Published online Jan 28, 2018. doi: 10.3748/wjg.v24.i4.494
Peer-review started: October 28, 2017
First decision: November 22, 20107
Revised: December 20, 2017
Accepted: December 27, 2017
Article in press: December 27, 2017
Published online: January 28, 2018
Processing time: 87 Days and 12 Hours
To investigate the relationship between glucose metabolism and glypican-3 (GPC3) expression in hepatocellular carcinoma (HCC).
Immunohistochemical staining of pathological samples for GPC3 and glucose transporter 1 (GLUT1), and whole-body 18F-FDG PET/CT for measuring tumour glucose uptake were performed in 55 newly diagnosed HCC patients. The maximum standard uptake value (SUVmax) and tumour-to-non-tumourous liver uptake (T/NT) ratio were used to quantify 18F-FDG uptake. In vitro18F-FDG uptake assay of GPC3-expressing HepG2 and non-GPC3-expressing RH7777 cells was used to examine the effect of GPC3 in cellular glucose metabolism. The relationships between GPC3 expression and 18F-FDG uptake, GLUT1 expression, tumour differentiation, and other clinical indicators were analysed using Spearman rank correlation, univariate and multiple logistic regression analyses.
Positive GPC3 expression was observed in 67.3% of HCC patients, including 75.0% of those with well or moderately differentiated HCC and 36.4% of those with poorly differentiated HCC. There was an inverse relationship between GPC3 expression and SUVmax (Spearman correlation coefficient = -0.281, P = 0.038) and a positive relationship between GLUT1 expression and SUVmax (Spearman correlation coefficient = 0.681, P < 0.001) in patients with HCC. Univariate analysis showed that two glucose metabolic parameters (SUVmax and T/NT ratio), tumour differentiation, lymph node metastasis, and TNM stage were all significantly associated with GPC3 expression (P < 0.05), whereas GLUT1 expression, sex, age, tumour size, intrahepatic lesion number, and distant metastasis showed no statistical association (P > 0.05). Further multivariate analysis revealed that only the T/N ratio was significantly correlated with GPC3 expression in patients with HCC (P < 0.05). In vitro assay revealed that the uptake of 18F-FDG in GPC3-expressing HepG2 cells was significantly lower than that of non-GPC3-expressing RH7777 cells (t = -20.352, P < 0.001).
The present study demonstrated that GPC3 expression is inversely associated with glucose metabolism, suggesting that GPC3 may play a role in regulating glucose metabolism in HCC.
Core tip: The present study demonstrated that glypican-3 (GPC3) was positively expressed in 67.3% of hepatocellular carcinoma (HCC) patients. GPC3 expression is found to be inversely associated with the glucose metabolism of HCC tumours in the patient study. Multivariate analysis revealed that only the glucose metabolism was significantly correlated with GPC3 expression (P < 0.05), but not GLUT1 expression, tumour differentiation, or other clinical indicators (P < 0.05). Low glucose metabolism was also observed in positive GPC3-expressing HepG2 cells in cellular uptake assay. Therefore, we suggested that GPC3 may play a role in regulating glucose metabolism in HCC.
- Citation: Li YC, Yang CS, Zhou WL, Li HS, Han YJ, Wang QS, Wu HB. Low glucose metabolism in hepatocellular carcinoma with GPC3 expression. World J Gastroenterol 2018; 24(4): 494-503
- URL: https://www.wjgnet.com/1007-9327/full/v24/i4/494.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i4.494
Hepatocellular carcinoma (HCC) is one of the most common and fatal malignancies worldwide and is especially prevalent in China. The outcome of patients with HCC is poor, with a low five-year survival rate of 25%-39%[1]. Surgical resection remains the standard treatment for early stage HCC[2,3]. Unfortunately, most patients present with advanced stage HCC at diagnosis, and there are few treatment options for them since current systemic therapy often cannot effectively control advanced stage HCC[1,2]. Therefore, it is of utmost importance to develop a technique that can accurately diagnose early stage HCC as well as an effective treatment that can control advanced stage HCC.
Glypican-3 (GPC3) is a member of the glypican family of heparin sulfate proteoglycans (HSPGs). This protein has been reported to be highly expressed in HCC, but not in normal liver tissue, cirrhosis tissue, or paracancerous tissue[4-6]. GPC3 plays an important role in regulating malignant transformation and promoting the growth of HCC by stimulating the canonical Wnt signalling pathway[7]. Therefore, GPC3 is suggested to be an important target for diagnosis and therapy. Recently, positron emission tomography (PET) imaging using a 89Zr-conjugated monoclonal antibody or a F(ab′)2 fragment directed against GPC3 was shown to successfully enable the non-invasive quantification and visualization of tumour GPC3 expression in vivo[8-10], which has potential to be a specific probe for HCC detection. In addition, GPC3-targeted therapies are emerging as novel molecular treatments for HCC patients[11-15].
Malignant cells require accelerated glycolysis to generate ATP, in order to meet their high energy demands for cell proliferation and survival. Accelerated glycolysis has been widely confirmed to be a common biological phenomenon in malignant tumours by positron emission tomography combined with computed tomography (PET/CT) using 2-[fluorine-18]-fluoro-2-deoxy-D-glucose (18F-FDG), a glucose analogue[16-18]. However, glucose metabolism varies greatly in HCC. Low glucose metabolism was often observed in well- and moderately differentiated HCC[19-20]. Previous studies have revealed that low 18F-FDG uptake was correlated with high FDG-6-phosphatase activity, low expression of GLUT1 or GLUT2, and high expression of P-glycoprotein[21-22]. However, Cho et al[23] found that GPC3 may also be an important regulator for glucose metabolism in HCC. They reported that GPC3 could bind to GLUT1 and decrease glucose uptake by HCC cells. Nevertheless, this phenomenon has not yet been confirmed in patients. Therefore, we performed the present study to elucidate their relationship in HCC patients. This work may contribute to a better understanding of the biological role of GPC3 in HCC and could be useful to predict the potential utility of GPC3 targeted imaging in the clinic.
This study included 55 patients (46 males, 9 females; mean age: 52.9 years [range: 18-78 years]) with newly diagnosed HCC who underwent 18F-FDG PET/CT for staging before local hepatectomy or biopsy at Nanfang Hospital from August 2013 to October 2017. The inclusion criteria were as follows: (1) final diagnosis of HCC established by pathologic examination; (2) no adjuvant therapy administered before the PET/CT scans; and (3) available GPC3 and GLUT1 immunohistochemical staining. A total of 55 patients met the criteria and were enrolled in this study.
18F-FDG PET/CT scans were performed using a Biograph mCTx scanner (Siemens, Germany). The patients were instructed to fast for at least 6 h, and their blood glucose levels were monitored with a glucometer prior to 18F-FDG injection. All the patients had blood glucose levels below 7.0 mmol/L. 18F-FDG was manufactured using a tracer synthesis system (TRACERlab FXFDG; GE Healthcare, United States) and had a > 95% radiochemical purity. Approximately 60 min after the intravenous injection of 318-524 MBq (8.6-14.2 mCi, 150 μCi/kg) 18F-FDG, whole-body PET/CT was performed at our centre according to established protocols[24].
The acquired PET and CT images were registered and analysed using the syngo MI workplace (Siemens, Germany). All the PET/CT images were independently read by two nuclear medicine physicians with over five years of experience. Both physicians were blinded to the findings of other imaging modalities and the GPC3 expression data. For visual analysis, tumours with higher 18F-FDG uptake than that of non-tumour liver tissue were considered PET positive. For the semi-quantitative analysis, a region of interest (ROI) was drawn along the margin of the HCC lesion to measure the SUVmax, which was used to quantify glucose metabolism. We also calculated the tumour-to-non-tumourous liver uptake (T/NT) ratio by dividing the tumour SUVmax by the SUVmean of the non-tumourous liver tissue, which was measured by automated computation of the average SUVmean of three 1-cm ROIs in non-tumourous liver tissue, two in the right lobe and one in the left lobe, using the syngo MI workplace (Siemens, Germany)[25]. For lesions without obvious 18F-FDG uptake, the ROI was drawn on CT images and copied to the corresponding region on the PET images in order to measure the SUVmax and T/NT ratio. Non-contrast-enhanced CT images obtained from PET/CT were reviewed by two experienced radiologists.
HCC tissue samples were acquired via biopsy or surgical resection. Paraffin-embedded tissue sections were deparaffinized with xylene and rehydrated in a graded series of ethanol solutions. Antigen retrieval was performed by heating the slides twice in 0.01 mol/L sodium citrate buffer, pH 6.0, in a microwave oven (13 min, 850 W). Endogenous peroxidase was then blocked with 0.3% H2O2 in methanol for 15 min at room temperature. Immunohistochemical staining was performed by incubating the slides with a mouse anti-GPC3 antibody (sc-65443 1G12; Santa Cruz Inc., Santa Cruz, CA, United States) or rabbit anti-GLUT1 antibody (ZA-0471; ZSGB-BIO, China) at a dilution of 1:100 at 4 °C overnight. Serial sections were stained with a horseradish peroxidase enzyme-labelled polymer conjugated to anti-mouse/rabbit immunoglobulins, according to the instructions of the Chemmate EnVision/Mo&Rb Detection Kit (GK500705, Gene Tech Company Limited, Shanghai, China).
The total GPC3 immunostaining score was calculated based on the percent positivity of stained tumour cells and the staining intensity. The percent positivity was scored as 0 (< 5%), 1 (5%-10%), 2 (11%-50%), or 3 (> 50%). The staining intensity was scored as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (strong staining). The percent positivity and staining intensity were determined in a double-blinded manner. The GPC3 expression score based on membrane and cytoplasmic immunostaining was calculated as percent positivity score × staining intensity score and ranged from 0 to 9. The GPC3 expression level was defined as -, 0; 1+, 1-2; 2+, 3-5; or 3+, 6-9[26].
Glucose transporter-1 expression in tumour cells was evaluated using a semiquantitative scoring method: score 0 = absence of immunostaining; score 1 = 1%-10% of cells stained; score 2 = 10%-50% of cells stained; and score 3 = > 50% of cells stained. No account was taken for the intensity of staining and necrotic areas were excluded from the evaluation[27]. An immunoreactive score above 2 was defined as high GLUT1 expression, while a score of 0 or 1 was defined as low expression.
In vitro assay was performed to evaluate the effect of GPC3 expression on the cellular glucose metabolism. GPC3-expressing HepG2 and non-GPC3-expressing RH7777 cells[8-10] were seeded into 12-well plates at a density of 5 × 105 cells per well for overnight incubation. Cells were rinsed three times with phosphate buffered saline (PBS), followed by the addition of 18F-FDG (111 kBq/well) to the cultured wells in quadruplicate. After incubation at 37 °C for 60 min, cells were rinsed three times with PBS and lysed with NaOH sodium dodecyl sulfate (SDS) (0.2 mol/L NaOH, 1% SDS). The cell lysate was collected and the cell-associated radioactivity was then measured using a gamma counter (GC-1200, USTC Chuangxin Co. Ltd. Zonkia Branch, China). The cell uptake was normalized with inputted radioactivity. Experiments were conducted in quadruplicate[28].
All statistical analyses were performed using SPSS version 20.0. Differences in glucose metabolic parameters (SUVmax, T/NT ratio) between groups were compared using the t-test (unpaired). GPC3 positive rates were compared using the crosstabs χ2 test. Spearman rank correlation was used to determine the association between GPC3 expression, GLUT1 expression, and glucose metabolism. Univariate and multiple logistic regression analyses were used to analyse the association between GPC3 expression and 18F-FDG uptake, GLUT1, histopathological diagnosis, and other clinical parameters. A P-value < 0.05 indicated statistical significance.
Of the 55 included patients, 44 (80.0%) were diagnosed with well or moderately differentiated HCC, and 11 (20.0%) were diagnosed with poorly differentiated HCC. Immunohistochemical analysis showed that the expression of GPC3 was positive in 67.3% (37/55) of patients, including 75.0% (33/44) of those with well or moderately differentiated HCC and 36.4% (4/11) of those with poorly differentiated HCC patients. The GPC3 expression score was 3+ in 34.5% (19/55) of the patients, 2+ in 14.5% (8/55), 1+ in 18.2% (10/55), and 0 in 32.7% (18/55). Twenty percent (11/55) of tumours had high GLUT1 expression and 80% (44/55) tumours had low GLUT1 expression. Multiple intrahepatic lesions were found in 18 (32.7%) patients, and solitary lesions were observed in 37 (67.3%) patients. Most intrahepatic lesions (69.1%) were larger than 5 cm in diameter. The disease was categorized into TNM stage I in 29 patients, TNM stage II in 4, TNM stage III in 6, and TNM stage IV in 16. Other related clinical information is shown in Table 1.
| Variable | GPC3 expression | χ2or t | P value | |
| Negative | Positive | |||
| Gender | 1.458 | 0.227 | ||
| Male | 13 (28.3) | 33 (71.7) | ||
| Female | 5 (55.6) | 4 (44.4) | ||
| Age (yr) | 0.014 | 0.907 | ||
| < 50 | 7 (31.8) | 15 (68.2) | ||
| ≥ 50 | 11 (33.3) | 22 (66.7) | ||
| Tumour differentiation | 4.341 | 0.037 | ||
| Well or moderate | 11 (25.0) | 33 (75.0) | ||
| Poor | 7 (63.6) | 4 (36.4) | ||
| Tumour size (cm) | 2.542 | 0.111 | ||
| < 5 | 3 (17.6) | 14 (82.4) | ||
| ≥ 5 | 15 (39.5) | 23 (60.5) | ||
| 18F-FDG | 3.135 | 0.77 | ||
| Positive | 15 (40.5) | 22 (59.5) | ||
| Negative | 3 (16.7) | 15 (83.3) | ||
| Intrahepatic lesion number | 0.461 | 0.497 | ||
| Solitary | 11 (29.7) | 26 (70.3) | ||
| Multiple | 7 (38.9) | 11 (61.1) | ||
| Lymph node metastasis | 4.341 | 0.037 | ||
| Positive | 7 (63.7) | 4 (36.4) | ||
| Negative | 11 (25.0) | 33 (75.0) | ||
| Distant metastasis | 0.836 | 0.361 | ||
| Positive | 5 (50.0) | 5(50.0) | ||
| Negative | 13 (28.9) | 32(71.1) | ||
| TNM stage | 4.969 | 0.026 | ||
| I-II | 7 (21.2) | 26 (78.8) | ||
| III-IV | 11 (50.0) | 11 (50.0) | ||
| Serum AFP (μg/L) | 2.645 | 0.104 | ||
| < 20 | 11 (44.0) | 14 (56.0) | ||
| ≥ 20 | 7 (23.3) | 23 (76.7) | ||
| HBV infection | 0.836 | 0.361 | ||
| Positive | 13 (28.9) | 32 (71.1) | ||
| Negative | 5 (50.0) | 5 (50.0) | ||
| Liver cirrhosis | 0.445 | 0.505 | ||
| Positive | 10 (29.4) | 24 (70.6) | ||
| Negative | 8 (38.1) | 13 (61.9) | ||
| GLUT1 | 1.863 | 0.172 | ||
| High | 6 (54.5) | 5 (45.5) | ||
| Low | 12 (27.3) | 32 (72.7) | ||
| SUVmax | 9.56 ± 5.95 | 6.01 ± 3.55 | 2.341 | 0.028 |
| T/N ratio | 4.52 ± 2.92 | 2.62 ± 1.55 | 2.597 | 0.017 |
HCC lesions were noted to be positive for 18F-FDG PET/CT in 37 (67.3%) patients and negative in 18 (32.7%) patients by the visual analysis. The SUVmax for primary tumours ranged from 2.07 to 18.60 (7.17 ± 4.73) and the T/NT ratio ranged from 0.86 to 10.0 (3.24 ± 2.26). In the lesions with negative 18F-FDG uptake, GPC3 expression was positive in 15/18 (83.3%) patients. Combining 18F-FDG uptake with GPC3 expression, the total positivity reached 94.5% (52/55).
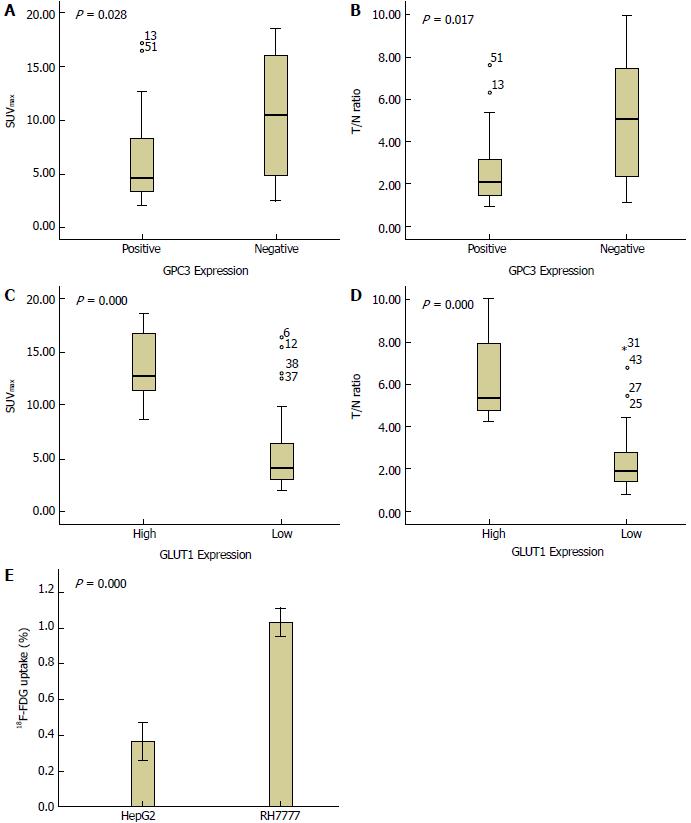
There was an inverse relationship between GPC3 expression and 18F-FDG uptake (SUVmax: Spearman correlation coefficient = -0.281, P = 0.038; T/NT ratio: Spearman correlation coefficient = -0.303, P = 0.025), and 18F-FDG uptake in HCC lesions with GPC3 positivity was significantly lower than that of lesions with GPC3 negativity (SUVmax: 6.01 ± 3.55 vs 9.56 ± 5.95, t = -2.341, P = 0.028; T/NT ratio: 2.62 ± 1.55 vs 4.52 ± 2.92, t = -2.597, P = 0.017) (Figure 1A and B, Figure 2). On the contrary, a positive association was found between GLUT1 expression and 18F-FDG uptake (SUVmax: Spearman correlation coefficient = 0.681, P < 0.001; T/NT ratio: Spearman correlation coefficient = 0.616, P < 0.001). 18F-FDG uptake in the high GLUT1 expression group was significantly higher than that in the low GLUT1 expression group (SUVmax: 13.58 ± 3.44 vs 5.57 ± 3.49, t = 6.898, P < 0.001; T/NT ratio: 6.38 ± 1.91 vs 2.46 ± 1.55, t = 6.307, P < 0.001) (Figure 1C and D). We then investigated the relationship between GPC3 and GLUT1 expression. Low GLUT1 expression was found in 86.5% of GPC3-positive tumours and in 66.7% of GPC3-negative tumours, respectively. Although there was an inverse trend of relationship between GPC3 and GLUT1 expression, it did not reach statistical significance (Spearman correlation coefficient = -0.232, P = 0.088).
There were significant differences in SUVmax and T/NT ratio between different degrees of tumour differentiation. Poorly differentiated HCC had a significantly higher SUVmax and T/NT ratio than well- or moderately differentiated HCC (SUVmax: 10.96 ± 6.08 vs 6.22 ± 3.86, t = 2.465, P = 0.030; T/NT ratio: 5.16 ± 3.06 vs 2.76 ± 1.74, t = 2.499, P = 0.028, respectively).
SUVmax, T/NT ratio, tumour differentiation, and other clinical factors were analysed for their relationship with GPC3 expression. Univariate analysis showed that two glucose metabolic parameters (SUVmax and T/NT ratio), tumour differentiation, lymph node metastasis, and TNM stage were all significantly associated with GPC3 positivity in HCC patients (P < 0.05) (Table 1). Low 18F-FDG uptake was observed in GPC3-positive HCCs. Well- or moderately differentiated HCCs also showed a significantly higher GPC3 positive rate than poorly differentiated HCC tumours (75.0% vs 36.4%, χ2 = 4.341, P = 0.037). Similar trends were observed for lymph node metastasis and TNM stage. Higher GPC3 positivity rates were found in patients with no lymph node metastasis and those with TNM stage I-II disease, than in patients with lymph node metastasis and TNM stage III-IV disease, respectively (75.0% vs 36.4%, χ2 = 4.341, P = 0.037 for lymph node status ; 78.8% vs 50.0%, χ2 = 4.969, P = 0.026 for TNM stage). Other clinical factors, such as GLUT1 expression, sex, age, tumour size, 18F-FDG positivity, intrahepatic lesion number, distant metastasis, HBV infection, and liver cirrhosis, were not significantly related to GPC3 expression (P > 0.05) (Table 1).
The five factors (two glucose metabolic parameters, tumour differentiation, lymph node metastasis, and TNM stage) that showed a significant relationship with GPC3 expression on univariate analysis were further analysed using multivariate analysis. The multivariate analysis demonstrated that only T/N ratio was significantly correlated with GPC3 expression in patients with HCC (P = 0.007, OR = 1.479, 95.0%CI: 1.113-1.964), while SUVmax, tumour differentiation, lymph node metastasis, and TNM stage had no significant association (P > 0.05).
To evaluate the effect of GPC3 expression on the glucose metabolism, GPC3-expressing HepG2 cells and non-GPC3-expressing RH7777 cells were incubated with 18F-FDG for 60 min and the cellular uptake was measured. The results revealed that HepG2 cells had a significantly lower 18F-FDG uptake than that of RH7777 cells (0.37% ± 0.05 % vs 1.03% ± 0.04% of inputted radioactivity, t = -20.352, P < 0.001) (Figure 1E), which is consistent with the findings in the patient study.
18F-FDG PET/CT has often been used to non-invasively evaluate tumour glycolysis in vivo by measuring the uptake of 18F-FDG, a glucose analogue[29-33]. This radiotracer is transported into cells via glucose transporters (GLUTs) and is then phosphorylated to 18F-FDG-6-phosphate by the rate-limiting glycolytic enzyme hexokinase type 2. 18F-FDG-6-phosphate then becomes trapped within cells[29-33]. High 18F-FDG uptake is indicative of accelerated glycolysis. Although 18F-FDG is consistently taken up intensively by a variety of cancers, 18F-FDG accumulation in HCCs appears to be variable. It is well established that 18F-FDG uptake by well and moderately differentiated HCCs is low, whereas 18F-FDG uptake by poorly differentiated HCC is high[34-36]. On the contrary, 11C-acetate and 11C-choline, which are probes for lipid metabolism, have been reported to be intensively taken up by well- and moderately differentiated HCCs[37,38], indicating that low glucose metabolism and high lipid metabolism are the specific energy metabolism patterns of low grade HCC. In the present study, we found that SUVmax was actually lower in well- or moderately differentiated HCC than in poorly differentiated HCC, which consolidated the above views[34-36]. Low 18F-FDG uptake has also been found to correlate with low expression of GLUT1 or GLUT2 and high expression of P-glycoprotein[21,22]. Our study confirmed the above findings that low GLUT1 expressing tumours actually had a significantly low 18F-FDG uptake than that of high GLUT1 expressing tumours (P < 0.001).
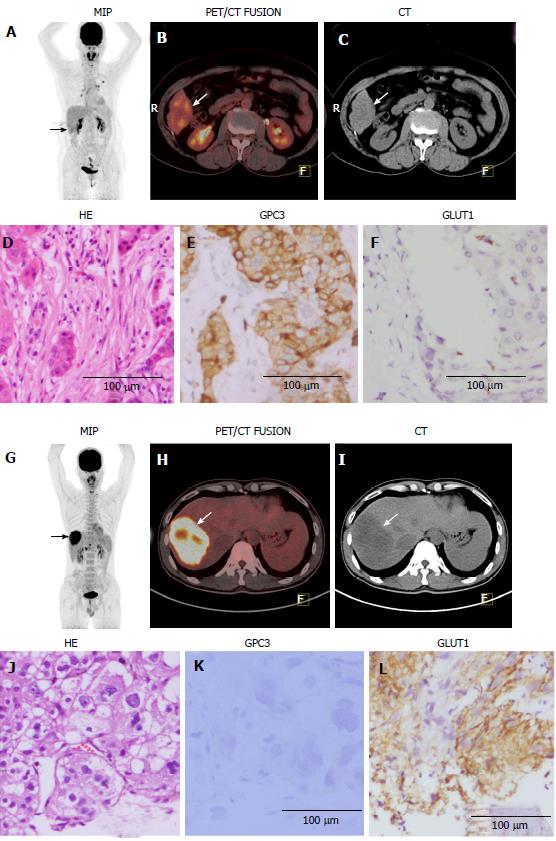
In the present study, for the first time, we found the phenomenon that low glucose metabolism also occurred in the HCCs with positive GPC3 expression, not only in the patient study, but also in the in vitro cellular uptake assay. In the patient study, an inverse association was noted between GPC3 expression and 18F-FDG uptake (P < 0.05). 18F-FDG uptake in HCC lesions with GPC3 positivity was significantly lower than that of lesions with GPC3 negativity (SUVmax: 6.01 ± 3.55 vs 9.56 ± 5.95, t = -2.341, P = 0.028; T/NT ratio: 2.62 ± 1.55 vs 4.52 ± 2.92, t = -2.597, P = 0.017). Furthermore, multivariate analysis revealed that only the glucose metabolism was significantly correlated with GPC3 expression (P < 0.05), but not other clinical factors. In vitro cellular uptake assay also revealed that GPC3-expressing HepG2 cells had a low 18F-FDG uptake than non-GPC3-expressing RH7777 cells (0.37 ± 0.05% vs 1.03 ± 0.04% of inputted radioactivity, t = -20.352, P < 0.001). Consistent with these findings, we observed that GPC3 expression was highly expressed in well- or moderately differentiated HCCs, which always have low 18F-FDG uptake[34-36]. Therefore, our study implied that GPC3 may be another underlying factor that contributes to the complex 18F-FDG uptake characteristics in HCCs. Cho et al[23] reported that GPC3 could bind to GLUT1 with an equilibrium dissociation constant (Kd) of 1.61 nmol/L and decrease glucose uptake by HCC cells, which might be helpful to explain this phenomenon. However, in the present study, low GLUT1 expression was found in most (86.5%) of GPC3-positive tumours. In addition, although an inverse trend of relationship was observed between GPC3 and GLUT1 expression, their association did not reach statistical significance (Spearman correlation coefficient = -0.232, P = 0.088). Therefore, the present study had no enough evidence to identify that GPC3 inversely regulates the glucose via GLUT1 and further basic research is warranted to uncover the mechanism.
Both SUVmax and T/NT ratio can be used to quantify 18F-FDG uptake in tumours, however, in the present study, multivariate analysis revealed that only the T/NT ratio was significantly correlated with GPC3 expression (P < 0.05), but not the SUVmax (P > 0.05). A rational explanation for this result is that T/NT ratio can be more accurate to define 18F-FDG uptake in HCC since it is not influenced by serum glucose level, the uptake period, or measurement variation, which often make the measurement of SUVmax inaccurate[25].
GPC3 is currently under consideration as a potential molecular therapeutic target for HCC[11-15]. GPC3-targeted treatments that utilize siRNA or anti-GPC3 antibodies have shown potential in altering cell migration, metastasis, and invasion, and in inhibiting xenograft tumour growth[13,15,39,40]. A GPC3-derived peptide vaccine has also been tested in a phase II study as an adjuvant therapy for HCC[41]. GPC3-targeted PET imaging might be useful for the non-invasive analysis of GPC3 expression in HCC patients and for selecting those suitable for GPC3-targeted therapy. The present study also indicated that GPC3-targeted PET imaging might be helpful for detection of early stage HCC, which often presents with a low uptake of 18F-FDG and appears as well- and moderately differentiated HCC in pathology tests. In the present study, we demonstrated that GPC3 expression was positive in most (75.0%) of the well- or moderately differentiated HCC tumours. More importantly, in the lesions with negative 18F-FDG uptake, GPC3 expression was positive in 15/18 (83.3%) patients. Combining 18F-FDG uptake with GPC3 expression, the total positivity reached 94.5% (52/55). Therefore, we propose that GPC3-targeted PET imaging may improve diagnostic sensitivity for early stage HCC and can serve as an effective complement to 18F-FDG imaging for diagnosing HCC.
There are some limitations to the present study. First, the sample size of patients was small, especially the number of poorly differentiated HCC patients, which may cause the results of this study to fail to reflect the real correlation between GPC3 and glucose metabolism. Second, this was a retrospective study, and thus, there may have been a certain degree of bias.
Glypican-3 (GPC3) is a cell surface proteoglycan overexpressed in most hepatocellular carcinomas (HCCs), but not in normal liver tissue, cirrhosis tissue, or paracancerous tissue. Therefore, GPC3 is suggested to be an important target for diagnosis and therapy. Elucidating the relationship between GPC3 expression and glucose metabolism may contribute to a better understanding of the biological role of GPC3 in regulating glucose metabolism. In addition, the research also could be useful to predict the potential utility of GPC3-targeted imaging in the clinic.
In this study, we investigated the relationship between GPC3 expression and glucose metabolism in HCC with an aim to uncover how GPC3 regulates the glucose metabolism in HCCs and predict the potential utility of GPC3-targeted imaging in the clinic.
This study aimed to investigate the relationship between glucose metabolism and GPC3 expression in HCC.
A retrospective analysis was performed on 55 HCC patients who had undergone 18F-FDG PET/CT before therapy. Tumour SUVmax and T/N ratio were used to quantify 18F-FDG uptake. The relationship between 18F-FDG uptake and expression of GPC3 and glucose transporter 1 (GLUT1) was analyzed by immunohistochemical analysis. In vitro cellular 18F-FDG uptake was also measured in GPC3-expressing HepG2 and non-GPC3-expressing RH7777 cells to determine the effect of GPC3 on glucose metabolism. The relationships between GPC3 expression and 18F-FDG uptake, GLUT1 expression, tumour differentiation, and other clinical indicators were analysed using spearman rank correlation, and univariate and multiple logistic regression analyses.
In the present study, we found a phenomenon that the glucose metabolism in the GPC3-expressing HCC tumours is low in the patient study. 18F-FDG uptake in HCC lesions with GPC3 positivity was significantly lower than that of lesions with GPC3 negativity (SUVmax: 6.01 ± 3.55 vs 9.56 ± 5.95, t = -2.341, P = 0.028; T/NT ratio: 2.62 ± 1.55 vs 4.52 ± 2.92, t = -2.597, P = 0.017). Furthermore, multivariate analysis revealed that only the glucose metabolism was significantly correlated with GPC3 expression (P < 0.05), but not other clinical factors. In in vitro cellular uptake experiments, GPC3-expressing HepG2 cells were also found to have low 18F-FDG uptake than that of non-GPC3-expressing RH7777 cells (0.37% ± 0.05% vs 1.03% ± 0.04% of inputted radioactivity, t = -20.352, P < 0.001). Although an inverse trend of relationship was observed between GPC3 and GLUT1 expression, their association did not reach statistical significance (Spearman correlation coefficient = -0.232, P = 0.088).
GPC3 was reported to play an important role in regulating malignant transformation and promoting the growth of HCC by stimulating the canonical Wnt signalling pathway. Besides, glucose is very important for malignant cell survival and proliferation. Both of them are very important for tumour growth. Therefore, we suggested that there might be a correlation between GPC3 and tumour glucose metabolism. We used 18F-FDG PET/CT for non-invasively measuring tumour glucose uptake in vivo in HCC patients and 18F-FDG uptake assay to measure the cellular glucose metabolism. In conclusion, the expression of GPC3 was observed to be positive in 67.3% (37/55) of HCC patients. The patient study and in vitro cellular uptake assay demonstrated that the glucose metabolism is inversely correlated with the expression of GPC3 in HCC. These results implied that GPC3 may be another underlying factor that contributes to the complex 18F-FDG uptake characteristics in HCCs. We believe that it is helpful for clarifying the mechanism of anti-GPC3 treatment by uncovering how GPC3 regulates the glucose metabolism in HCC. In addition, we found that GPC3 expression was positive in 15/18 (83.3%) of the lesions with negative 18F-FDG uptake. Combining 18F-FDG uptake with GPC3 expression, the total positivity reached 94.5% (52/55). Therefore, we propose that GPC3-targeted PET imaging may improve diagnostic sensitivity for early stage HCC and can serve as an effective complement to 18F-FDG imaging for diagnosing HCC.
For the future research, we want to investigate the mechanism concerning how GPC3 regulates the glucose and lipid metabolism in HCC. In the previous study, we found 11C-choline, as a probe of lipid metabolism, could be highly taken up by well- and moderately differentiated HCC. So, we deduce that GPC3 may have a potential to promote the lipid metabolism in HCC, which may conversely reduce the glucose metabolism. We want to do further basic research confirm this hypothesis.
ACKNOWLEDGEMENTS
We thank our colleagues at the Nanfang PET Center for manufacturing the radiopharmaceutical reagents and performing the PET/CT scans. We are also grateful to our colleagues in the Department of Hepatobiliary and Pathology, Nanfang Hospital, for providing follow-up data and pathologic diagnoses.
| 1. | Thomas MB, Zhu AX. Hepatocellular carcinoma: the need for progress. J Clin Oncol. 2005;23:2892-2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 305] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 2. | Tsim NC, Frampton AE, Habib NA, Jiao LR. Surgical treatment for liver cancer. World J Gastroenterol. 2010;16:927-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Yang JD, Roberts LR. Hepatocellular carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 2010;7:448-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 1067] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 4. | Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 700] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 5. | Zhu ZW, Friess H, Wang L, Abou-Shady M, Zimmermann A, Lander AD, Korc M, Kleeff J, Büchler MW. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 2001;48:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 225] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 6. | Zhang L, Liu H, Sun L, Li N, Ding H, Zheng J. Glypican-3 as a potential differential diagnosis marker for hepatocellular carcinoma: a tissue microarray-based study. Acta Histochem. 2012;114:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Gao W, Kim H, Feng M, Phung Y, Xavier CP, Rubin JS, Ho M. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology. 2014;60:576-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 8. | Sham JG, Kievit FM, Grierson JR, Miyaoka RS, Yeh MM, Zhang M, Yeung RS, Minoshima S, Park JO. Glypican-3-targeted 89Zr PET imaging of hepatocellular carcinoma. J Nucl Med. 2014;55:799-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 9. | Yang X, Liu H, Sun CK, Natarajan A, Hu X, Wang X, Allegretta M, Guttmann RD, Gambhir SS, Chua MS. Imaging of hepatocellular carcinoma patient-derived xenografts using ⁸⁹Zr-labeled anti-glypican-3 monoclonal antibody. Biomaterials. 2014;35:6964-6971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Sham JG, Kievit FM, Grierson JR, Chiarelli PA, Miyaoka RS, Zhang M, Yeung RS, Minoshima S, Park JO. Glypican-3-targeting F(ab’)2 for 89Zr PET of hepatocellular carcinoma. J Nucl Med. 2014;55:2032-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H, Sugo I, Ohizumi I, Aburatani H, Hamakubo T. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008;68:9832-9838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Nakano K, Orita T, Nezu J, Yoshino T, Ohizumi I, Sugimoto M, Furugaki K, Kinoshita Y, Ishiguro T, Hamakubo T. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;378:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Wang L, Yao M, Pan LH, Qian Q, Yao DF. Glypican-3 is a biomarker and a therapeutic target of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14:361-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Fleming BD, Ho M. Glypican-3 Targeting Immunotoxins for the Treatment of Liver Cancer. Toxins (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Yu D, Dong Z, Yao M, Wu W, Yan M, Yan X, Qiu L, Chen J, Sai W, Yao D. Targeted glypican-3 gene transcription inhibited the proliferation of human hepatoma cells by specific short hairpin RNA. Tumour Biol. 2013;34:661-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Pauwels EK, Coumou AW, Kostkiewicz M, Kairemo K. [¹⁸F]fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography imaging in oncology: initial staging and evaluation of cancer therapy. Med Princ Pract. 2013;22:427-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Weber WA, Petersen V, Schmidt B, Tyndale-Hines L, Link T, Peschel C, Schwaiger M. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21:2651-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 354] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Higashi K, Ueda Y, Yagishita M, Arisaka Y, Sakurai A, Oguchi M, Seki H, Nambu Y, Tonami H, Yamamoto I. FDG PET measurement of the proliferative potential of non-small cell lung cancer. J Nucl Med. 2000;41:85-92. [PubMed] |
| 19. | Wudel LJ Jr, Delbeke D, Morris D, Rice M, Washington MK, Shyr Y, Pinson CW, Chapman WC. The role of [18F]fluorodeoxyglucose positron emission tomography imaging in the evaluation of hepatocellular carcinoma. Am Surg. 2003;69:117-124; discussion 124-126. [PubMed] |
| 20. | Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, Collins BT, Di Bisceglie AM. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 295] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Seo S, Hatano E, Higashi T, Nakajima A, Nakamoto Y, Tada M, Tamaki N, Iwaisako K, Kitamura K, Ikai I. P-glycoprotein expression affects 18F-fluorodeoxyglucose accumulation in hepatocellular carcinoma in vivo and in vitro. Int J Oncol. 2009;34:1303-1312. [PubMed] |
| 22. | Lee JD, Yang WI, Park YN, Kim KS, Choi JS, Yun M, Ko D, Kim TS, Cho AE, Kim HM. Different glucose uptake and glycolytic mechanisms between hepatocellular carcinoma and intrahepatic mass-forming cholangiocarcinoma with increased (18)F-FDG uptake. J Nucl Med. 2005;46:1753-1759. [PubMed] |
| 23. | Cho HS, Ahn JM, Han HJ, Cho JY. Glypican 3 binds to GLUT1 and decreases glucose transport activity in hepatocellular carcinoma cells. J Cell Biochem. 2010;111:1252-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Ren YY, Li YC, Wu HB, Wang QS, Han YJ, Zhou WL, Li HS. Whole-body 18F-FDG PET/CT for M staging in the patient with newly diagnosed nasopharyngeal carcinoma: Who needs? Eur J Radiol. 2017;89:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Lee JW, Hwang SH, Kim HJ, Kim D, Cho A, Yun M. Volumetric parameters on FDG PET can predict early intrahepatic recurrence-free survival in patients with hepatocellular carcinoma after curative surgical resection. Eur J Nucl Med Mol Imaging. 2017;44:1984-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Ning S, Bin C, Na H, Peng S, Yi D, Xiang-hua Y, Fang-yin Z, Da-yong Z, Rong-cheng L. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol Biol Rep. 2012;39:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Cooper R, Sarioğlu S, Sökmen S, Füzün M, Küpelioğlu A, Valentine H, Görken IB, Airley R, West C. Glucose transporter-1 (GLUT-1): a potential marker of prognosis in rectal carcinoma? Br J Cancer. 2003;89:870-876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Li ZB, Wu Z, Chen K, Ryu EK, Chen X. 18F-labeled BBN-RGD heterodimer for prostate cancer imaging. J Nucl Med. 2008;49:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med. 2009;50:1820-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 30. | Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49 Suppl 2:24S-42S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 468] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 31. | Zhao S, Kuge Y, Mochizuki T, Takahashi T, Nakada K, Sato M, Takei T, Tamaki N. Biologic correlates of intratumoral heterogeneity in 18F-FDG distribution with regional expression of glucose transporters and hexokinase-II in experimental tumor. J Nucl Med. 2005;46:675-682. [PubMed] |
| 32. | Medina RA, Owen GI. Glucose transporters: expression, regulation and cancer. Biol Res. 2002;35:9-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 342] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 33. | Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 907] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 34. | Torizuka T, Tamaki N, Inokuma T, Magata Y, Sasayama S, Yonekura Y, Tanaka A, Yamaoka Y, Yamamoto K, Konishi J. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med. 1995;36:1811-1817. [PubMed] |
| 35. | Okazumi S, Isono K, Enomoto K, Kikuchi T, Ozaki M, Yamamoto H, Hayashi H, Asano T, Ryu M. Evaluation of liver tumors using fluorine-18-fluorodeoxyglucose PET: characterization of tumor and assessment of effect of treatment. J Nucl Med. 1992;33:333-339. [PubMed] |
| 36. | Yamamoto Y, Nishiyama Y, Kameyama R, Okano K, Kashiwagi H, Deguchi A, Kaji M, Ohkawa M. Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. J Nucl Med. 2008;49:1245-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Wu HB, Wang QS, Li BY, Li HS, Zhou WL, Wang QY. F-18 FDG in conjunction with 11C-choline PET/CT in the diagnosis of hepatocellular carcinoma. Clin Nucl Med. 2011;36:1092-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 38. | Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213-221. [PubMed] |
| 39. | Yao M, Wang L, Dong Z, Qian Q, Shi Y, Yu D, Wang S, Zheng W, Yao D. Glypican-3 as an emerging molecular target for hepatocellular carcinoma gene therapy. Tumour Biol. 2014;35:5857-5868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Xu C, Lee SA, Chen X. RNA interference as therapeutics for hepatocellular carcinoma. Recent Pat Anticancer Drug Discov. 2011;6:106-115. [PubMed] |
| 41. | Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, Nobuoka D, Gotohda N, Takahashi S, Kato Y. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5:e1129483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Demirci E, Goral V, Papa S, Tsuchiya A S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ