Published online Oct 28, 2018. doi: 10.3748/wjg.v24.i40.4548
Peer-review started: August 7, 2018
First decision: August 27, 2018
Revised: September 20, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: October 28, 2018
Processing time: 80 Days and 22.2 Hours
At present, the best rescue therapy for Helicobacter pylori (H. pylori) infection following failure of first-line eradication remains unclear. The Maastricht V/Florence Consensus Report recommends bismuth quadruple therapy, or fluoroquinolone-amoxicillin triple/quadruple therapy as the second-line therapy for H. pylori infection. Meta-analyses have shown that bismuth quadruple therapy and levofloxacin-amoxicillin triple therapy have comparable eradication rates, while the former has more adverse effects than the latter. There are no significant differences between the eradication rates of levofloxacin-amoxicillin triple and quadruple therapies. However, the eradication rates of both levofloxacin-containing treatments are suboptimal. An important caveat of levofloxacin-amoxicillin triple or quadruple therapy is poor eradication efficacy in the presence of fluoroquinolone resistance. High-dose dual therapy is an emerging second-line therapy and has an eradication efficacy comparable with levofloxacin-amoxicillin triple therapy. Recently, a 10-d tetracycline-levofloxacin (TL) quadruple therapy comprised of a proton pump inhibitor, bismuth, tetracycline and levofloxacin has been developed, which achieves a markedly higher eradication rate compared with levofloxacin-amoxicillin triple therapy (98% vs 69%) in patients with failure of standard triple, bismuth quadruple or non-bismuth quadruple therapy. The present article reviews current second-line anti-H. pylori regimens and treatment algorisms. In conclusion, bismuth quadruple therapy, levofloxacin-amoxicillin triple/quadruple therapy, high-dose dual therapy and TL quadruple therapy can be used as second-line treatment for H. pylori infection. Current evidence suggests that 10-d TL quadruple therapy is a simple and effective regimen, and has the potential to become a universal rescue treatment following eradication failure by all first-line eradication regimens for H. pylori infection.
Core tip: The present article reviews current second-line anti-Helicobacter pylori (H. pylori) regimens. Bismuth quadruple therapy and levofloxacin-amoxicillin triple therapy have comparable eradication rates in the rescue treatment of H. pylori infection, while the former has more adverse effects than the latter. High-dose dual therapy has an eradication rate comparable with levofloxacin-amoxicillin triple therapy. Ten-day tetracycline-levofloxacin quadruple therapy achieves a markedly higher eradication rate compared with levofloxacin-amoxicillin triple therapy (98% vs 69%) in patients with failure of standard triple, bismuth quadruple or non-bismuth quadruple therapy. In conclusion, tetracycline-levofloxacin quadruple therapy has the potential to become a universal second-line treatment for H. pylori infection.
INTRODUCTION
Helicobacter pylori (H. pylori) infects > 50% of humans globally. It is a major cause of chronic gastritis, peptic ulcer disease, gastric adenocarcinoma and gastric mucosa-associated lymphoid tissue lymphoma[1,2]. With the rising prevalence of global antibiotic resistance, the eradication rate of H. pylori with standard triple therapy has decreased to < 80% worldwide[3]. Although there are other emerging 1st-line therapies, including bismuth quadruple therapy and non-bismuth quadruple (sequential, concomitant or hybrid) therapy, which can increase the eradication rate, H. pylori eradication still fails in 3%-24% of infected patients[4-7]. At present, the optimal choice for second-line anti-H. pylori therapy has not been well established. The present article aims to review and update the current options for second-line therapy against H. pylori infections.
ANTIBIOTIC RESISTANCE IN ANTI-H. PYLORI THERAPY
Causes of treatment failure of anti-H. pylori therapies include antibiotic resistant bacteria, poor patient compliance, low gastric pH and a high bacterial load. Among these reasons, antibiotic resistance is the main factor which determines the efficacy of an eradication therapy[8]. Primary resistance to amoxicillin is either null or < 1% in most countries[9]. In contrast, the rate of primary clarithromycin-resistance ranges from 49% (Spain) to 1% (the Netherlands) worldwide[10]. High primary resistance to clarithromycin and low resistance to metronidazole have been observed in Japan; moderate resistance to clarithromycin and high resistance to metronidazole were reported in South Korea; and high primary resistance to both clarithromycin and metronidazole was observed in China[11]. High primary resistance to both clarithromycin and metronidazole has also been reported in some other countries, such as Italy, Spain, Mexico and Vietnam. Low clarithromycin resistance is generally observed in northern Europe, including the Netherlands, Sweden and Ireland[10,11].
In patients who experience eradication failure following standard triple therapy, the rates of drug resistance to clarithromycin, metronidazole, levofloxacin, amoxicillin and tetracycline are 65%-75%, 30%-56%, 26%-37%, 0%-6.1% and 0%-10%, respectively[12-16]. Whereas for patients who experience failure of non-bismuth quadruple therapy, the rates of drug resistance to clarithromycin, metronidazole, levofloxacin, amoxicillin and tetracycline are 75%, 75%, 25%, 0%, and 0%, respectively[17,18]. This data implies that amoxicillin, tetracycline and levofloxacin are good choices of antibiotics for rescue treatment of H. pylori infection.
Point mutations play a primary role in the antimicrobial resistance of H. pylori, and different mutations involving the rdxA gene have been identified in metronidazole resistant strains[19]. Resistance to clarithromycin in H. pylori is commonly caused by point mutations in the rrl gene encoding two 23S rRNA nucleotides, namely 2142 and 2143[20]. Another mechanism associated with the development of clarithromycin resistance is the efflux pump system[21,22]. Fluoroquinolone acts on the site of the type A DNA gyrase enzyme, which is encoded by the gyrA gene, to inhibit DNA cleavage and rejoining[23]. Gene mutations in gyrA are associated with fluoroquinolone resistance. In particular double mutations at both N87 and D91 in gyrA have been reported to increase fluoroquinolone resistance[24].
UPDATED SECOND-LINE THERAPIES
Current updated second-line therapies include bismuth quadruple therapy, fluoroquinolone-amoxicillin triple therapy, fluoroquinolone-amoxicillin quadruple therapy, tetracycline-levofloxacin (TL) quadruple therapy and high-dose dual therapy.
- Citation: Lin TF, Hsu PI. Second-line rescue treatment of Helicobacter pylori infection: Where are we now? World J Gastroenterol 2018; 24(40): 4548-4553
- URL: https://www.wjgnet.com/1007-9327/full/v24/i40/4548.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i40.4548
Bismuth quadruple therapy consists of a proton pump inhibitor (PPI), bismuth, metronidazole and tetracycline (Table 1). The standard regimen comprises PPI twice daily, colloidal bismuth subcitrate 120 mg four times daily, tetracycline 500 mg four times daily and metronidazole 500 mg three times daily for 10 to 14 d. A pool analysis demonstrated that bismuth quadruple therapy fails in 5%-63% of patients as a second-line therapy and achieves a mean 76% eradication rate[25-27]. Its efficacy is related to metronidazole resistance in H. pylori strains and the duration of the regimens[28]. Meta-analysis of randomized controlled trials of bismuth quadruple therapy as a rescue treatment after failure of clarithromycin triple therapy revealed a significantly higher eradication rate for the 14-d regimen compared with the 7-d regimen[29]. Therefore, it is reasonable to encourage a 14-d regimen duration for bismuth quadruple therapy when used as a second-line treatment for H. pylori infection.
| Regimen | Drug | Duration of therapy | |||||
| PPI | Bismuth | Levo | Amox | Tetra | Metro | ||
| Bismuth-containing quadruple therapy | SD, b.i.d. | 120 mg, q.i.d. | 500 mg, q.i.d. | 500 mg, t.i.d. | 10-14 d | ||
| Levofloxacin-containing triple therapy | SD, b.i.d. | 500 mg, q.d. | 1 g, b.i.d. | 10-14 d | |||
| Levofloxacin-amoxicillin quadruple therapy | SD, b.i.d. | 120 mg, q.i.d. | 500 mg, q.d. | 1 g, b.i.d. | 10-14 d | ||
| Tetracycline-levofloxacin quadruple therapy | SD, b.i.d. | 120 mg, q.i.d. | 500 mg, q.d. | 500 mg, q.i.d. | 10 d | ||
| High-dose dual therapy | SD, q.i.d. | 750 mg, b.i.d. | 14 d | ||||
The most commonly used fluoroquinolone-based triple therapy is composed of levofloxacin 500 mg daily, amoxicillin 1 g twice daily and a PPI (standard dose) twice daily for 10 to 14 d (Table 1). Meta-analyses revealed that levofloxacin-amoxicillin triple therapy and bismuth quadruple therapy had comparable eradication rates, whereas the former had fewer adverse effects than the latter[30]. A systemic review and meta-analysis revealed that levofloxacin-amoxicillin triple therapy achieved an overall eradication rate of 78% after failure of a non-bismuth quadruple therapy[31]. It was similarly effective after failure of sequential and concomitant therapies (81% vs 78%, respectively), and the cure rate of levofloxacin-amoxicillin triple therapy following hybrid therapy was 50%.
An important drawback of levofloxacin-amoxicillin triple therapy is poor eradication efficacy in the presence of fluoroquinolone resistance. Bismuth salts have a synergistic effect on antibiotics and have been used to increase eradication rates[32]. The Maastricht V/Florence Consensus Report also recommended the application of fluoroquinolone-amoxicillin quadruple therapy as a second-line therapy for H. pylori infection[31]. Levofloxacin-amoxicillin quadruple therapy is composed of levofloxacin 500 mg daily, amoxicillin 1 g twice daily, PPI (standard dose) twice daily and bismuth 240 mg twice daily for 10 to 14 d (Table 1). A randomized controlled trial showed there were no significant differences between the eradication rates of second-line 14-d levofloxacin-amoxicillin quadruple therapy and 14-d levofloxacin-amoxicillin triple therapy (87% vs 83%, respectively)[33]. However, the former had a higher eradication rate for levofloxacin-resistant strains than the latter (71% vs 37%)[33].
Recently, Hsu et al[17] developed a novel TL quadruple therapy as a rescue treatment for H. pylori infection. It consists of esomeprazole 40 mg twice daily, tripotassium dicitrato bismuthate 120 mg four times daily, tetracycline 500 mg four times daily, and levofloxacin 500 mg once daily for 10 d (Table 1). The simple regimen maintains a high eradication rate for H. pylori strains with levofloxacin resistance[17]. A randomized control study showed that as a second-line anti-H. pylori treatment, 10-d of TL quadruple therapy achieved a much higher eradication rate compared with 10-d levofloxacin triple therapy containing esomeprazole, amoxicillin and levofloxacin (98% vs 68%, respectively)[34]. Subgroup analysis revealed that the former was superior to the latter in patients with failure of either standard triple therapy (100% vs 75%) or non-bismuth quadruple therapy (95% vs 53%). There were only 7 patients recruited into the study with eradication failure by bismuth quadruple therapy as a first-line treatment, and both TL quadruple and levofloxacin-amoxicillin triple therapies had a 100% eradication rate in this subgroup of patients. The data suggests that 10-d TL quadruple therapy is a good option for second-line treatment after failure of standard triple, concomitant and bismuth quadruple therapies.
High-dose dual therapy is another emerging second-line treatment for H. pylori infection[35]. The new therapy consists of high-dose PPI and amoxicillin (Table 1), which keep the intragastric pH higher than 6.5 regardless of CYP2C19 genotype[36], and maintain a steady plasma concentration of amoxicillin above the minimal inhibitory concentration for H. pylori[37]. A randomized control trial from Taiwan revealed that 14-d high-dose dual therapy achieved a higher eradication rate than 10-d sequential therapy as a second-line treatment for H. pylori infection (89% vs 52%), and had an eradication rate comparable with 7-d levofloxacin-amoxicillin triple therapy (79%)[35]. Another randomized controlled trial from Germany demonstrated that 14-d high-dose dual therapy and 14-d bismuth quadruple therapy had comparable efficacies as a rescue treatment for H. pylori infection (76% vs 81%, respectively)[38].
TREATMENT ALGORISM
After a first failure of H. pylori treatment, if an endoscopy is arranged, the Maastricht V/Florence Consensus Report recommends antimicrobial susceptibility testing (AST)[31] to enable tailoring of the rescue eradication therapy. However, AST is not routinely performed in clinical practice due to the invasiveness of the endoscopy procedure, the availablity of laboratory culture facilities and cost considerations. If AST data are not available, 10-d TL quadruple therapy can be used as a rescue treatment since it achieves an eradication rate of > 90% following failure of standard triple, concomitant and bismuth quadruple therapies. In addition, the novel 10-d TL quadruple regimen can maintain a high eradication rate (> 90%) for H. pylori strains with levofloxacin resistance[34]. However, the choice of second line rescue regimen also depends on regional factors. In Japan, PPI-containing triple therapy with metronidazole and amoxicillin is the standard second line regimen and is covered under Japan’s national health insurance. This second-line therapy can also achieve an eradication rate of around 90% because metronidazole resistance rate is relatively low in Japan.
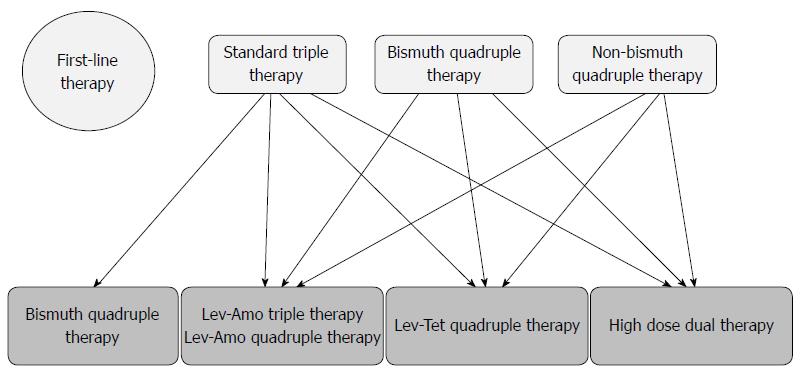
According to the Maastricht V/Florence Consensus Report[31], bismuth-containing quadruple therapy, fluoroquinolone-containing triple therapy or fluoroquinolone-amoxicillin quadruple therapy are recommended following failure of standard triple therapy. As TL quadruple therapy achieves a higher eradication rate than levofloxacin triple therapy, and high-dose dual therapy has a comparable eradication rate with levofloxacin-amoxicillin triple therapy in patients with failure of standard triple therapy[34,35], both TL quadruple and high-dose dual therapies can be recommended as a rescue regimen following failure of standard triple therapy (Figure 1).
The Maastricht V/Florence Consensus Report recommends bismuth quadruple therapy, levofloxacin-amoxicillin triple therapy and levofloxacin-amoxicillin quadruple therapy as rescue treatments after failure of a non-bismuth quadruple therapy[31]. As 10-d TL quadruple therapy is superior to 10-d levofloxacin-amoxicillin triple therapy, it is reasonable to recommend TL-quadruple therapy as the rescue treatment for patients with eradication failure by non-bismuth quadruple therapy (Figure 1).
According to the Maastricht V/Florence Consensus Report[31], fluoroquinolone-containing triple or fluoroquinolone-amoxicillin quadruple therapy can be recommended for patients with eradication failure by bismuth quadruple therapy for H. pylori infection. As both TL quadruple and levofloxacin-amoxicillin triple therapies achieved a 100% cure rate in this setting. TL quadruple therapy may also be considered as an option for the rescue treatment of bismuth quadruple therapy (Figure 1).
CONCLUSION
The current updated second-line therapies include bismuth quadruple therapy, fluoroquinolone-amoxicillin triple therapy, fluoroquinolone-amoxicillin quadruple therapy, TL quadruple therapy and high-dose dual therapy. Ten-day TL quadruple therapy has great potential to become a universal rescue treatment following eradication failure by all first-line eradication regimens for H. pylori infection, and warrants further investigation.
| 1. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1934] [Article Influence: 80.6] [Reference Citation Analysis (3)] |
| 2. | Zucca E, Dreyling M; ESMO Guidelines Working Group. Gastric marginal zone lymphoma of MALT type: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:113-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Camargo MC, García A, Riquelme A, Otero W, Camargo CA, Hernandez-García T, Candia R, Bruce MG, Rabkin CS. The problem of Helicobacter pylori resistance to antibiotics: a systematic review in Latin America. Am J Gastroenterol. 2014;109:485-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 4. | Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069-79; quiz 1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 5. | Hsu PI, Wu DC, Wu JY, Graham DY. Is there a benefit to extending the duration of Helicobacter pylori sequential therapy to 14 days? Helicobacter. 2011;16:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Hsu PI, Wu DC, Wu JY, Graham DY. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter. 2011;16:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 7. | Kao SS, Chen WC, Hsu PI, Lai KH, Yu HC, Cheng HH, Peng NJ, Lin CK, Chan HH, Tsai WL. 7-Day Nonbismuth-Containing Concomitant Therapy Achieves a High Eradication Rate for Helicobacter pylori in Taiwan. Gastroenterol Res Pract. 2012;2012:463985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 750] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 9. | Kobayashi I, Murakami K, Kato M, Kato S, Azuma T, Takahashi S, Uemura N, Katsuyama T, Fukuda Y, Haruma K. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007;45:4006-4010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011;17:3971-3975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 107] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Suzuki H, Mori H. World trends for H. pylori eradication therapy and gastric cancer prevention strategy by H. pylori test-and-treat. J Gastroenterol. 2018;53:354-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Wu IT, Chuah SK, Lee CH, Liang CM, Lu LS, Kuo YH, Yen YH, Hu ML, Chou YP, Yang SC. Five-year sequential changes in secondary antibiotic resistance of Helicobacter pylori in Taiwan. World J Gastroenterol. 2015;21:10669-10674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Kuo CH, Hu HM, Kuo FC, Hsu PI, Chen A, Yu FJ, Tsai PY, Wu IC, Wang SW, Li CJ. Efficacy of levofloxacin-based rescue therapy for Helicobacter pylori infection after standard triple therapy: a randomized controlled trial. J Antimicrob Chemother. 2009;63:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Rendell-Baker L. Nineteenth-century resuscitation apparatus. Anaesthesia. 1981;36:1058-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Liou JM, Bair MJ, Chen CC, Lee YC, Chen MJ, Chen CC, Tseng CH, Fang YJ, Lee JY, Yang TH. Levofloxacin Sequential Therapy vs Levofloxacin Triple Therapy in the Second-Line Treatment of Helicobacter pylori: A Randomized Trial. Am J Gastroenterol. 2016;111:381-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Cao Z, Chen Q, Zhang W, Liang X, Liao J, Liu W, Xiao S, Lu H. Fourteen-day optimized levofloxacin-based therapy versus classical quadruple therapy for Helicobacter pylori treatment failures: a randomized clinical trial. Scand J Gastroenterol. 2015;50:1185-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Hsu PI, Chen WC, Tsay FW, Shih CA, Kao SS, Wang HM, Yu HC, Lai KH, Tseng HH, Peng NJ, Chen A, Kuo CH, Wu DC; Taiwan Acid-Related Disease (TARD) Study Group. Ten-day Quadruple therapy comprising proton-pump inhibitor, bismuth, tetracycline, and levofloxacin achieves a high eradication rate for Helicobacter pylori infection after failure of sequential therapy. Helicobacter. 2014;19:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Gisbert JP, Molina-Infante J, Marin AC, Vinagre G, Barrio J, McNicholl AG. Second-line rescue triple therapy with levofloxacin after failure of non-bismuth quadruple "sequential" or "concomitant" treatment to eradicate H. pylori infection. Scand J Gastroenterol. 2013;48:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Masaoka T, Suzuki H, Kurabayashi K, Nomoto Y, Nishizawa T, Mori M, Hibi T. Could frameshift mutations in the frxA and rdxA genes of Helicobacter pylori be a marker for metronidazole resistance? Aliment Pharmacol Ther. 2006;24 Suppl 4:81-87. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Arslan N, Yılmaz Ö, Demiray-Gürbüz E. Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World J Gastroenterol. 2017;23:2854-2869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (3)] |
| 21. | Bina JE, Alm RA, Uria-Nickelsen M, Thomas SR, Trust TJ, Hancock RE. Helicobacter pylori uptake and efflux: basis for intrinsic susceptibility to antibiotics in vitro. Antimicrob Agents Chemother. 2000;44:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Hirata K, Suzuki H, Nishizawa T, Tsugawa H, Muraoka H, Saito Y, Matsuzaki J, Hibi T. Contribution of efflux pumps to clarithromycin resistance in Helicobacter pylori. J Gastroenterol Hepatol. 2010;25 Suppl 1:S75-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Nishizawa T, Suzuki H. Mechanisms of Helicobacter pylori antibiotic resistance and molecular testing. Front Mol Biosci. 2014;1:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Mori H, Suzuki H, Matsuzaki J, Masaoka T, Kanai T. Acquisition of double mutation in gyrA caused high resistance to sitafloxacin in Helicobacter pylori after unsuccessful eradication with sitafloxacin-containing regimens. United European Gastroenterol J. 2018;6:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Wu DC, Hsu PI, Tseng HH, Tsay FW, Lai KH, Kuo CH, Wang SW, Chen A. Helicobacter pylori infection: a randomized, controlled study comparing 2 rescue therapies after failure of standard triple therapies. Medicine (Baltimore). 2011;90:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 26. | Gisbert JP. "Rescue" regimens after Helicobacter pylori treatment failure. World J Gastroenterol. 2008;14:5385-5402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 27. | Hojo M, Miwa H, Nagahara A, Sato N. Pooled analysis on the efficacy of the second-line treatment regimens for Helicobacter pylori infection. Scand J Gastroenterol. 2001;36:690-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Lee BH, Kim N, Hwang TJ, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, Lee DH, Jung HC. Bismuth-containing quadruple therapy as second-line treatment for Helicobacter pylori infection: effect of treatment duration and antibiotic resistance on the eradication rate in Korea. Helicobacter. 2010;15:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 1069] [Article Influence: 118.8] [Reference Citation Analysis (1)] |
| 30. | Di Caro S, Fini L, Daoud Y, Grizzi F, Gasbarrini A, De Lorenzo A, Di Renzo L, McCartney S, Bloom S. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastroenterol. 2012;18:5669-5678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 2085] [Article Influence: 231.7] [Reference Citation Analysis (1)] |
| 32. | Goodwin CS, Marshall BJ, Blincow ED, Wilson DH, Blackbourn S, Phillips M. Prevention of nitroimidazole resistance in Campylobacter pylori by coadministration of colloidal bismuth subcitrate: clinical and in vitro studies. J Clin Pathol. 1988;41:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 143] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Liao J, Zheng Q, Liang X, Zhang W, Sun Q, Liu W, Xiao S, Graham DY, Lu H. Effect of fluoroquinolone resistance on 14-day levofloxacin triple and triple plus bismuth quadruple therapy. Helicobacter. 2013;18:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Hsu PI, Tsai FW, Kao SS, Hsu WH, Cheng JS, Peng NJ, Tsai KW, Hu HM, Wang YK, Chuah SK. Ten-Day Quadruple Therapy Comprising Proton Pump Inhibitor, Bismuth, Tetracycline, and Levofloxacin is More Effective than Standard Levofloxacin Triple Therapy in the Second-Line Treatment of Helicobacter pylori Infection: A Randomized Controlled Trial. Am J Gastroenterol. 2017;112:1374-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Yang JC, Lin CJ, Wang HL, Chen JD, Kao JY, Shun CT, Lu CW, Lin BR, Shieh MJ, Chang MC. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2015;13:895-905.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 36. | Sugimoto M, Furuta T, Shirai N, Kajimura M, Hishida A, Sakurai M, Ohashi K, Ishizaki T. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2004;76:290-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1-10; quiz 11-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2187] [Cited by in RCA: 2239] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 38. | Miehlke S, Kirsch C, Schneider-Brachert W, Haferland C, Neumeyer M, Bästlein E, Papke J, Jacobs E, Vieth M, Stolte M. A prospective, randomized study of quadruple therapy and high-dose dual therapy for treatment of Helicobacter pylori resistant to both metronidazole and clarithromycin. Helicobacter. 2003;8:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Nishida T, Suzuki H S- Editor: Ma RY L- Editor: A E- Editor: Bian YN