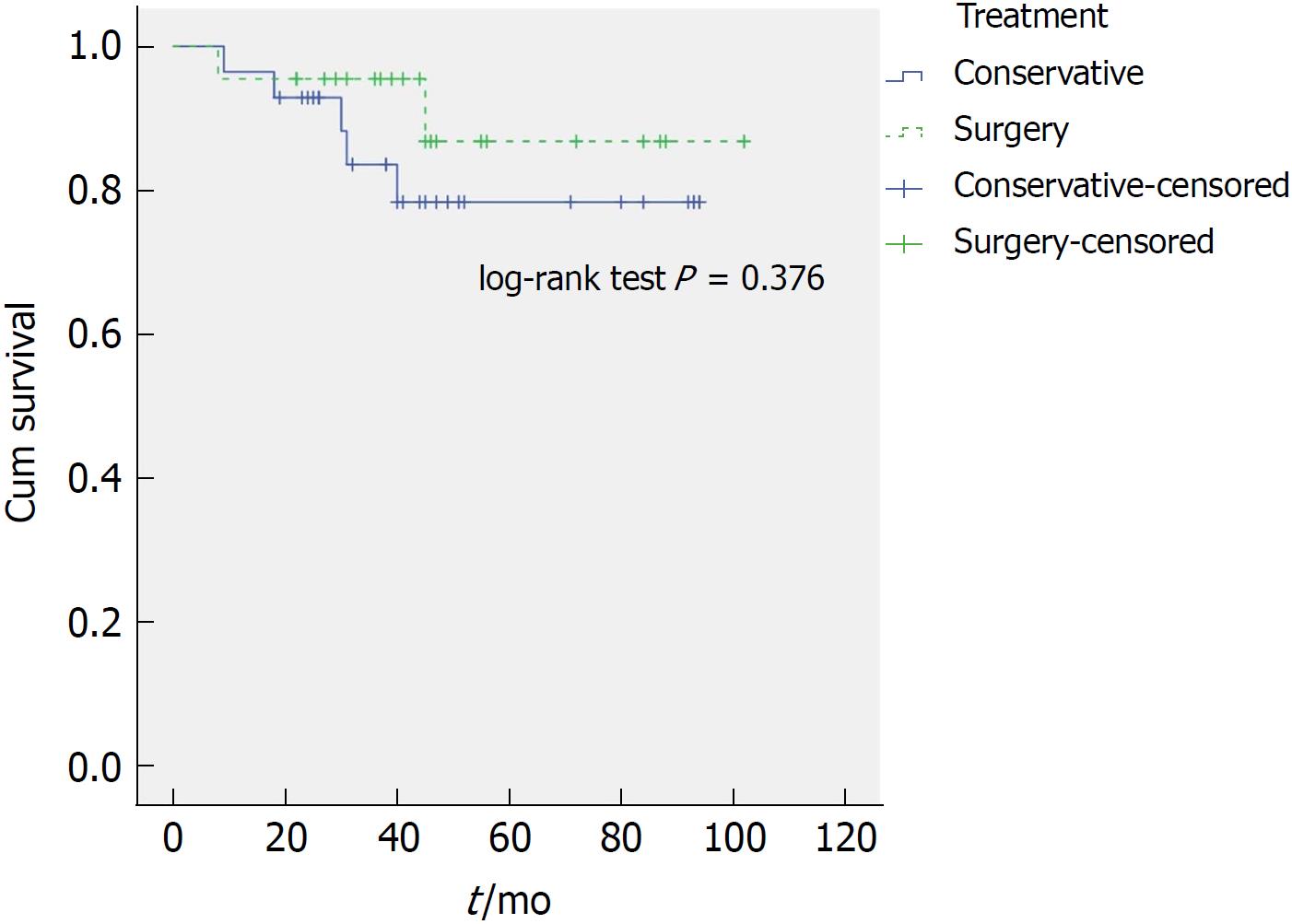
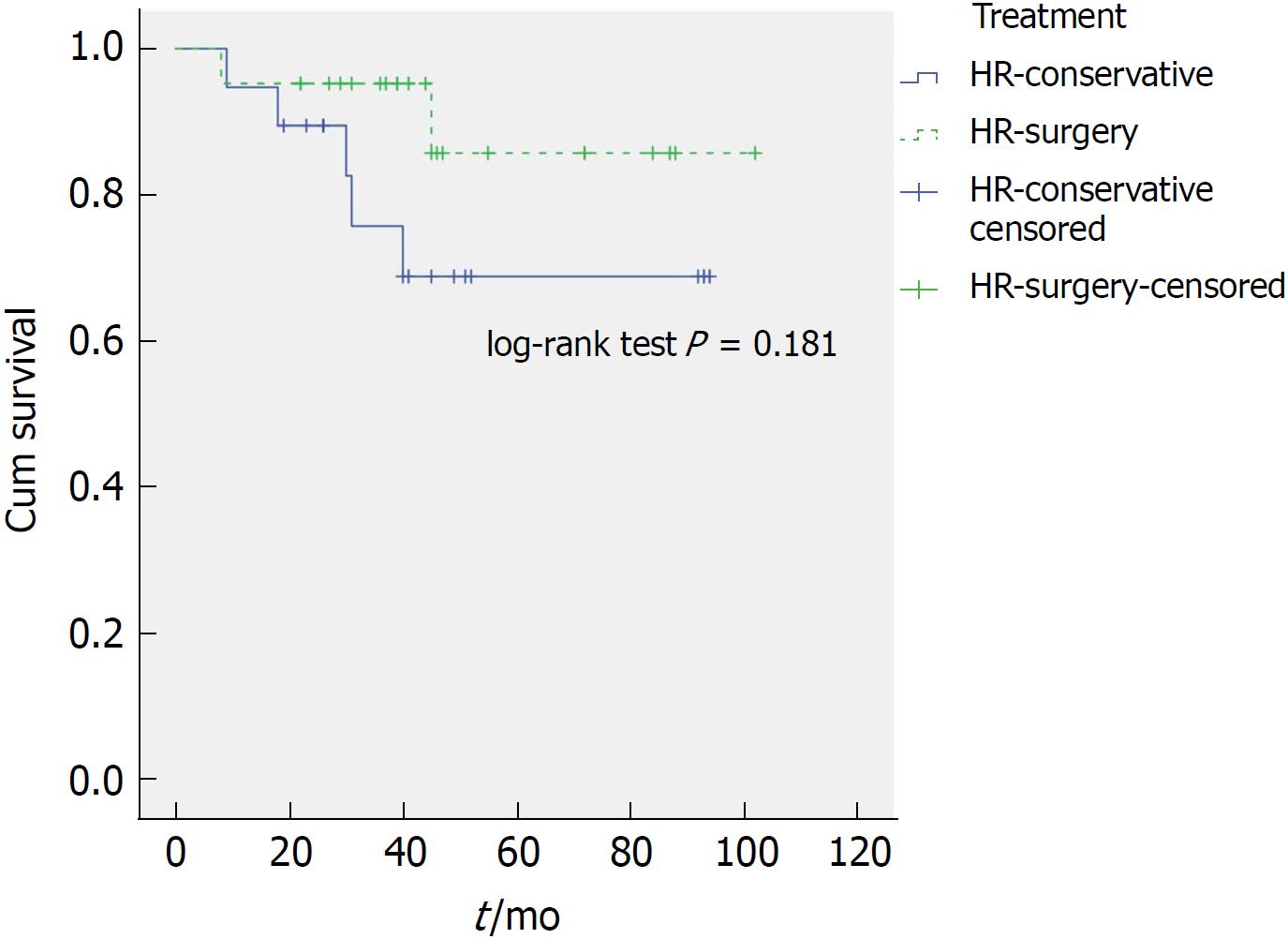
Within the HR group 14 of 47 patients (30%) died during the follow-up period. Of these, 7 died of OAC. We found no significant difference for OAC-related deaths during the follow-up period when comparing treatment modality. 5/38 patients treated conservatively (13%) died of OAC whilst 2/22 patients (9%) of those treated surgically died of OAC (P = 0.636). Importantly, OAC-related survival time remains comparable (P = 0.436) when one compares only the HR patients that underwent surgery (2/22) to the HR patients that received conservative management (5/26). The median overall survival of patients in the surgery group compared to patients in the conservative group was better, 45 mo (IQR 32-56; range 8-102) and 39 mo (IQR 24-50; range 3-94), respectively (P = 0.019). However, after excluding patients who died of non-OAC-related causes (disease-specific survival), the difference was no longer statistically significant (P = 0.376) (Figure 3). Patients in HR-surgery group similarly had a better overall survival compared to patients in HR-conservative group: Median of 44 mo (IQR 31-55, range 8-102) and 36 mo (IQR 23-50, range 3-94), respectively (P = 0.012). Again, after excluding patients who died of non-OAC-related causes, the difference was no longer statistically significant (P = 0.181) (Figure 4).
During the follow-up period 3/60 (5%) patients developed a local recurrence, 1 patient from the LR group and 2 patients from the HR group. The patient within the LR group had undergone an oesophagectomy as baseline therapy. The surgical resection specimen demonstrated residual HGD and the patient developed a T1a M3 tumour at the anastomosis after 47 mo. This was successfully resected by EMR and the patient has survived to 50 mo at the end of the study. In the HR group, two patients with an R1 EMR specimen who were treated conservatively developed T1a and T1bSm1 tumours at the site of previous resection after 6 and 10 mo, respectively. Both tumours were successfully resected by EMR. One of these individuals was also found to have LNM and received CRT. Both of these patients were alive at last follow-up.
One of 12 patients within the LR-conservative group received CRT following MDT discussion. 9/12 patients remain alive with those who have died doing so from a cause unrelated to OAC (renal cancer, ischaemic heart disease and a cerebrovascular accident). 13 of 26 (50%) patients within the HR-conservative group received CRT. Of those who received CRT 7 have died although 5 (38%) of those were due to OAC. Of those who did not receive CRT 5 patients have died, although none of these deaths were due to OAC.
This single centre retrospective analysis of 60 patients with T1b OAC suggests that patients with Sm1 tumours without high-risk histopathological markers can be treated endoscopically with good long-term outcomes. This is in keeping with other recently published studies. Within this LR group, none of the 13 patients had or developed LNM within the follow-up period. 12 patients were treated conservatively and the one patient who had surgical treatment was found to have a local recurrence, successfully endoscopically resected, 47 mo after the initial EMR. These data support the emerging treatment strategy that patients with LR T1b lesions should be offered EET and conservative treatment.
Of the patients with HR lesions that underwent surgical resection, 4 (18%) were found to have LNM within their resection specimens. All 4 of these were G3 tumours with either LVI or were LVIX. Of those who had the combination of both of these high-risk histopathological features 50% (4/8) developed LNM suggesting that these patients are high-risk and EET carries higher risk of LNM and recurrence. None of the Sm1 tumours with only one high-risk histopathological feature had LNM. Although the numbers are too small to recommend changes to the existing treatment strategy, this data suggest that endoscopic resection and conservative management may be an appropriate approach for some patients categorised as high-risk where only one high-risk pathological feature is confirmed. This is highlighted by the fact that only 23% of HR tumours treated conservatively developed LNM during the follow-up period. Further prospective RCT studies of surgical versus conservative treatment strategies with higher patient numbers are required to explore this potential change in treatment paradigm.
Our data on LR and HR T1b cancers and clinical outcomes are similar to the limited published series on this patient cohort. Manner et al[16] examined 72 patients with pT1bSm1 pathology on EMR specimens retrospectively over a 14 year period form their centre. These authors also differentiated LR and HR lesions based on criteria similar to our cohort. In the LR patients (n = 49) only one patient developed LNM (2%) at follow-up and in the HR group (n = 22) only two patients developed LNM (8.7%). In a similar study, Scholvinck et al[18] analysed 69 patients with pT1b cancers, with their LR group having no LMN detected at follow-up, whereas the HR group displayed an overall LNM rate of 16% which is similar to our series.
Clearly the decision-making in offering patients conservative treatment with EET or more radical therapy with surgery is complex and multifaceted with factors such as a patient’s fitness for surgery and patient preference influencing the final decision. The main advantage of surgery is that the entire organ and draining lymph nodes are cleared minimising LNM risk. However, even in high-volume expert centres, the 30-d mortality from oesophagectomy ranges between 2% and 5 %. There were no surgery-related deaths in our cohort, but the argument for approaching LR T1b OAC with endoscopic therapy is based on the published data where the rate on LNM is lower than that of surgical mortality.
There was no statistically significant disease-specific survival benefit for patients treated surgically compared to patients treated conservatively in our study. Even in the high-risk patient group, although a trend toward better survival of surgery patients was observed, this was not statistically significant. Furthermore, OAC-related deaths were comparable in both groups. Here, we should highlight the high mortality of patients in our cohort due to conditions not related to OAC. There were 26% of patients in the conservative group who died of non-OAC-related disease (none in the surgery group) and only 13% died of OAC. It is important not to over-treat cancer in these patients. Furthermore, we observed that 2 out of 4 patients died of OAC despite oesophagectomy and removal of positive local lymph nodes. Thus, in these data, surgery did not always cure T1b OAC. One possible explanation is that the tumour had spread to lymph nodes outside the surgical resection field. Alternatively, a recently published study on metastatic colorectal cancer proposes a model that some distant metastases might arise independently of lymph node metastases[21]. If this were true for OAC, oesophagectomy with nodal clearance would not prevent metastatic disease due to haematogenous cancer spread. In this case (neoadjuvant) chemotherapy might be the preferred treatment.
An unexpected feature in our cohort was a high percentage (84%) of incomplete (R1) endoscopic mucosal resections. This was at least partly due to our histopathologic criteria of at least 1 mm clear resection margin, which is difficult to achieve by EMR technique with tumours invading relatively thin oesophageal submucosal layer of approximately 1-1.5 mm. Thus, the percentage of R1 endoscopic resections in our cohort might have been overestimated. The evidence supporting this is that 32% of all patients in the surgery group (7/22) who were classified as R1 on EMR had no residual cancer identified in the surgical resection specimen. In retrospect, even though none of these patients developed metastatic disease, surgery should not be considered over-treatment as all of these tumours, apart from 1, demonstrated other high-risk features. Possible overestimation of R1 endoscopic resections could also explain the relatively low rate of local recurrence and metastatic disease in patients with R1 EMR, who did not undergo additional resection (surgical or endoscopic).
In conclusion, endoscopic therapy appears to be equal if not preferable to surgery in submucosal OAC with low-risk histopathologic features. In high-risk OAC, a conservative approach is a viable alternative to surgery, especially for selected patients and those with poor performance status. Further prospective studies are required to provide solid clinical evidence on this important issue.
This study provides long-term outcome data on patients with submucosal oesophageal adenocarcinoma.
The optimal management of submucosal oesophageal adenocarcinoma is not clearly defined. Data suggests endoscopic therapy may be a viable alternative to surgery and thus radically change the treatment paradigm.
To analyse our data from a large tertiary specialist centre on the management of patients with submucosal oesophageal adenocarcinoma in order to support the potential for endoscopic therapy for these patients. In addition, we feel our work promotes the need for a large-scale multi-centre trial exploring endoscopic therapy for submucosal lesions.
This was a retrospective cohort study that uniquely offers long-term outcomes on patients with high-risk and low-risk oesophageal submucosal lesions who received surgery and endoscopic therapy.
Lymph node metastases were detected in 18% of patients who had undergone conservative management. There was no statistically significant difference in tumour-related deaths between those treated surgically or conservatively and disease-specific survival time was also comparable between the two treatment strategies.
This study provides supporting data for the potential of endoscopic therapy in the management of submucosal oesophageal adenocarcinoma. In particular, the work suggests that endoscopic therapy may be a viable alternative to surgery in selected patients. This work could support a change to the treatment strategy for submucosal lesions.
Low-risk submucosal lesions can be safely treated endoscopically whilst our research suggests that endoscopic therapy may be a viable option for high-risk lesions. This study supports the need for a large-scale multicentre study addressing this uncertainty in the treatment paradigm.
| 1. | Pech O, May A, Manner H, Behrens A, Pohl J, Weferling M, Hartmann U, Manner N, Huijsmans J, Gossner L. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146:652-660.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 323] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 2. | Haidry RJ, Dunn JM, Butt MA, Burnell MG, Gupta A, Green S, Miah H, Smart HL, Bhandari P, Smith LA. Radiofrequency ablation and endoscopic mucosal resection for dysplastic barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology. 2013;145:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Haidry RJ, Butt MA, Dunn JM, Gupta A, Lipman G, Smart HL, Bhandari P, Smith L, Willert R, Fullarton G. Improvement over time in outcomes for patients undergoing endoscopic therapy for Barrett’s oesophagus-related neoplasia: 6-year experience from the first 500 patients treated in the UK patient registry. Gut. 2015;64:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Luigiano C, Iabichino G, Eusebi LH, Arena M, Consolo P, Morace C, Opocher E, Mangiavillano B. Outcomes of Radiofrequency Ablation for Dysplastic Barrett’s Esophagus: A Comprehensive Review. Gastroenterol Res Pract. 2016;2016:4249510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 909] [Article Influence: 75.8] [Reference Citation Analysis (1)] |
| 6. | Weusten B, Bisschops R, Coron E, Dinis-Ribeiro M, Dumonceau JM, Esteban JM, Hassan C, Pech O, Repici A, Bergman J. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 386] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 7. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1081] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 8. | Hölscher AH, Bollschweiler E, Schneider PM, Siewert JR. Early adenocarcinoma in Barrett’s oesophagus. Br J Surg. 1997;84:1470-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 93] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Rice TW, Zuccaro G Jr, Adelstein DJ, Rybicki LA, Blackstone EH, Goldblum JR. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg. 1998;65:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 261] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Stein HJ, Feith M, Bruecher BL, Naehrig J, Sarbia M, Siewert JR. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566-573; discussion 573-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 325] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 11. | Ancona E, Rampado S, Cassaro M, Battaglia G, Ruol A, Castoro C, Portale G, Cavallin F, Rugge M. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol. 2008;15:3278-3288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Badreddine RJ, Prasad GA, Lewis JT, Lutzke LS, Borkenhagen LS, Dunagan KT, Wang KK. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin Gastroenterol Hepatol. 2010;8:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Leers JM, DeMeester SR, Oezcelik A, Klipfel N, Ayazi S, Abate E, Zehetner J, Lipham JC, Chan L, Hagen JA. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 14. | Braghetto I, Csendes A, Cardemil G, Burdiles P, Korn O, Valladares H. Open transthoracic or transhiatal esophagectomy versus minimally invasive esophagectomy in terms of morbidity, mortality and survival. Surg Endosc. 2006;20:1681-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Fotis D, Doukas M, Wijnhoven BP, Didden P, Biermann K, Bruno MJ, Koch AD. Submucosal invasion and risk of lymph node invasion in early Barrett’s cancer: potential impact of different classification systems on patient management. United European Gastroenterol J. 2015;3:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Manner H, Pech O, Heldmann Y, May A, Pauthner M, Lorenz D, Fisseler-Eckhoff A, Stolte M, Vieth M, Ell C. The frequency of lymph node metastasis in early-stage adenocarcinoma of the esophagus with incipient submucosal invasion (pT1b sm1) depending on histological risk patterns. Surg Endosc. 2015;29:1888-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Chadwick G, Varagunam M, Brand C, Cromwell D, Maynard M, Riley S, Crosby T, Michalowski J. National Oesophago-Gastric Cancer Audit 2016. R Coll Surg Engl. 2016;. |
| 18. | Schölvinck D, Künzli H, Meijer S, Seldenrijk K, van Berge Henegouwen M, Bergman J, Weusten B. Management of patients with T1b esophageal adenocarcinoma: a retrospective cohort study on patient management and risk of metastatic disease. Surg Endosc. 2016;30:4102-4113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Manner H, Wetzka J, May A, Pauthner M, Pech O, Fisseler-Eckhoff A, Stolte M, Vieth M, Lorenz D, Ell C. Early-stage adenocarcinoma of the esophagus with mid to deep submucosal invasion (pT1b sm2-3): the frequency of lymph-node metastasis depends on macroscopic and histological risk patterns. Dis Esophagus. 2017;30:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Boys JA, Worrell SG, Chandrasoma P, Vallone JG, Maru DM, Zhang L, Blackmon SH, Dickinson KJ, Dunst CM, Hofstetter WL. Can the Risk of Lymph Node Metastases Be Gauged in Endoscopically Resected Submucosal Esophageal Adenocarcinomas? A Multi-Center Study. J Gastrointest Surg. 2016;20:6-12; discussion 12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, Cai T, Clevers H, Swanton C, Nowak MA. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 393] [Article Influence: 43.7] [Reference Citation Analysis (15)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bratlie SO, Dinç T S- Editor: Ma RY L- Editor: A E- Editor: Yin SY