Published online Oct 28, 2018. doi: 10.3748/wjg.v24.i40.4606
Peer-review started: July 23, 2018
First decision: August 25, 2018
Revised: September 6, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: October 28, 2018
Processing time: 99 Days and 15.2 Hours
To investigate survival rate and incidence of hepatocellular carcinoma (HCC) in patients with decompensated cirrhosis in the antiviral era.
We used the Korean Health Insurance Review and Assessment. Korea’s health insurance system is a public single-payer system. The study population consisted of 286871 patients who were prescribed hepatitis B antiviral therapy for the first time between 2007 and 2014 in accordance with the insurance guidelines. Overall, 48365 antiviral treatment-naïve patients treated between 2008 and 2009 were included, and each had a follow-up period ≥ 5 years. Data were analyzed for the 1st decompensated chronic hepatitis B (CHB) and treatment-naïve patients (n = 7166).
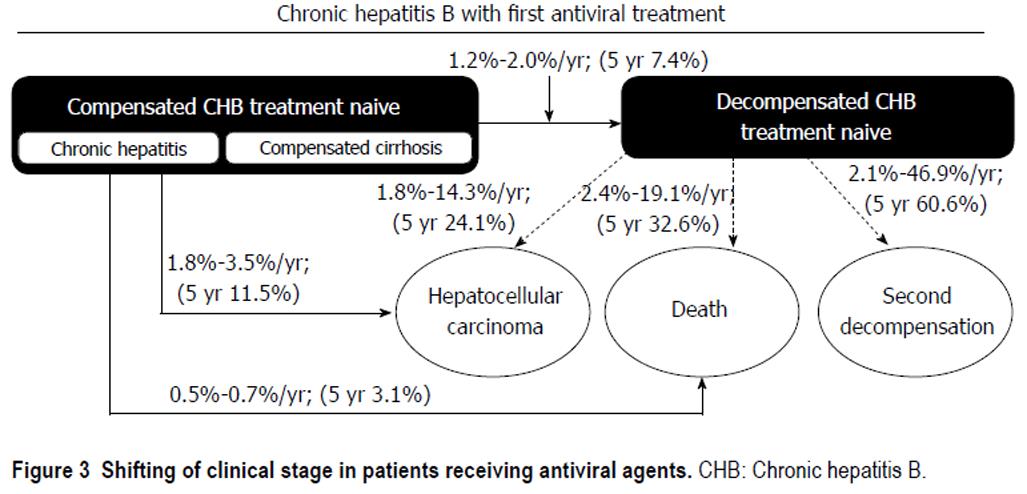
The mean patient age was 43.5 years. The annual mortality rates were 2.4%-19.1%, and 5-year cumulative mortality rate was 32.6% in 1st decompensated CHB treatment-naïve subjects. But the annual mortality rates sharply decreased to 3.4% (2.4%-4.9%, 2-5 year) after one year of antiviral treatment. Incidence of HCC at first year was 14.3%, the annual incidence of HCC decreased to 2.5% (1.8%-3.7%, 2-5 year) after one year. 5-year cumulative incidence of HCC was 24.1%. Recurrence rate of decompensated event was 46.9% at first year, but the annual incidence of second decompensation events in decompensated CHB treatment-naïve patients was 3.4% (2.1%-5.4%, 2-5 year) after one year antiviral treatment. 5-year cumulative recurrence rate of decompensated events was 60.6%. Meanwhile, 5-year cumulative mortality rate was 3.1%, and 5-year cumulative incidence of HCC was 11.5% in compensated CHB treatment-naïve patients.
Long term outcome of decompensated cirrhosis treated with antiviral agent improved much, and incidence of hepatocellular carcinoma and mortality sharply decreased after one year treatment.
Core tip: It is well known that antiviral treatment improves clinical outcomes of chronic hepatitis B-associated decompensated cirrhosis. However, long term and large scale clinical data regarding survival rate, and incidence of hepatocellular carcinoma in patients with decompensated cirrhosis in the antiviral era are lacking. We investigated the survival rate and incidence of hepatocellular carcinoma (HCC) in patients with decompensated cirrhosis by using the Health Insurance Review and Assessment database. Long term outcome of treating hepatitis B-associated decompensated cirrhosis using antiviral agents improved much compare to previous reports. Cumulative mortality rate and incidence of HCC was sharply decreased after one year antiviral treatment.
- Citation: Ju YC, Jun DW, Choi J, Saeed WK, Lee HY, Oh HW. Long term outcome of antiviral therapy in patients with hepatitis B associated decompensated cirrhosis. World J Gastroenterol 2018; 24(40): 4606-4614
- URL: https://www.wjgnet.com/1007-9327/full/v24/i40/4606.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i40.4606
There are many studies on the natural course of chronic hepatitis B (CHB)[1,2]. A population-based cohort study reported an 11% annual incidence of decompensated complications in patients with compensated chronic liver disease[1]. In patients with decompensated liver disease, the risk of hepatocellular carcinoma (HCC) and mortality increases by 7%-8% and up to 20%-50% annually, respectively[2].
It is clear that antiviral therapy reduces liver disease progression and mortality in decompensated CHB patients[3-5]. However, clinical data for long-term survival rate, the incidence of HCC, and the recurrence of decompensated events in patients with decompensated cirrhosis receiving antiviral agents are still lacking in the antiviral era.
A landmark study of clinical outcomes of CHB-associated decompensated cirrhosis was recently published[6,7]. Jang et al[6] evaluated the effects of antiviral therapy on mortality rate in decompensated cirrhosis patients and reported a 5-year survival rate of 423 decompensated cirrhosis patients of 59.7%. However, the sample size was small, and the follow-up period was short. Moreover, all patients were treated in a tertiary hospital setting and the selection bias was unclear. There are several meta-analyses on the effects of antiviral agents in patients with CHB-associated decompensated cirrhosis[8-11]. However, only three studies to date have included > 100 patients for > 1 year[6,12,13], and few studies have used a highly potent viral nucleos(t)ide analog (entecavir or tenofovir).
Here we used a nationwide database to investigate the long-term mortality rate and incidence of HCC in patients with CHB-associated decompensated cirrhosis who received antiviral agents.
This study used insurance reimbursement claims data provided by the Health Insurance Review and Assessment (HIRA). The health insurance claims data are generated when a health care provider submits a reimbursement claim to HIRA for payment of the portion of the medical services provided to the patient covered by the National Health Insurance. Korea’s health insurance system is a public single-payer system. 97.2% of the total population in Korean people is enrolled in national insurance system. Health care providers are automatically eligible and obliged to treat patients for services covered under the system. The system is controlled by the government[14].
This retrospective cohort study used HIRA claims data from January 2007 to December 2014. The provided data were approved by the National Health Insurance Service following a review process, and the data were released with an encrypted number according to the disclosure principle. The study was conducted with approval from the institutional review board (IRB approval: HYUH 2017-04-006).
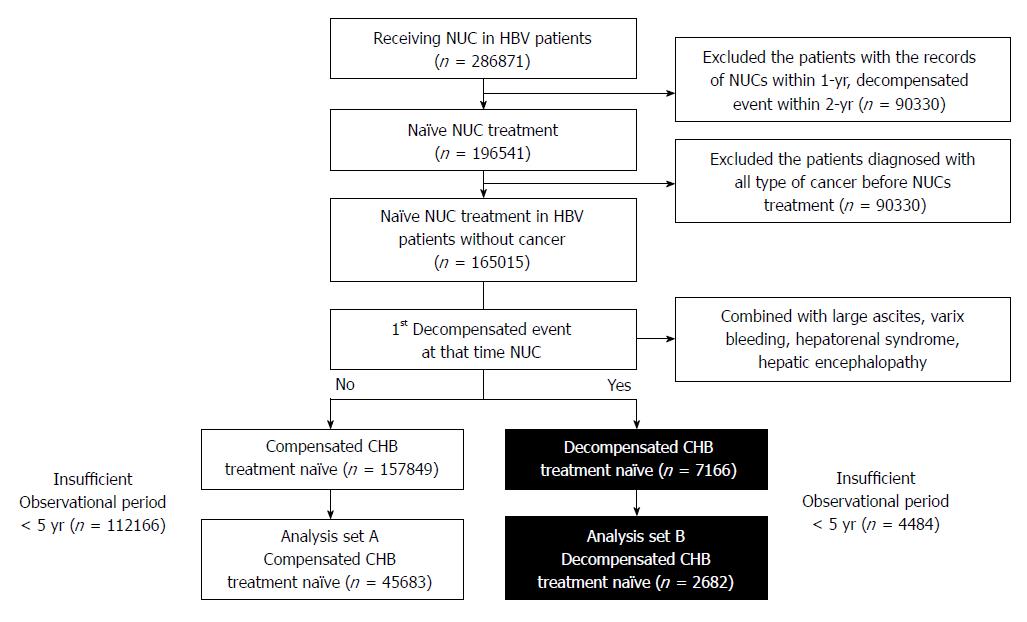
The study population consisted of 286871 patients who were prescribed hepatitis B antiviral therapy for the first time between 2007 and 2014 in accordance with the insurance guidelines. Overall, 48365 antiviral treatment-naïve patients treated between 2008 and 2009 were included, and each had a follow-up period ≥ 5 years.
The inclusion criteria were as follows: (1) CHB patients who were prescribed nucleos(t)ide analogs for the first time under the national reimbursement policy. The HIRA reimbursement criteria were as follows: hepatitis B surface antigen (HBsAg)-positive for ≥ 6 mo, aminotransferase activity ≥ 80 U/L, and baseline hepatitis B e antigen (HBeAg)-positive with hepatitis B virus (HBV)-DNA ≥ 100000 copies/mL or HBeAg-negative with HBV-DNA ≥ 10000 copies/mL; (2) prescribed oral antiviral agents for > 90 d; and (3) an observational period for ≥ 5 years after the initial use of an oral antiviral agent. All subjects were nucleos(t)ide analog-naïve patients.
Patients meeting any of the following criteria were excluded: (1) prescription of oral antiviral agents (lamivudine, clevudine, adefovir, telbivudine, entecavir, or tenofovir) within the prior 1 year under the reimbursement system; (2) decompensated event (tense ascites, variceal bleeding, hepatorenal syndrome, or hepatic encephalopathy) using the operational definition within the prior 2 years before nucleos(t)ide analog treatment; (3) diagnosis of any type of cancer before antiviral treatment; and (4) observational period < 5 years after nucleos(t)ide analogs treatment.
“Compensated CHB treatment-naïve” was defined as having never developed a decompensated event within the prior 2 years before starting an antiviral agent. Both CHB and compensated cirrhosis were included. “Decompensated CHB treatment-naïve” was defined as having never developed a decompensated event within 2 years before starting the antiviral agent but at the start of antiviral treatment. Diagnosis of HCC is strictly regulated by HIRA, because government pays 95% of the bill. HCC can be diagnosed, if the typical hallmark (hypervascularity in the arterial phase and washout in the portal or delayed phase) is identified on one or more (two or more) imaging techniques (dynamic computed tomography, dynamic magnetic resonance imaging, gadolinium-ethoxybenzyldiethylenetriamine pentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging magnetic resonance imaging)[15].
The operational definitions were as follows. Naïve nucleos(t)ide analog treatment was defined as having no record of prescribed antiviral agents through insurance reimbursement 1 year before the first administration of an antiviral agent. Decompensated CHB treatment-naïve was defined as the removal of ≥ 3 L on paracentesis, endoscopic band ligation (or sclerotherapy) or drug therapy for variceal bleeding, lactulose enema after admission for hepatic encephalopathy, and treatment after admission for hepatorenal syndrome. The first decompensated event was defined as a complication such as tense ascites, variceal bleeding, hepatic encephalopathy, and/or hepatorenal syndrome at the start of antiviral treatment, but there were no decompensated events within 2 years before antiviral treatment. Tense ascites was defined as the need for paracentesis (C8050, C8051, and Q2470) while using an antiviral agent. Variceal bleeding was defined as the need for sclerotherapy, variceal ligation (Q2430, Q2431, Q2432, Q2433, Q2434, Q2435, Q2436, Q2437, Q2438, Q7631, Q7632, Q7633, and Q7634), or drug therapy for esophageal variceal bleeding (vasopressin, terlipressin, somatostatin, or octreotide) while on antiviral therapy. Hepatic encephalopathy was defined as administration of a lactulose enema (M0076) while using an antiviral agent. Hepatorenal syndrome was defined as co-administration of terlipressin and albumin while under antiviral therapy. Death was defined as HIRA code (clinical outcome code DGRSLT_TP_CD “4: Death”).
Based on the operational definitions, the appropriateness of the data extracted from the HIRA was assessed as follows. The databases from tertiary hospitals covering 6 years (January 2009 to December 2014) were used to review the patients’ medical records corresponding to the operational definitions to confirm agreement with the operational definitions (IRB approval: HYUH 2015-09-017). Overall, 133 patients had data corresponding to the definition of decompensated cirrhosis; 120 patients, tense ascites; 40 patients, variceal bleeding; 51 patients, hepatic encephalopathy; and 5 patients, hepatorenal syndrome.
The primary endpoint was mortality rate. The secondary endpoint was the incidence of decompensated cirrhosis-associated complications and HCC.
The t-test and chi-square tests were used to assess demographic differences based on sex and differences in biochemical data. Kaplan-Meier analysis was used to assess survival rate and re-bleeding frequency. The statistical analysis was performed using SAS software (version 9.4; SAS Institute, Inc., Cary, NC, United States).
A total of 286871 CHB patients were prescribed nucleos(t)ide analogs under the reimbursement policy (HBsAg-positive, baseline HBeAg-positive with HBV-DNA ≥ 100000 copies/mL or HBeAg-negative with HBV-DNA ≥ 10000 copies/mL, and aminotransferase level ≥ 80 IU/L) between 2007 and 2014 (Figure 1). A total of 196541 treatment-naïve patients who were never prescribed oral antiviral agents (lamivudine, clevudine, adefovir, telbivudine, entecavir, or tenofovir) within the previous 1 year were selected. Only those with an observation period ≥ 5 years were included. Ultimately, 45683 compensated CHB treatment-naïve (of 157849) and 7166 decompensated CHB treatment-naïve subjects were selected (Figure 1). The 45683 patients included showed compensated liver disease, while 2682 patients had accompanying decompensated complications at initiation of nucleos(t)ide analog treatment (Table 1). Mean patient age was 43.5 years, and the patients took various antiviral agent (entecavir: 41.7%; lamivudine: 17.4%; clevudine: 6.3%; combination: 29.4%).
| Total (n = 48365) | Compensated HBV group1 (n = 45683) | Decompensated cirrhosis group(n = 2682) | aP value | |
| Sex (male) | 32691 (67.6) | 30881 (67.6) | 1810 (67.5) | 0.9214 |
| Age (yr) | 43.5 ± 12.3 | 43.0 ± 12.2 | 51.7 ± 11.5 | < 0.0001 |
| Nucleos(t)ide analog | ||||
| Entecavir | 20147 (41.7) | 18644 (40.8) | 1503 (56.0) | < 0.0001 |
| Lamivudine | 8411 (17.4) | 7937 (17.4) | 474 (17.7) | 0.7105 |
| Clevudine | 3029 (6.3) | 2898 (6.3) | 131 (4.9) | 0.0028 |
| Lamivudine + adefovir | 2525 (5.2) | 2415 (5.3) | 110 (4.1) | 0.0084 |
| Telbivudine | 32 (0.1) | 30 (0.1) | 2 (0.1) | 1.0000 |
| Others | 14221 (29.4) | 13759 (30.1) | 462 (17.2) | < 0.0001 |
| Medication compliance | ||||
| < 25% | 12793 (26.5) | 12045 (26.4) | 748 (27.9) | 0.0700 |
| 25%-50% | 6067 (12.5) | 5769 (12.6) | 298 (11.1) | 0.0229 |
| 50%-75% | 7075 (14.6) | 6686 (14.6) | 389 (14.5) | 0.8735 |
| > 75% | 22430 (46.4) | 21183 (46.4) | 1247 (46.5) | 0.9835 |
| Type of first decompensation | ||||
| Refractory ascites | 2022 (55.6) | 2022 (55.6) | ||
| Varix bleeding | 711 (19.6) | 711 (19.6) | ||
| Hepatorenal syndrome | 151 (4.2) | 151 (4.2) | ||
| Hepatic encephalopathy | 752 (20.7) | 752 (20.7) |
The validity of the operational definitions was tested as follows. The operational definitions were used to identify patients from the databases of tertiary hospitals (covering 6 years between 2009 and 2014), and their medical records were reviewed to assess the agreement with the operational definitions. Overall, 120 patients with tense ascites were identified according to the operational definitions. Among them, 107 (89.2%) had matching and 13 had non-matching medical records. Among the unmatched 13 patients, five underwent postoperative paracentesis, six underwent paracentesis due to multiple organ failure caused by sepsis, and two underwent paracentesis because of malignant condition. A total of 40 patients with variceal bleeding were extracted according to the operational definition; of them, 38 (95.0%) had matching and 2 had non-matching medical records. Both non-matching cases involved peptic ulcer disease being misdiagnosed as variceal bleeding. A total of 51 hepatic encephalopathy cases were extracted according to the operational definition; of them, 49 (96.0%) had matching and 2 had non-matching medical records. Finally, five patients with hepatorenal syndrome were extracted based on the operational definition, and all had matching medical records.
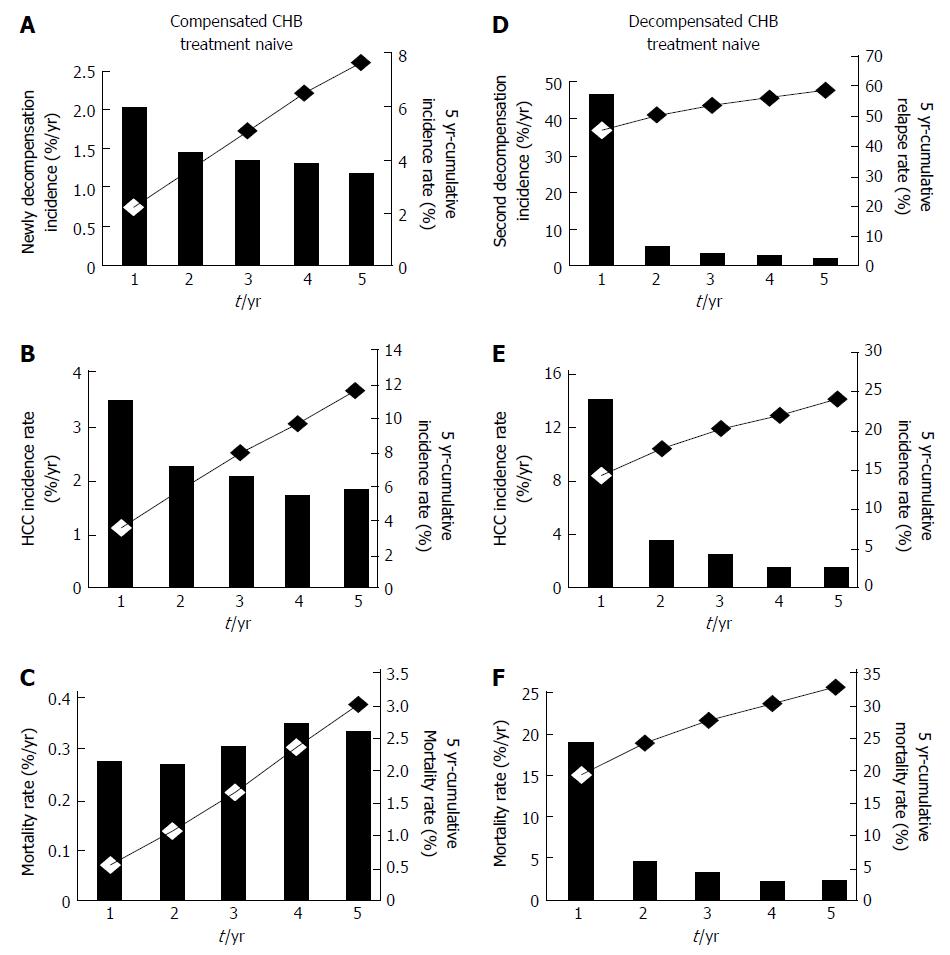
In compensated CHB treatment-naïve patients, the 5-year cumulative incidence of various complications was 7.4%, while the annual incidence of the first onset of decompensated complications after using an antiviral agent was 1.2%-2.0% (Table 2). The frequency of decompensated complications decreased significantly as the duration of antiviral agent use increased (P for trend, < 0.0001) (Figure 2A).
| Compensated chronic hepatitis B (n = 45683) | Decompensated cirrhosis (n = 2682) | |||||||||||||||||
| Newly decompensated incidence(n = 3401) | Hepatoma incidence(n = 5268) | Mortality incidence(n = 1415) | Second decompensated incidence(n = 1626) | Hepatoma incidence(n = 1295) | Mortality incidence(n = 874) | |||||||||||||
| Event | Percent | Accumulation (%) | Event | Percent | Accumulation (%) | Event | Percent | Accumulation (%) | Event | Percent | Accumulation (%) | Event | Percent | Accumulation (%) | Event | Percent | Accumulation (%) | |
| < 1 yr | 923 | 2.02% | 2.02% | 1602 | 3.51% | 3.51% | 253 | 0.55% | 0.55% | 1257 | 46.87% | 46.87% | 765 | 14.26% | 14.26% | 513 | 19.13% | 19.13% |
| 1-2 yr | 662 | 1.45% | 3.47% | 1053 | 2.31% | 5.81% | 249 | 0.55% | 1.10% | 144 | 5.37% | 52.24% | 200 | 3.73% | 17.99% | 132 | 4.92% | 24.05% |
| 2-3 yr | 634 | 1.39% | 4.86% | 947 | 2.07% | 7.88% | 280 | 0.61% | 1.71% | 99 | 3.69% | 55.93% | 140 | 2.61% | 20.60% | 94 | 3.50% | 27.55% |
| 3-4 yr | 631 | 1.38% | 6.24% | 802 | 1.76% | 9.64% | 324 | 0.71% | 2.42% | 71 | 2.65% | 58.58% | 94 | 1.75% | 22.35% | 65 | 2.42% | 29.98% |
| 4-5 yr | 551 | 1.21% | 7.44% | 864 | 1.89% | 11.53% | 309 | 0.68% | 3.10% | 55 | 2.05% | 60.63% | 96 | 1.79% | 24.14% | 70 | 2.61% | 32.59% |
| aP for trend | < 0.0001 | < 0.0001 | 0.0004 | < 0.0001 | < 0.0001 | < 0.0001 | ||||||||||||
The annual incidence of HCC was 1.8%-3.5%, and it decreased significantly during the period in which an antiviral agent was used (P for trend, < 0.0001) (Figure 2B). In compensated CHB treatment-naïve patients, the 5-year cumulative incidence of HCC was 11.5%, which was higher than that of decompensated complications (7.4%) (P < 0.0001). Finally, the annual mortality rate was 0.6%-0.7% (Figure 2C), whereas the 5-year cumulative mortality rate was 3.1% and the cumulative survival rate was 96.9%.
The annual incidence of a second decompensation event in decompensated CHB treatment patients was 2.1%-46.9%: It was highest within the first year (46.9%) but decreased over the antiviral treatment period (P for trend, < 0.0001) (Figure 2D). The 5-year cumulative recurrence rate was 60.6%.
The annual incidence of HCC was 1.8%-14.3%, and it decreased significantly as antiviral treatment period increased (P for trend, < 0.0001) (Figure 2E). The 5-year cumulative incidence of HCC was 24.1%. The development of HCC was high within the first year after antiviral agent use (14.3%) and decreased thereafter, but the incidence remained steady even 1 year after the start of antiviral therapy, showing an annual incidence of 2.5% (1.8%-3.7%, 2-5 year). Finally, the annual mortality rate was approximately 2.4%-19.1%, whereas the 5-year cumulative mortality rate was 32.6% and the cumulative survival rate was 67.4%.
In compensated CHB treatment-naïve patients, the 5-year cumulative incidences of HCC and decompensated complications were 11.5% and 7.4%, respectively. In CHB-associated decompensated cirrhosis patients, the 5-year cumulative incidence of HCC was 24.1%, whereas the mortality rate was 32.6% after antiviral therapy (Figure 3).
This study used national database that was compiled from physician reimbursement claims for medical services. There is inherent weakness when someone uses such database which relies on local and regional practices in order to generate a reimbursement claim. Therefore, we tried to used performance or drug medication code rather than diagnosis code. Use of antiviral agents, albumin, vasoactive drugs (vasopressin, terlipressin, somatostatin, or octreotide) are strictly regulated by government. All above drugs have very narrow indication and regulated strictly, because of high price. All health care providers should submit a supporting laboratory data or documents that meet reimbursement criteria. Because government pays 95% of total medical expenses in case of HCC, diagnose of HCC verified by the government according to strict criteria.[15] So we tried to use diagnostic code (ICD-10) and popular medication, such as diuretics and oral lactulose relies on local and regional practices in order to generate reimbursement. Another challenge when use reimbursement data is baseline disease severity is quite heterogeneous, because laboratory data was not visible. To correct the disease severity of baseline only first decompensated cirrhosis patients and antiviral treatment-naïve patients were enrolled. Baseline alanine aminotransferase ≥ 80 IU with HBV-DNA ≥ 100000 copies/mL (in HBeAg-negative patients) or HBV-DNA ≥ 10000 copies/mL (in HBeAg-positive patients) should be clarified by HIRA.
Several studies have examined the natural course of CHB in patients without antiviral therapy[2,16,17]. Previous reports showed 5-year survival rate of decompensated cirrhosis at 14%-35% under supportive treatment[5,18]. There are two Asian studies[19,20]. Five-year survival rate was 19% in 102 untreated decompensated cirrhosis patients[20]. In our study, 5-year cumulative survival rate was 67.4% in 1st decompensated CHB treatment-naïve subjects. Median survival was only 2 years, and the 2-year possibility of survival was 57% in patients with CHB-associated decompensated cirrhosis in a meta-analysis[17]. The 1-year possibility of death of patients with clinical stage 3 disease (ascites ± varix) was 20%, while the 1-year death rate was 57% in patients with clinical stage 4 disease (variceal bleeding ± ascites)[16].
Data of the clinical course of CHB-associated decompensated cirrhosis with antiviral agent use are sparse. Antiviral treatment was effective in improving survival rate in decompensated cirrhosis[18]. Two-year survival rate of 70 decompensated patients was 83% in entecavir treatment group[21]. In our study, 2-year cumulative survival rate was 76.0% in 1st decompensated CHB treatment-naïve subjects. Das et al[20] showed the clinical course of patients with CHB-associated decompensated cirrhosis from 1998 to 2008. Of the 253 patients, 151 (59.7%) received antiviral treatment. The 5-year survival rate was 21% in the antiviral treatment group and 19% in the untreated group, quite low compared to our results (21% vs 67.4%, respectively). The reason might be differences in baseline characteristics and their use of low-potent antiviral agents. The dominant antiviral agent was lamivudine (88%) in Das’s study, whereas most of our patients were prescribed entecavir (41.7%) or combination treatment (29.4%). Jang et al[6] evaluated the effects of antiviral therapy on mortality rate in decompensated cirrhosis patients and reported a 5-year survival rate of 423 decompensated cirrhosis patients of 59.7%. Although we used very strict operational definitions, the 5-year survival rate (61.4%) of decompensated CHB treatment-naïve patients was comparable to that of Jang’s results (59.7%). The definition of tense ascites used in the present study was relatively restricted to cases in which paracentesis of > 3 L was performed. Thus, patients with mild-grade ascites, which can be controlled by diuretics, were excluded from our study. This is the largest cohort study to date to analyze the clinical outcomes of antiviral therapy in decompensated CHB patients. In our study, 7166 decompensated treatment-naïve patients were enrolled.
Until now, the annual incidence of HCC in patients with compensated cirrhosis was known to be 7%-8%[2]. Antiviral therapy reduced the incidence of HCC in compensated liver disease patients[3,4,22]. In the present study, in compensated CHB patients on antiviral therapy, the annual incidence and 5-year cumulative incidence of HCC were 1.7%-3.5% and 11.5%, respectively. It seems low compared to those of previous studies[3,4,22], but the incidence of HCC looked higher than that of first decompensated complications (7.4%). This means that, in compensated CHB patients, clinicians should be more alert to HCC than to newly developing decompensated complications.
There is little room for debate about whether antiviral therapy can reduce the incidence of HCC in patients with compensated CHB, but no studies have reported whether it can reduce the incidence of HCC in patients with decompensated cirrhosis. In present study, the 5-year cumulative incidence of HCC in patients with decompensated cirrhosis and on antiviral therapy was 24.1%. Interestingly, antiviral therapy after the onset of decompensated complications reduced the incidence of HCC from 14.26% in the first year to 3.73%, 2.61%, 1.75%, and 1.79% in the following years. However, additional studies are needed to determine whether such a drastic decrease in the incidence of HCC is a result of antiviral therapy itself or a result of modifying one’s lifestyle, for example, abstaining from alcohol (Figure 2).
The limitations of the present study are as follows. First, the present study used data that were specific to insurance reimbursement claims; thus, the data lacked accurate information about the patients’ disease severity and blood test results (HBV-DNA polymerase chain reaction, liver enzymes, alpha-fetoprotein, etc.). To minimize this problem, we included treatment-naïve patients (all baseline HBeAg-positive patients with HBV-DNA ≥ 100000 copies/mL or HBeAg-negative patients with HBV-DNA ≥ 10000 copies/mL and alanine aminotransferase or aspartate aminotransferase level ≥ 80 IU/L). Second, mismatching operational definitions are possible. In our study, the concordance rate of the operational definition of uncontrolled ascites was 89.1%, which means that approximately 10% of cases did not match. We tried to validate the operational definitions in various clinical settings, because pattern of prescribing antiviral agent might be quite different depending on the clinical settings. We investigated two different sized hospitals; one is tertiary hospital and the other is secondary hospital. Although we tried to validate the operational definitions using real world data from two different hospitals, we thought 133 patients were not enough to validate for 7166 decompensated patients’ data. Third, the definition of “compensated CHB” is more lenient as it included both CHB and early compensated cirrhosis patients, while “decompensated CHB” is very strict, as it only includes “patients with large amounts of ascites” but generally “ascites is a feature of decompensation.” Patients with moderate ascites who used diuretics and had mild variceal bleeding with no drug therapy were excluded from the study. Consequently, the decompensated complications defined in the present study may have been underestimated compared to those in actual patients with decompensated cirrhosis. Fourth, the present study used diagnosis codes to diagnose HCC. Because the diagnosis of HCC was based on dynamic contrast-enhanced computed tomography or magnetic resonance imaging findings without a biopsy, the numbers may have been overestimated compared to the actual incidence of HCC. Fifth, given that it was impossible to check the medical and radiological records of all patients, those with both CHB and compensated cirrhosis were labeled compensated CHB treatment-naïve. As a result, some patients with compensated cirrhosis and only a small amount of ascites may have been included in our patients with compensated liver disease. Finally, this study did not consider the patients’ alcohol consumption history. In patients with hepatitis B, alcohol consumption is known to increase the incidence of HCC. However, the present study was unable to identify the patients’ alcohol consumption and drinking habits.
In conclusion, in compensated CHB patients, the 5-year cumulative incidences of decompensated complications and HCC were 7.4% and 11.5%, respectively. In decompensated CHB patients, the annual and 5-year cumulative incidences of HCC were 1.8%-14.3% and 24.1%, respectively. Moreover, the annual and 5-year cumulative mortality rates were approximately 2.6%-19.1% and 32.6%, respectively.
According to a population-based cohort study, the annual incidence of decompensated complications was 11% in patients with compensated chronic liver disease. It is clear that antiviral therapy reduces liver disease progression and mortality in decompensated chronic hepatitis B (CHB) patients. However, clinical data for long-term survival rate, the incidence of hepatocellular carcinoma (HCC), and the recurrence of decompensated events in patients with decompensated cirrhosis treated with antiviral agents are still lacking in the antiviral era
Several studies have examined the natural course of CHB in patients without antiviral therapy. Data of the clinical course of CHB-associated decompensated cirrhosis with antiviral agent use are sparse. In this study, we tried to investigate the survival rate and incidence of HCC in patients with decompensated cirrhosis in the antiviral era.
The primary objective was mortality rate and the secondary objectives were the incidence of decompensated cirrhosis-associated complications and HCC.
The data source of this study was the insurance reimbursement claims data provided by the Korean Health Insurance Review and Assessment (HIRA). Overall, 48365 antiviral treatment-naïve patients treated between 2008 and 2009 were included, and each had a follow-up period ≥ 5 years. Naïve nucleos(t)ide analog treatment and the decompensated complications were defined with the operational definitions. The appropriateness of the data extracted from the HIRA was assessed and the validation the operational definitions was conducted in two different sized hospitals.
The 45683 patients included showed compensated liver disease, while 2682 patients had accompanying decompensated complications at initiation of nucleos(t)ide analog treatment. Mean patient age was 43.5 years. In compensated CHB treatment-naïve patients, the 5-year cumulative incidence of various complications was 7.4%, while the annual incidence of the first onset of decompensated complications after using an antiviral agent was 1.2%-2.0%. The 5-year cumulative incidence of HCC in compensated CHB treatment-naïve patients was 11.5%, which was higher than that of decompensated complications (7.4%). In decompensated CHB treatment-naïve patients, the annual incidence of a second decompensation event in decompensated CHB treatment patients was 2.1%-46.9%: It was highest within the first year (46.9%). The 5-year cumulative incidence of HCC in decompensated CHB treatment patients was 24.1%. The 5-year cumulative mortality rate was 32.6% and the cumulative survival rate was 67.4%.
This study used national database that was compiled from physician reimbursement claims for medical services. There would be several limitations, but we proposed the new methodology when using a long term and large scale clinical database such as HIRA. According to the present study, we suggested that clinicians should be more alert to HCC than to newly developing decompensated complications. Interestingly, antiviral therapy after the onset of decompensated complications reduced the incidence of HCC from 14.26% in the first year to 3.73%, 2.61%, 1.75%, and 1.79% in the following years. However, additional studies are needed to determine. In conclusion, long term outcome of treating hepatitis B-associated decompensated cirrhosis using antiviral agents improved much compare to previous reports. Incidence of cumulative mortality rate and hepatocellular carcinoma was sharply decreased after one year antiviral treatment.
We investigated the mortality rate and the incidence of decompensated cirrhosis-associated complications and HCC in the antiviral era. We hope the data suggested in this study would be helpful for the future study of comparing antiviral agents.
| 1. | Fleming KM, Aithal GP, Card TR, West J. The rate of decompensation and clinical progression of disease in people with cirrhosis: a cohort study. Aliment Pharmacol Ther. 2010;32:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Fattovich G, Giustina G, Schalm SW, Hadziyannis S, Sanchez-Tapias J, Almasio P, Christensen E, Krogsgaard K, Degos F, Carneiro de Moura M. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology. 1995;21:77-82. [PubMed] |
| 3. | Tada T, Kumada T, Toyoda H, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Kitabtake S, Ito T. Long-term prognosis of patients with hepatitis B infection: causes of death and utility of nucleos(t)ide analogue therapy. J Gastroenterol. 2015;50:795-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Lim YS, Han S, Heo NY, Shim JH, Lee HC, Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014;147:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 5. | Chu CM, Liaw YF. Hepatitis B virus-related cirrhosis: natural history and treatment. Semin Liver Dis. 2006;26:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Jang JW, Choi JY, Kim YS, Woo HY, Choi SK, Lee CH, Kim TY, Sohn JH, Tak WY, Han KH. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology. 2015;61:1809-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Jang JW, Choi JY, Kim YS, Yoo JJ, Woo HY, Choi SK, Jun CH, Lee CH, Sohn JH, Tak WY. Effects of Virologic Response to Treatment on Short- and Long-term Outcomes of Patients With Chronic Hepatitis B Virus Infection and Decompensated Cirrhosis. Clin Gastroenterol Hepatol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Wang FY, Li B, Li Y, Liu H, Qu WD, Xu HW, Qi JN, Qin CY. Entecavir for Patients with Hepatitis B Decompensated Cirrhosis in China: a meta-analysis. Sci Rep. 2016;6:32722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Peng H, Liu J, Yang M, Tong S, Yin W, Tang H, Hu P, Hu H, Ren H. Efficacy of lamivudine combined with adefovir dipivoxil versus entecavir monotherapy in patients with hepatitis B-associated decompensated cirrhosis: A meta-analysis. J Clin Pharmacol. 2014;54:189-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Ye XG, Su QM. Effects of entecavir and lamivudine for hepatitis B decompensated cirrhosis: meta-analysis. World J Gastroenterol. 2013;19:6665-6678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (2)] |
| 11. | Singal AK, Fontana RJ. Meta-analysis: oral anti-viral agents in adults with decompensated hepatitis B virus cirrhosis. Aliment Pharmacol Ther. 2012;35:674-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Fontana RJ, Keeffe EB, Carey W, Fried M, Reddy R, Kowdley KV, Soldevila-Pico C, McClure LA, Lok AS; National Institutes of Health Hepatitis B Virus Orthotopic Liver Transplantation Study Group. Effect of lamivudine treatment on survival of 309 North American patients awaiting liver transplantation for chronic hepatitis B. Liver Transpl. 2002;8:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Fontana RJ, Hann HW, Perrillo RP, Vierling JM, Wright T, Rakela J, Anschuetz G, Davis R, Gardner SD, Brown NA. Determinants of early mortality in patients with decompensated chronic hepatitis B treated with antiviral therapy. Gastroenterology. 2002;123:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 180] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 14. | Health Insurance Review and Assessment. Accessed July 22, 2018. Available from: URL: http://www.hira.or.kr/eng/main.do. |
| 15. | Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut Liver. 2015;9:267-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, Tinè F, Giannuoli G, Traina M, Vizzini G. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 393] [Article Influence: 32.8] [Reference Citation Analysis (1)] |
| 17. | D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2215] [Article Influence: 110.8] [Reference Citation Analysis (3)] |
| 18. | Peng CY, Chien RN, Liaw YF. Hepatitis B virus-related decompensated liver cirrhosis: benefits of antiviral therapy. J Hepatol. 2012;57:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 19. | Hui AY, Chan HL, Leung NW, Hung LC, Chan FK, Sung JJ. Survival and prognostic indicators in patients with hepatitis B virus-related cirrhosis after onset of hepatic decompensation. J Clin Gastroenterol. 2002;34:569-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Das K, Das K, Datta S, Pal S, Hembram JR, Dhali GK, Santra A, Chowdhury A. Course of disease and survival after onset of decompensation in hepatitis B virus-related cirrhosis. Liver Int. 2010;30:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Shim JH, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Efficacy of entecavir in treatment-naïve patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2010;52:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 22. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 555] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kanda T, Kim DJ, Niu ZS, Sirin G S- Editor: Ma RY L- Editor: A E- Editor: Bian YN