Published online Oct 28, 2018. doi: 10.3748/wjg.v24.i40.4586
Peer-review started: July 2, 2018
First decision: July 18, 2018
Revised: August 14, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: October 28, 2018
Processing time: 119 Days and 3.1 Hours
To investigate second primary malignancy (SPM) risk after radiotherapy in rectal cancer survivors
We used Taiwan’s National Health Insurance Research Database to identify rectal cancer patients between 1996 and 2011. Surgery-alone, preoperative short course, preoperative long course, and post-operative radiotherapy groups were defined. The overall and site-specific SPM incidence rates were compared among the radiotherapy groups by multivariate Cox regression, taking chemotherapy and comorbidities into account. Sensitivity tests were performed for attained-year adjustment and long-term survivors analysis.
A total of 28220 patients were analyzed. The 10-year cumulative SPM incidence was 7.8% [95% confidence interval (CI): 7.2%-8.2%] using a competing risk model. The most common sites of SPM were the lung, liver, and prostate. Radiotherapy was not associated with increased SPM risk in multi-variate Cox model (hazard ratio = 1.05, 95%CI: 0.91-1.21, P = 0.494). The SPM hazard remained unchanged in 10-year-survivors. In addition, no SPM risk difference was found between the preoperative radiotherapy and postoperative radiotherapy groups.
In this large population-based cohort study, we demonstrated that radiotherapy had no increase in SPM.
Core tip: Developing a second primary malignancy (SPM) after radiotherapy represents a major problem for long-term cancer survivors. In this large population-based study, no increased risk of developing SPM was found in rectal cancer patients who received pelvic radiotherapy in their initial treatment after carefully adjusted basline confounders. Also, the SPM risk remained the same among the preoperative long-course, preoperative short-course, and postoperative radiotherapy groups. However, rectal cancer survivors, similarly to other cancer survivors, are burdened with an overall higher probability of developing a second primary cancer. Life-long follow-up is recommended.
- Citation: Wang TH, Liu CJ, Chao TF, Chen TJ, Hu YW. Second primary malignancy risk after radiotherapy in rectal cancer survivors. World J Gastroenterol 2018; 24(40): 4586-4595
- URL: https://www.wjgnet.com/1007-9327/full/v24/i40/4586.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i40.4586
Thanks to the progress in early detection and treatment, rectal cancer survival has increased steadily over time[1]. Death rates due to colorectal cancer have declined by approximately 3% per year during the past decade[2]. Undoubtedly, radiotherapy has an established role in the multi-modal treatment of this disease[3,4]. However, radiotherapy may be related to several late adverse effects, which represents a major problem for long-term cancer survivors[5]. One of these effects, the risk of developing a second primary malignancy (SPM), has received greater attention in clinical practice. Rectal cancer survivors have a 4%-8% higher background rate of SPM compared with the normal population[6,7]. This higher rate may reflect the patients’ genetic backgrounds, cancer-related treatments, lifestyles, and environmental risk factors[8]. Although several studies have investigated the relationship between radiotherapy and SPM in rectal cancer patients, the conclusions have been diverse[9-12]. Most studies have only addressed the initial treatment, which leads to results that are affected by potential confounders, such as comorbidities and other treatments during follow-up. Furthermore, whether preoperative long-course radiotherapy, preoperative short-course radiotherapy, or postoperative radiotherapy has a different contribution in increasing SPM risk is not clear. Here, we used Taiwan’s National Health Insurance Research Database (NHIRD), which provides detailed diagnosis and treatment data, to assess the association between SPM and radiotherapy, taking chemotherapy and comorbidities into account.
Taiwan’s National Health Insurance, established in 1995, covers the comprehensive medical care of > 99% Taiwanese residents[13]. Taiwan’s NHIRD provides encrypted nationwide data for health research, including inpatient and outpatient diagnoses, claimed procedures and drug prescriptions. The Registry of Catastrophic Illness Database (RCID), a subpart of the NHIRD, provides information on patients with a confirmed malignancy. The certification of both first primary rectal cancer and SPM requires tissue pathologic proof for peer review. This study was exempted from full review by the Institutional Review Board (No. 2016-05-007BC).
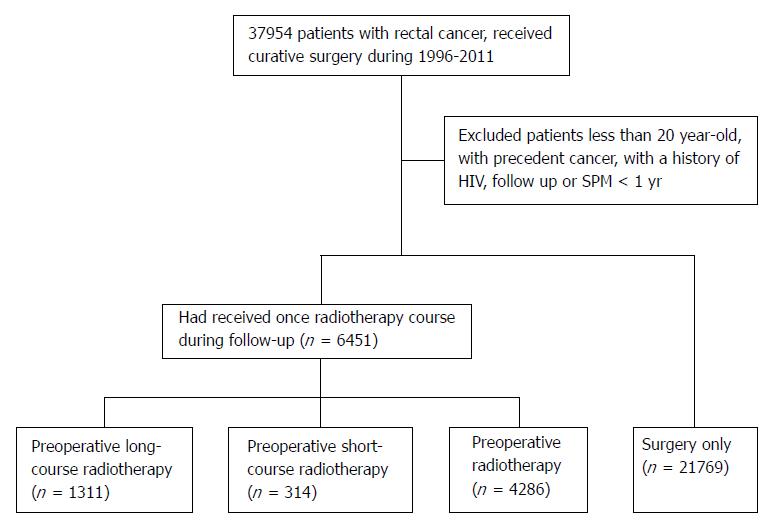
The cohort was composed of patients aged 20 years or older who were diagnosed with a first primary rectal cancer (ICD-9-CM 154.0 and 154.1) from the RCID between Jan 1, 1996, and Dec 31, 2011. Because there is a lag time between radiation and SPM, we excluded patients who had SPMs within the first year of treatment or survived less than one year after treatment[14]. We also excluded patients with HIV infection. Because synchronous and metachronous colorectal cancers (CRCs) were difficult to distinguished, second primary CRCs were not analyzed. We also excluded neoplasms of the small intestine to avoid misclassification. The follow-up time for each individual began one year after the initial treatment and ended on the date of diagnosis of any SPM, death, or the end of study (Dec 31, 2011), whichever came first.
The patients were classified into four groups. The surgery-only group was composed of patients who underwent radical rectal surgery, such as abdominoperineal resection of the rectum, low anterior resection, local excision, transsacral rectosigmoidectomy, or posterior resection of the rectum, and who never received radiotherapy within the follow-up time. The postoperative radiotherapy group was composed of patients who underwent radical rectal surgery followed by radiotherapy within one year after surgery (considering that the radiotherapy may have been administered after 6 mo of chemotherapy). The preoperative radiotherapy group was composed of patients who received radiotherapy within 6 mo prior to radical rectal surgery. The preoperative radiotherapy group was further categorized into the short-course and the long-course radiotherapy groups according to their radiotherapy regimen, judging by claimed radiation portals. The exact dose of radiation used was not available in the NHIRD. However, the typical radiation regimen for preoperative long-course radiotherapy and postoperative radiotherapy is 45-50.4 Gy in 25-28 fractions, while 25 Gy in 5 fractions is used for preoperative short course radiotherapy. Patients who received incomplete radiotherapy regimens or re-irradiation during the follow-up period were excluded.
We collected all cancer treatment information within the first 2 years after diagnosis, including surgery, radiation, and chemotherapy. The surgery procedures were coded using ICD-9-CM codes. The chemotherapy agents were classified by their Anatomical Therapeutic Chemical (ATC) code. Chemotherapy administered after and within one year of an SPM was omitted due to possible treatment of a second cancer. Demographic data such as age at rectal cancer diagnosis, year of diagnosis, attained age and year of SPM diagnosis, sex, and comorbidities, including autoimmune diseases, chronic obstructive pulmonary disease (COPD), diabetes mellitus (DM), dyslipidemia, end-stage renal disease (ESRD), liver cirrhosis, and hypertension (HTN), were collected from the NHIRD.
Because death could be considered a competing event to SPM during follow-up, a competing-risk model was used to estimate the cumulative incidence of SPM in each radiotherapy group. We used univariate and multivariate Cox proportional hazards models to identify possible risk factors for SPM development. The final Cox proportional hazard model was used to assess the significant difference between the relative risk of an SPM across the four groups after adjustment for age at and year of rectal cancer diagnosis, sex, chemotherapy, and comorbidities. A two-sided P-value less than 0.05 was considered statistically significant.
The data processing was performed with Microsoft SQL Server 2012 (Microsoft Corp., Redmond, WA, United States). All analyses were computed in R (version R-2.15.3; http://www.r-project.org). The cmprsk library in R was used for competing-risk analyses.
In addition to the final Cox model, a SPM attained-calendar-year stratified Cox proportional hazards model was tested to assess for adjusted radiotherapy effects. Subgroup analyses were also undertaken to investigate the consistency of the conclusion among different subpopulations. We generated Cox models in patients who survived more than 5 years and more than 10 years.
We identified a total of 28220 eligible rectal cancer patients based on our criteria. There were 21769, 1311, 314, and 4826 patients in the surgery-only, preoperative long-course, preoperative short-course, and postoperative radiotherapy groups, respectively. The cohort selection flow chart is shown in Figure 1.
The median follow-up for all patients was 5.2 years (range: 1 to 16.0 years) and was 5.5 years (range, 1 to 15.3 years) in the surgery-only group, 4.2 years (range, 1 to 13.2 years) in the preoperative long-course group, 4.1 years (range, 1 to 10.5 years) in the preoperative short-course group, and 4.3 years (range, 1 to 16.0 years) in the postoperative radiotherapy group. The patients in the radiotherapy group were slightly younger (mean age 61 years vs 66 years in those without radiotherapy), had a more recent diagnosis year (median year 2006 versus 2004 in those without radiotherapy), and had a higher chance of receiving chemotherapy (92% vs 56% in those without radiotherapy). The most commonly used chemotherapy agents were fluorouracil, tegafur/uracil, oxaliplatin, irinotecan, and capecitabine. Table 1 summarizes the patient and treatment characteristics.
| All patients | Surgery-only | All radiotherapy | Postoperative | Preoperative | Long | Short | |
| Patient number | 28220 | 21769 | 6451 | 4826 | 1625 | 1311 | 314 |
| Male (%) | 16297 (58%) | 12323 (57%) | 3974 (62%) | 2940 (61%) | 1034 (64%) | 831 (63%) | 203 (65%) |
| Median follow-up (IQR), yr | 5.19 (5.02) | 5.47 (5.18) | 4.25 (3.98) | 4.29 (4.10) | 4.16 (3.61) | 4.18 (3.76) | 4.10 (3.01) |
| Median rectal cancer diagnosis age (IQR) | 65 (18) | 66 (18) | 62 (17) | 62 (18) | 61 (19) | 60 (18) | 64 (18) |
| Median rectal cancer diagnosis year (IQR) | 2005 (7) | 2004 (6) | 2006 (6) | 2005 (6) | 2007 (5) | 2007 (5) | 2007 (4) |
| Surgery | |||||||
| LAR | 20416 | 16253 | 4163 | 2953 | 1210 | 950 | 260 |
| APR | 6285 | 4453 | 1832 | 1471 | 361 | 311 | 50 |
| Other surgery | 1519 | 1063 | 456 | 402 | 54 | 50 | 4 |
| Chemotherapy | |||||||
| All chemotherapy (%) | 18236 (65%) | 12310 (57%) | 5926 (92%) | 4445 (92%) | 1481 (91%) | 1276 (97%) | 205 (65%) |
| Fluorouracil | 12063 | 7399 | 4664 | 3428 | 1236 | 1105 | 131 |
| Tegafur | 11324 | 8139 | 3185 | 2547 | 638 | 517 | 121 |
| Oxaliplatin | 4033 | 2460 | 1573 | 1273 | 300 | 262 | 38 |
| Irinotecan | 3273 | 2020 | 1253 | 1069 | 184 | 151 | 33 |
| Capecitabine | 2620 | 1632 | 988 | 773 | 215 | 185 | 30 |
| Comorbidities | |||||||
| DM | 10802 | 8560 | 2242 | 1696 | 546 | 427 | 119 |
| Hypertension | 18096 | 14438 | 3658 | 2742 | 916 | 720 | 196 |
| Liver cirrhosis | 1521 | 1227 | 294 | 225 | 69 | 46 | 23 |
| Autoimmune disease | 1763 | 1372 | 391 | 298 | 93 | 76 | 17 |
| End stage renal disease | 5456 | 4402 | 1054 | 825 | 229 | 168 | 61 |
| COPD | 10762 | 8709 | 2053 | 1585 | 468 | 368 | 100 |
| Dyslipidemia | 11695 | 9246 | 2449 | 1779 | 670 | 534 | 136 |
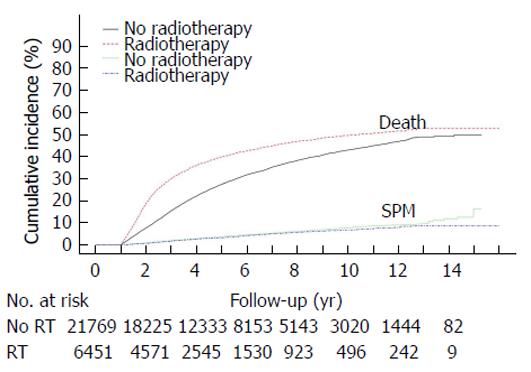
During the follow-up period, 1270 of the 28220 patients (4.5%) developed a SPM. In the surgery-only group, 1056 patients (8.6%) developed a second cancer, compared with 49 (3.7%) in the preoperative long-course group, 10 (3.2%) in the preoperative short-course group, and 182 (3.2%) in the postoperative radiotherapy group. The most common sites of SPM were lung (n = 284), liver (n = 183), and prostate (n = 129). The distributions of the SPMs in each group are listed in Table 2. The cumulative incidences of SPM and mortality rate are shown in Figure 2. Death is a strong competitor for SPM in both non-irradiated and irradiated patients. The cumulative incidence of mortality is higher in the irradiated patients because these patients generally had more advanced disease. The estimated cumulative incidence of SPM in the competing-risk model at the 5 year, 10 year, and 15 year marks was 3.7% (95%CI: 3.4%-3.9%), 7.8% (95%CI: 7.2%-8.2%), and 12.4% (95%CI: 10.5%-14.6%) in the surgery-only group and 3.2% (95%CI: 2.7%-3.7%), 6.7% (95%CI: 5.8%-7.6%), and 8.3% (95%CI: 7.1%-9.7%) in the irradiated groups, respectively.
| All patients | Surgery-only | Long | Short | Post | |
| All SPM | 1270 (100) | 1056 (100) | 49 (100) | 10 (100) | 155 (100) |
| Head and neck | 89 (7) | 69 (6.5) | 7 (14.3) | 2 (20) | 11 (7.1) |
| Esophagus | 31 (2.4) | 28 (2.7) | 0 (0) | 0 (0) | 3 (1.9) |
| Stomach | 82 (6.5) | 68 (6.4) | 4 (8.2) | 1 (10) | 9 (5.8) |
| Liver | 183 (14.4) | 162 (15.3) | 3 (6.1) | 3 (30) | 15 (9.7) |
| Pancreas | 31 (2.4) | 26 (2.5) | 1 (2) | 0 (0) | 4 (2.6) |
| Lung | 284 (22.4) | 224 (21.2) | 16 (32.7) | 0 (0) | 44 (28.4) |
| Bone | 17 (1.3) | 14 (1.3) | 0 (0) | 0 (0) | 3 (1.9) |
| Skin | 31 (2.4) | 23 (2.2) | 1 (2) | 2 (20) | 5 (3.2) |
| Breast | 82 (6.5) | 71 (6.7) | 3 (6.1) | 0 (0) | 8 (5.2) |
| Cervix | 18 (1.4) | 17 (1.6) | 0 (0) | 0 (0) | 1 (0.6) |
| Uterus | 15 (1.2) | 10 (0.9) | 2 (4.1) | 0 (0) | 3 (1.9) |
| Ovary | 10 (0.8) | 10 (0.9) | 0 (0) | 0 (0) | 0 (0) |
| Prostate | 129 (10.2) | 116 (11) | 2 (4.1) | 1 (10) | 10 (6.5) |
| Bladder | 83 (6.5) | 63 (6) | 2 (4.1) | 0 (0) | 18 (11.6) |
| Kidney | 45 (3.5) | 40 (3.8) | 1 (2) | 1 (10) | 3 (1.9) |
| Thyroid | 18 (1.4) | 15 (1.4) | 0 (0) | 0 (0) | 3 (1.9) |
| Hematologic | 59 (4.6) | 46 (4.4) | 3 (6.1) | 0 (0) | 10 (6.5) |
| Others | 63 (5) | 54 (5.1) | 4 (8.2) | 0 (0) | 5 (3.2) |
A univariate Cox regression model was used to test the potential risk factors for SPM. The results showed that male sex, age, liver cirrhosis, autoimmune disease, and COPD were significantly associated with a higher risk for SPMs, while dyslipidemia was significantly associated with a lower risk for SPMs. Chemotherapy and radiotherapy were not significantly associated with SPMs, although preoperative long-course radiotherapy had a trend toward increasing risk [hazard ratio (HR) = 1.25, 95%CI: 0.97-1.62; P = 0.090]. To better clarify the risk of radiotherapy for SPM, the final Cox regression model contained the covariates gender, age at and year of rectal cancer diagnosis, the use of radiotherapy, the use of chemotherapy, DM, HTN, liver cirrhosis, autoimmune disease, COPD, ESRD, and dyslipidemia. In multi-variate analysis, age (HR = 1.02 per one-year increment, 95%CI: 1.01-1.02; P < 0.001), male sex (HR = 1.47, 95%CI: 1.32-1.65; P < 0.001), DM (HR = 1.14, 95%CI: 1.02-1.28; P = 0.027), liver cirrhosis (HR = 2.40, 95%CI: 2.03-2.82; P < 0.001), and COPD (HR = 1.19, 95%CI: 1.06-1.33; P = 0.003) were significantly associated with a higher risk for SPMs. Hypertension (HR = 0.86, 95%CI: 0.75-0.97; P = 0.017) and dyslipidemia (HR = 0.85, 95%CI: 0.76-0.95; P = 0.006) were significantly associated with a lower risk for SPMs (Table 3). Again, no significantly elevated HR was observed among the different radiotherapy groups compared with the surgery-alone group.
| Univariate Cox regression | Multi-variate Cox regression | |||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Sex(M) | 1.57 (1.40-1.75)a | < 0.001 | 1.47 (1.32-1.65)a | < 0.001 |
| Diagnosis age (1 yr increment) | 1.02 (1.01-1.02)a | < 0.001 | 1.02 (1.01-1.02)a | < 0.001 |
| Diagnosis year | 1.06 (1.04-1.08)a | < 0.001 | 1.06 (1.04-1.08)a | < 0.001 |
| Chemotherapy | 0.95 (0.86-1.06) | 0.371 | 0.97 (0.87-1.08) | 0.562 |
| DM | 1.11 (0.99-1.23) | 0.062 | 1.14 (1.02-1.28)a | 0.027 |
| Hypertension | 1.01 (0.90-1.13) | 0.849 | 0.86 (0.75-0.97)a | 0.017 |
| Liver cirrhosis | 2.47 (2.10-2.90)a | < 0.001 | 2.40 (2.03-2.82)a | < 0.001 |
| Rheumatologic disease | 0.78 (0.62-0.99)a | 0.038 | 0.81 (0.64-1.03) | 0.080 |
| End stage renal disease | 1.01 (0.89-1.16) | 0.828 | 0.91 (0.80-1.05) | 0.192 |
| COPD | 1.33 (1.20-1.48)a | < 0.001 | 1.19 (1.06-1.33)a | 0.003 |
| Dyslipidemia | 0.87 (0.78-0.96)a | 0.008 | 0.85 (0.76-0.95)a | 0.006 |
| Radiotherapy1 | 1.04 (0.90-1.19) | 0.625 | 1.05 (0.91-1.21) | 0.494 |
| Long course RT1 | 1.25 (0.97-1.62) | 0.090 | 1.28 (0.98-1.67)2 | 0.071 |
| Short course RT1 | 1.01 (0.56-1.83) | 0.976 | 0.91 (0.50-1.64)2 | 0.742 |
| Post-OP RT1 | 0.98 (0.84-1.15) | 0.801 | 1.01 (0.86-1.18)2 | 0.941 |
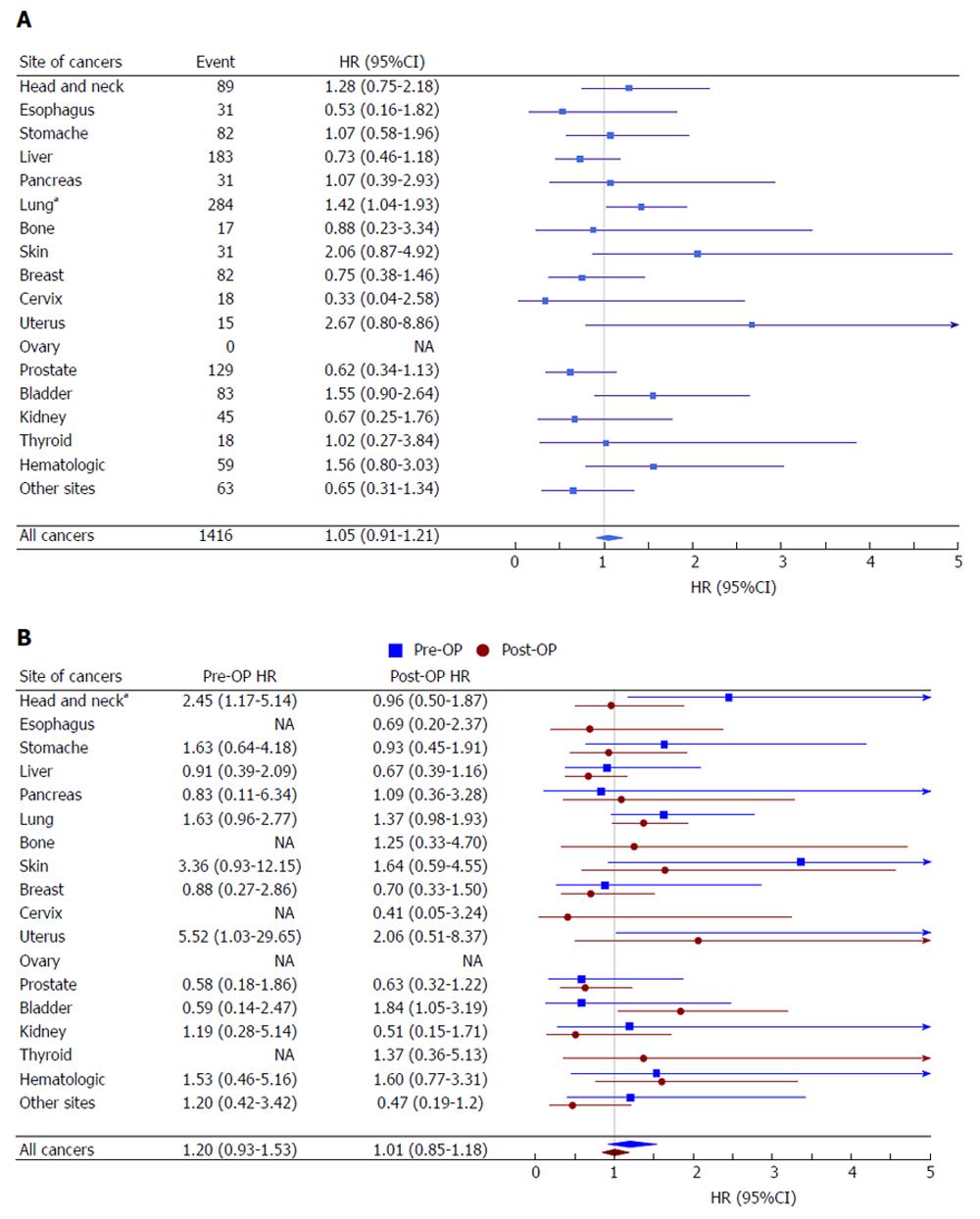
A similar covariate-adjusted Cox model was applied to the individual SPM sites. Compared with the surgery-only group, a significantly increased HR for SPM in the radiotherapy group was only evident for lung cancer (HR = 1.42, 95%CI: 1.04-1.93; P < 0.001). The risk of bladder, uterus, skin, and hematologic cancer was elevated in irradiated patients, but the difference was not statistically significant. Irradiated patient also had less prostate and liver cancer, but again, the difference was not statistically significant (Figure 3A). We further compared the preoperative and postoperative radiotherapy groups. Due to relatively few events in each of the preoperative long/short-course groups, we combined these two groups in the second primary sites analysis. Among all SPM, the HR of the preoperative and postoperative groups compared with the surgery-only group was 1.20 (95%CI: 0.93-1.53) and 1.01 (95%CI: 0.85-1.18), respectively. Across the second cancer sites, the risk associated with radiotherapy was generally consistent between the preoperative and postoperative groups, except that patients in the preoperative radiotherapy group had a higher risk of head and neck cancers (P = 0.042) (Figure 3B).
A stratified Cox proportional hazards model showed the HR of radiotherapy remained unchanged after considering second primary cancer attained year (Supplementary Table 1). There were 12064 patients surviving without a SPM after 5 years of follow-up, and 3516 patients after 10 years. The HR of radiotherapy in all patients, > 5 year survivors, and > 10 year survivors was 1.05 (95%CI: 0.91-1.21), 1.17 (95%CI: 0.92-1.47), and 1.03 (95%CI: 0.56-1.89), respectively. None of these HRs was statistically significant, as listed in Supplementary Table 2.
The aim of radiotherapy in rectal cancer is to reduce the recurrence risk, and this benefit is well documented[15]. Clinical practice has shifted from postoperative chemoradiotherapy to preoperative radiotherapy as encouraging results with preoperative radiotherapy have emerged over the last decade[4]. Still, there is debate regarding short-course preoperative radiation and the more conventional approach of long-course neoadjuvant chemoradiation. The reported efficacy of these two regimens is comparable, yet there appears to be more late gastrointestinal toxicity in short-course studies[16]. Whether different radiotherapy regimens result in different SPM risks has not been investigated. Our results showed no differences in overall SPM probability between patients in each radiotherapy regimen. To our knowledge, this is the first report to directly compare the risk of SPM among preoperative long-course radiotherapy, preoperative short-course radiotherapy, and postoperative radiotherapy.
Four previous studies have addressed the issue of SPM after rectal irradiation. Birgisson et al[12] analyzed pooled data from the Uppsala Trial and the Swedish Rectal Cancer Trial, and they reported an overall relative risk of 1.85 for developing a second cancer in irradiated patients. However, their results were limited by the relatively small cohort size. More recently, Martling et al[17]analyzed Swedish ColoRectal Cancer Registry data and reported no increased risk of second primary cancer following RT for rectal cancer within or outside of the irradiated volume up to 20 years of follow-up. Two groups have taken advantage of the large Surveillance, Epidemiology, and End Results (SEER) registry database to exam this issue, but their efforts yielded opposite results. It is noteworthy that neither of the SEER-based studies reported the radiotherapy regimen. Kendal et al[11] used Kaplan-Meier and Cox analyses and demonstrated no significant difference in SPM occurrence between irradiated and non-irradiated cohorts, comprising a total of 20910 patients. In a subpart of Berrington’s comprehensive study, they reported that the relative risk was 1.15 in irradiated patients using a Poisson regression analysis. Although radiation-induced malignancy is a stochastic effect and risk increases in a linear-quadratic fashion with dose and exposure at younger ages, they found neither a dose response nor a correlation with patient’s age at rectal cancer diagnosis. This lack may harm the validity of the casual association. In addition, the two SEER studies may have been negatively affected by occult confounding factors. For example, certain comorbidities may have a strong correlation to SPM. Liver cirrhosis is strongly associated with hepatocellular carcinoma. COPD is not only linked with smoking history but also acts as an independent risk factor for lung cancer[18]. In the present study, we demonstrated that several comorbidities were significantly associated with SPM on multivariate analysis. Any conclusion regarding radiotherapy made without adjustment for these factors is vulnerable to bias. Finally, Wiltink examined the Total Mesorectal Excision trial data[10]. They used a competing-risk model and Gray’s test and found that the 10-year SPM rates were 14.8% and 15.3% in patients with and without radiotherapy, respectively. No significant difference was noted. The competing-risk model is more accurate in estimating SPM probability than the Kaplan-Meier model in that the competing circumstance is death. However, for etiological research, a proportional cause-specific hazards model may be more appropriate than the competing-risk model[19]. Here, we used competing-risk model to report the cumulative incidence of SPM and applied a Cox model to compare the HRs for different treatment groups.
Another limitation of these four studies is the lack of chemotherapy analysis. Chemotherapy is associated with SPM risk, mainly leukemias but also solid tumors[14]. However, most data on chemotherapy are derived from studies on Hodgkin lymphoma[20,21] and breast cancer[22]. The association between chemotherapy and SPM in rectal cancers has not been studied. In our study, the use of chemotherapy was not associated with increased SPM. After controlling for chemotherapy and other comorbidities, we could assess the absolute excess risk of the radiotherapy effect. We found that the overall SPM risk did not increase in irradiated patients. Considering the diagnosis age of rectal cancer patients tends to be older, we would expect to find less radiation-induced cancers than in younger cancer patients[23]. In our sensitivity test, we added attained cancer year into the model and performed a subgroup analysis focused on long-term survivors. The absence of a radiotherapy effect was still in consistent in these analyses. Considering age is not an exclusive factor that affect surgical complication in colo-rectal cancer patients[24,25], we suggest irradiation should not be avoided either in the elderly rectal cancer patients.
In Berrington’s SEER study, the relative risk of second lung cancer in irradiated rectal cancer patients was 1.27[9]. Additionally, second primary lung cancer has been reported to increase after irradiation in prostate cancer patients[26,27]. In our second cancer site analysis, lung cancer was the only increased SPM subsite that was associated with radiotherapy. One reason for this relationship may be that lung cancer can be induced efficiently by relatively low doses of radiation, which has been shown in breast cancer and Hodgkin lymphoma survivors[28,29]. Another explanation is the possible uneven distribution of patients who smoke. Of the other specific solid tumor sites, both bladder carcinoma and uterus carcinoma showed non-significant increasing trend, which was broadly consistent with previous studies. We found that the risk of subsequent prostate cancer was decreased in irradiated patients, although again the difference was not statistically significant (HR = 0.62, 95%CI: 0.34-1.13). A recent meta-analysis supported this finding that radiotherapy for rectal cancer is associated with a decreased prostate cancer risk[30]. However, the mechanism is still unclear.
The strength of our study is that these data were derived from population-based registries, which permits a powerful evaluation of SPM risk according to a variety of relevant variables. By controlling for treatment and patient characteristics, we can minimize the potential for bias. We also performed a sensitivity analysis to test the robustness of our conclusions. Nonetheless, our study had several limitations. The main limitation was the relatively short mean follow-up. However, there were still more than 3000 patients followed up for more than 10 years. In the sensitivity analysis, the HR of radiotherapy in patients followed more than 5 or 10 years remained statistically insignificant. This conclusion is not likely to be altered after even longer follow-up periods. Second, the radiotherapy dose and volume were not available in the NHIRD, which made it impossible to analyze the radiotherapy dose response. Instead of the dose, we used radiation portals as a surrogate and applied strict criteria for the different radiotherapy regimens to ensure that the radiotherapy dose was consistent in each regimen group. Radiation techniques have evolved in the past decades, but we could not ascertain the radiotherapy technique information used for each patient. The use of the intensity-modulated radiation therapy (IMRT) technique may result in a greater volume of low-dose irradiated tissue and therefore more SPM[31]. However, the three-dimensional conformal radiation therapy (3DCRT) technique was still the standard treatment for rectal cancer during the study period. We also adjusted for the diagnosis year, which may have helped to eliminate this bias. Third, the lack of data on smoking and other lifestyle information likely suggests that there is residual confounding.
In the future, we advocate that study regards to SPM related to radiotherapy should carefully adjust comorbidities, chemotherapy, and use competing risk model to yield true effect of radiotherapy. Also studies should focused on the mechanisms by which radiation may produce carcinogenic changes, especially in SPM outside irradiation volume.
In conclusion, in this population-based study, no increased risk of developing SPM was found in rectal cancer patients who received pelvic radiotherapy in their initial treatment. The SPM risk remained the same among the preoperative long-course, preoperative short-course, and postoperative radiotherapy groups. Therefore, the SPM risk should not be a major consideration in treatment decisions. However, rectal cancer survivors, similarly to other cancer survivors, are burdened with an overall higher probability of developing a second primary cancer. Life-long follow-up is recommended.
Previous literature on second primary malignancy (SPM) risk after radiotherapy in rectal cancer survivors yielded controversial results. Also, lack of comorbidities, chemotherapy, and competing risk adjustment may cause biased conclusion. In addition, whether different radiotherapy regimens results in different SPM risk has not been investigated. In this study, we meticulously collected and analyzed all factors may contribute in SPM, and yielded true radiotherapy effect.
The risk of developing an SPM has received greater attention in clinical practice. Although several studies have investigated the relationship between radiotherapy and SPM in rectal cancer patients, the conclusions have been diverse.
To analyze true radiotherapy effect on developing an SPM in rectal cancer patients.
We used Taiwan’s National Health Insurance Research Database to identify rectal cancer patients between 1996 and 2011. The cohort was composed of patients aged 20 years or older who were diagnosed with a first primary rectal cancer. SPM risk was analyzed by competing risk model. The overall and site-specific SPM incidence rates were compared among the radiotherapy groups by multivariate Cox regression, taking chemotherapy and comorbidities into account. Sensitivity tests were performed for attained-year adjustment and long-term survivor analysis.
In this large-scale population-based cohort study, we found no increase of SPM due to radiotherapy in rectal patients. Different radiotherapy regimens results in same SPM risk. Factors that were significantly associated with a higher risk for SPMs included male sex, age, liver cirrhosis, autoimmune disease, and COPD. Compared with the surgery-only group, a significantly increased HR for SPM in the radiotherapy group was only evident for lung cancer (HR = 1.42, 95%CI: 1.04-1.93; P < 0.001). The risk of bladder, uterus, skin, and hematologic cancer was elevated in irradiated patients, but the difference was not statistically significant.
This study confirmed no increased risk of SPM due to radiotherapy in rectal patients. Many secondary malignancy may only reflect the patients’ genetic backgrounds, cancer-related treatments, lifestyles, and environmental risk factors. After careful confounder adjustment and appropriate statistical analysis, no radiotherapy effect on SPM can be drawn. This is an important conclusion to both patients and physicians.
Some comorbidities confounders have profound effects on developing secondary malignancy. Also, death is a strong competing risk need to handle. In future, we need to explore and investigate the mechanism of oncogenic effect of radiotherapy, especially in cancer outside radiation volume.
| 1. | De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol. 2014;15:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1472] [Cited by in RCA: 1392] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 2. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2083] [Article Influence: 173.6] [Reference Citation Analysis (2)] |
| 3. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1387] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 4. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1131] [Article Influence: 66.5] [Reference Citation Analysis (6)] |
| 5. | Birgisson H, Påhlman L, Gunnarsson U, Glimelius B. Late adverse effects of radiation therapy for rectal cancer - a systematic overview. Acta Oncol. 2007;46:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Phipps AI, Chan AT, Ogino S. Anatomic subsite of primary colorectal cancer and subsequent risk and distribution of second cancers. Cancer. 2013;119:3140-3147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Lee YT, Liu CJ, Hu YW, Teng CJ, Tzeng CH, Yeh CM, Chen TJ, Lin JK, Lin CC, Lan YT. Incidence of Second Primary Malignancies Following Colorectal Cancer: A Distinct Pattern of Occurrence Between Colon and Rectal Cancers and Association of Co-Morbidity with Second Primary Malignancies in a Population-Based Cohort of 98,876 Patients in Taiwan. Medicine (Baltimore). 2015;94:e1079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30:3734-3745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 242] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Berrington de Gonzalez A, Curtis RE, Kry SF, Gilbert E, Lamart S, Berg CD, Stovall M, Ron E. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12:353-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 374] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 10. | Wiltink LM, Nout RA, Fiocco M, Meershoek-Klein Kranenbarg E, Jürgenliemk-Schulz IM, Jobsen JJ, Nagtegaal ID, Rutten HJ, van de Velde CJ, Creutzberg CL. No Increased Risk of Second Cancer After Radiotherapy in Patients Treated for Rectal or Endometrial Cancer in the Randomized TME, PORTEC-1, and PORTEC-2 Trials. J Clin Oncol. 2015;33:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Kendal WS, Nicholas G. A population-based analysis of second primary cancers after irradiation for rectal cancer. Am J Clin Oncol. 2007;30:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Birgisson H, Påhlman L, Gunnarsson U, Glimelius B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J Clin Oncol. 2005;23:6126-6131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 199] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Cheng TM. Taiwan’s new national health insurance program: genesis and experience so far. Health Aff (Millwood). 2003;22:61-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 525] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 14. | Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15:2020-2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet. 2001;358:1291-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 663] [Article Influence: 26.5] [Reference Citation Analysis (12)] |
| 16. | Mohiuddin M, Marks J, Marks G. Management of rectal cancer: short- vs. long-course preoperative radiation. Int J Radiat Oncol Biol Phys. 2008;72:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Martling A, Smedby KE, Birgisson H, Olsson H, Granath F, Ekbom A, Glimelius B. Risk of second primary cancer in patients treated with radiotherapy for rectal cancer. Br J Surg. 2017;104:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 485] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 19. | Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28:2670-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 527] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 20. | Delwail V, Jais JP, Colonna P, Andrieu JM. Fifteen-year secondary leukaemia risk observed in 761 patients with Hodgkin’s disease prospectively treated by MOPP or ABVD chemotherapy plus high-dose irradiation. Br J Haematol. 2002;118:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | van Leeuwen FE, Klokman WJ, Hagenbeek A, Noyon R, van den Belt-Dusebout AW, van Kerkhoff EH, van Heerde P, Somers R. Second cancer risk following Hodgkin’s disease: a 20-year follow-up study. J Clin Oncol. 1994;12:312-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 307] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Curtis RE, Boice JD Jr, Stovall M, Bernstein L, Greenberg RS, Flannery JT, Schwartz AG, Weyer P, Moloney WC, Hoover RN. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med. 1992;326:1745-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 297] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | VanderWalde AM, Hurria A. Second malignancies among elderly survivors of cancer. Oncologist. 2011;16:1572-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Grosso G, Biondi A, Marventano S, Mistretta A, Calabrese G, Basile F. Major postoperative complications and survival for colon cancer elderly patients. BMC Surg. 2012;12 Suppl 1:S20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Biondi A, Vacante M, Ambrosino I, Cristaldi E, Pietrapertosa G, Basile F. Role of surgery for colorectal cancer in the elderly. World J Gastrointest Surg. 2016;8:606-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000;88:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 27. | Joung JY, Lim J, Oh CM, Jung KW, Cho H, Kim SH, Seo HK, Park WS, Chung J, Lee KH. Risk of Second Primary Cancer among Prostate Cancer Patients in Korea: A Population-Based Cohort Study. PLoS One. 2015;10:e0140693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Travis LB, Gospodarowicz M, Curtis RE, Clarke EA, Andersson M, Glimelius B, Joensuu T, Lynch CF, van Leeuwen FE, Holowaty E. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94:182-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 408] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 29. | Rubino C, de Vathaire F, Shamsaldin A, Labbe M, Lê MG. Radiation dose, chemotherapy, hormonal treatment and risk of second cancer after breast cancer treatment. Br J Cancer. 2003;89:840-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Lee YC, Hsieh CC, Li CY, Chuang JP, Lee JC. Secondary Cancers After Radiation Therapy for Primary Prostate or Rectal Cancer. World J Surg. 2016;40:895-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Zwahlen DR, Ruben JD, Jones P, Gagliardi F, Millar JL, Schneider U. Effect of intensity-modulated pelvic radiotherapy on second cancer risk in the postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2009;74:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Biondi A, Kin SH, Velenik V S- Editor: Gong ZM L- Editor: A E- Editor: Bian YN