Published online Oct 21, 2018. doi: 10.3748/wjg.v24.i39.4436
Peer-review started: May 9, 2018
First decision: June 15, 2018
Revised: September 3, 2018
Accepted: October 5, 2018
Article in press: October 5, 2018
Published online: October 21, 2018
Processing time: 162 Days and 9.1 Hours
Hepatocellular carcinoma (HCC) is now the second leading cause of cancer-related deaths globally and many patients have incurable disease. HCC predominantly occurs in the setting of liver cirrhosis and is a paradigm for inflammation-induced cancer. The causes of chronic liver disease promote the development of transformed or premalignant hepatocytes and predisposes to the development of HCC. For HCC to grow and progress it is now clear that it requires an immunosuppressive niche within the fibrogenic microenvironment of cirrhosis. The rationale for targeting this immunosuppression is supported by responses seen in recent trials with checkpoint inhibitors. With the impact of immunotherapy, HCC progression may be delayed and long term durable responses may be seen. This makes the management of the underlying liver cirrhosis in HCC even more crucial as studies demonstrate that measures of liver function are a major prognostic factor in HCC. In this review, we discuss the development of cancer in the setting of liver inflammation and fibrosis, reviewing the microenvironment that leads to this tumourigenic climate and the implications this has for patient management.
Core tip: In this review, we discuss the development of hepatocellular carcinoma in the setting of liver inflammation and fibrosis, reviewing the microenvironment that leads to this tumorigenic climate and the implications this has for patient management.
- Citation: O’Rourke JM, Sagar VM, Shah T, Shetty S. Carcinogenesis on the background of liver fibrosis: Implications for the management of hepatocellular cancer. World J Gastroenterol 2018; 24(39): 4436-4447
- URL: https://www.wjgnet.com/1007-9327/full/v24/i39/4436.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i39.4436
Hepatocellular carcinoma (HCC) is now the fifth most commonly diagnosed cancer in men worldwide, and in women it is ranked ninth. HCC is the second most common cause of cancer related deaths and is reported to have been responsible for nearly 745000 deaths in 2012[1]. Incidence rates are highest in Asia and Africa with Central Europe having intermediate rates[2]. Different risk factors predominate depending on the region of the world. In Africa and Asia infection with hepatitis B virus (HBV) and aflatoxin B1 exposure are the major risk factors. In developed countries the hepatitis C virus (HCV), alcohol and the metabolic syndrome have predominated[3].
Despite increasing knowledge on the aetiologies of cirrhosis and progress in diagnosing and managing risk factors, the incidence rates for HCC are increasing. In England, HCC incidence increased from 0.63 per 100000 in 1990 to 2.48 in 2009[4]. In the United States (US), HCC incidence increased by 4.5% (95%CI: 4.3-4.7) annually between 2000 and 2009 but only 0.7% annually (95%CI: 0.2-1.6) after that. The post 2009 slowing in overall rates, seen in the US, may represent a plateau created from increases in vaccination against HBV and improved chronic HBV antiviral treatment[5].
It is uncommon to see HCC in the absence of liver fibrosis but it does occur. Table 1 lists some of the aetiologies associated with non-cirrhotic HCC[6,7]. Chronic hepatitis B is a major risk factor for the development of HCC in the non-cirrhotic setting[8]. In Europe and the United States the 5-year cumulative incidence of developing HCC was found to be 1% in non-cirrhotic chronic HBV hepatitis. This incidence increased to 10% in HBV with cirrhosis[9]. Other causes of HCC in the non-cirrhotic setting include hereditary conditions for example porphyria and type 1 glycogen storage disease, metabolic syndromes and genotoxin exposure. Genotoxins are agents which damage the genetic information within a cell. For example, the aflatoxin B1, which is produced by Aspergillus flavus, is a pathogenic fungus and can lead to non-cirrhotic HCC induction[10]. The global epidemic of non-alcoholic fatty liver disease (NAFLD) which is characterised by macrovesicular steatosis can lead to cirrhosis. It is however observed that a significant proportion of patients with NAFLD develop HCC in the non-cirrhotic setting[11,12]. However worldwide at present the majority (70%-90%) of HCC cases occur on a background of cirrhosis[13].
| Viral | HBV |
| Metabolic | Porphyria |
| Type 1 glycogen storage disease | |
| NAFLD | |
| Α1 antitrypsin | |
| Haemochromatosis | |
| Type 1 hypercitrullinemia | |
| Genotoxins | Aflatoxin B1 |
| Congenital | Alagille syndrome |
| Congenital hepatic fibrosis | |
| Sex hormones | Anabolic steroids |
| Hepatic adenoma transformation | |
| Vascular | Hepatic vascular pathology, e.g., Budd Chiari |
When data from the World Health Organization (WHO) mortality database was examined by Ascione et al[14], the age-standardised death rate for liver cirrhosis in European countries between 1970 and 2010 showed cause for concern for the United Kingdom (UK), Finland, Ireland and Denmark. Looking at percentage change in mortality, the UK in those four decades showed a high increase (+284.8%), Finland, Ireland and Denmark also saw increases. However these countries were the exceptions and in all other countries in Europe there was a reduction in mortality for liver cirrhosis. The same database provided comparable data, between 1980-2010 with a 85.4% increase in death from HCC over this period[14]. The overall decrease in liver cirrhosis related deaths in Europe and the increasing mortality for HCC is confounding and concerning.
Cirrhosis mortality in the UK has been the subject of extensive discussion and patterns of alcohol consumption may account for the discrepancies between the UK and other parts of Europe. The rise in HCC cases in Europe over the last 30 years seems confounding when it is reported that in many countries mortality from cirrhosis is reducing. However, our knowledge and the management of chronic liver disease has over this timeframe improved, and it is suggested that with increased survival we are seeing increased development of HCC[14]. This would be in keeping with our knowledge that cirrhosis creates a microenvironment for tumour development and is considered a precursor for HCC.
Over 3 decades ago the 5-year survival for HCC was 3%. Despite improvements in earlier detection 5-year survival is less than 20% for this cancer[15].
Liver fibrosis is a risk factor for the development of HCC with up to 90% of cases occurring on the background of a cirrhotic liver[16] and is a leading cause of death in this population. The major global causes of liver disease which are associated with HCC include viral hepatitis, alcoholism and non-alcoholic steatohepatitis (NASH). The effects of HBV infection have started to decline due to increased use of antivirals and immunisation programs. It is hoped that in the age of new direct acting antiviral agents with time we will see a reduction in HCV associated cirrhosis. The impact of alcohol and the development of NASH cirrhosis will prove to be more challenging to prevent and cases are predicted to continue to rise.
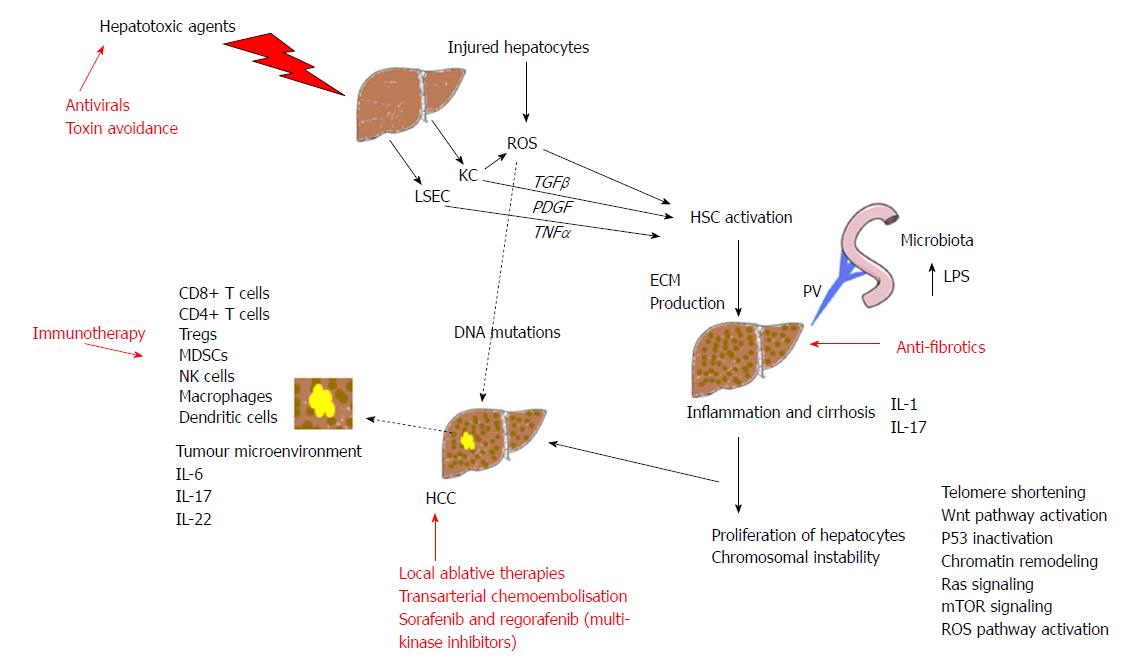
Fibrosis occurs when the liver is repeatedly and continuously injured. Liver volume is formed from 80% parenchymal and 20% non-parenchymal cells[17]. Hepatocytes are the parenchymal cells and they are the target for hepatotoxic agents. Damage to hepatocytes triggers the release of reactive oxygen species (ROS) and mediators of fibrosis inducing activation of hepatic stellate cells (HSCs). HSCs with phagocytic Kupffer cells (KCs) and liver sinusoidal endothelial cells (LSECs) are central players in fibrosis development[18]. The activation of HSCs, extracellular matrix (ECM) producing myofibroblasts, is said to be the key step in fibrosis development. Paracrine signals from injured hepatocytes and activated KCs play a prominent role in HSC activation. KCs also generate ROS in the liver and this enhances HSC activation and collagen synthesis leading to fibrosis[19,20].
In addition to the multitude of cells involved in the development of cirrhosis there are also several cytokines that have been identified to play significant roles. They include platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), tumour necrosis factor-α (TNF-α), interferon and interleukins (ILs). A variety of hepatotoxic agents can induce KC to synthesise PDGF[21] which binds to the HSC membrane and activates them. There are different isoforms of PDGF and two of these, PDGF B and D have been shown to have a role in activating HSCs leading to liver fibrosis[22]. TGF-β is the most potent stimulator of fibrogenesis and is produced by a variety of cells in the liver: HSCs, KCs, LSECs, and hepatocytes. The TGF-β family has multiple members and the one that has been implicated as a notable player in hepatic fibrosis is TGF-β1. It is reported to contribute not only to activation of HSCs but also the inhibition of ECM breakdown[23] and the induction of apoptosis of hepatocytes[24]. TNF-α has also been shown to activate HSCs to synthesise ECM[25], however, results from murine studies on TNF-α are complex and it appears to also have antifibrogenic effects in some reports[26]. ILs are expressed by many cells with the majority of ILs being produced by helper CD4 T lymphocytes. In the liver ILs have both pro-fibrogenic and antifibrogenic roles[18]. IL-1 can activate HSCs[27] and IL-17 has a role inducing fibrosis through the activation of HSCs and KCs[28]. ILs with antifibrogenic roles have been identified as IL-6, IL-10 and IL-22[29-31].
The inception and progression of HCC is described as being largely influenced by the microenvironment of the liver. This includes influences from chronic inflammation, liver remodelling, changes in genetics and cellular signalling. These pathways can be affected by chemical toxins, viruses, immune cells, hypoxia, ECM changes, microflora from the gastrointestinal tract and extra cellular microvesicles which carry altering signals, cytokines and oncogenic miRNAs.
Chronic inflammation and fibrosis are seen in the background of many HCCs and the most common aetiologies are viruses and ethanol. The immune mediated cell death seen in viral infections leads to increased production of ROS. This leads to increased hepatocellular oxidative stress which induces DNA mutations contributing to HCC development. Ethanol consumption is associated with increased ROS concentrations in hepatocytes resulting in hepatic DNA damage[32]. Chronic inflammation leads to increased proliferation of hepatocytes, shortening of telomeres and therefore chromosomal instability and a predisposition to malignant transformation[33]. Genomic alterations which have been identified in HCC and are considered to be drivers in progression include mutations affecting telomere maintenance, Wnt pathway activation, inactivation of p53, chromatin remodelling, Ras signalling, mechanistic target of rapamycin (mTOR) signalling and ROS pathway initiation[34].
Chronic inflammation can progress to fibrosis and cirrhosis and this in turn induces several further changes in the microenvironment. Firstly, it creates altered blood flow and hypoxic hepatocytes which produce reactive nitrogen species[35]. Areas of hypoxia in the liver parenchyma lead to changes in molecular signalling and we know that the response is to upregulate angiogenic factors including vascular endothelial growth factor (VEGF)[36]. In a tumour this facilitates angiogenesis and tumour growth. The hepatocytes may provide the genetic mutation but it is the unique surrounding microenvironment that enables the tumour to establish.
We have explained that chronic inflammation has effects on cytokine expression within the liver, ECM production by HSC, TNF-α receptors and also the mitogenic cytokine IL6 is significantly increased in advanced cirrhosis leading to a propensity towards cancer[37]. IL6 regulates immune cells and the growth of tumour cells[38] and this dual role therefore is an example of the association between the tumour and the microenvironment. The effects of IL6 are controlled by nuclear factor-κB signalling. Both pathways are altered in liver inflammation and hepatocarcinogenesis[39]. EGFR overexpression also promotes liver cancer progression when present in macrophages[40]. It has also been shown that hepatic stellate cells can promote the pro-tumourigenic change in macrophages[41]. The expansion of liver progenitor cells to replace hepatocytes, in the presence of cytokines and increases in oxidative stress promotes the accruing of mutations[42].
Hepatocytes have great potential to regenerate but we know this predisposes cells to malignant transformation[43]. The cell underlying the inception of HCC is also critical to understand. The human liver is not just made up of hepatocytes but also adult stem cells and progenitor cells which maybe potential cells of origin for cancer[44].
The gut microbiota has in recent years received much attention in many disease processes including liver disease. It has been described that the microbes in our bodies encompass 100 trillion cells, with the majority residing in the gut[45]. It is increasingly recognised that the gastrointestinal tract plays a pivotal role in liver diseases, including HCC. As we have previously described HCC usually occurs in inflamed and fibrotic livers and intensive immune cell infiltration is seen. Via the portal vein the liver is exposed to gut-derived bacterial products and in advanced liver diseases there is increased intestinal permeability to gut-derived bacterial products including lipopolysaccharides (LPS)[46]. Accumulation of LPS is said to contribute to HCC development by generating inflammatory reactions in the hepatic environment[47], activating KCs and endothelial cells to release pro-inflammatory cytokines which contributes to liver injury. Levels of LPS are increased in animal models of hepatocarcinogenesis and in patients with HCC[47-49]. Dapito et al[50] found that toll like receptor 4 activation by LPS contributed to driving inflammation and tumour progression and that gut sterilisation supressed hepatocarcinogenesis. To date studies have been on preclinical animal models but there is potential that manipulating the microbiome may one day be an option in the prevention and perhaps treatment of HCC[51].
Dysregulation of the immune system has been implicated in the pathogenesis of HCC. Changes in the innate and adaptive immune system makes the immune system tolerant to cancer and facilitates tumour progression. Understanding these processes is therefore essential to tailor therapeutic approaches. Key cells implicated include T lymphocytes, myeloid-derived suppressor cells (MDSCs), dendritic cells and natural killer (NK) cells[52]. The innate immune system key players are dendritic cells, macrophages, MDSCs and NK cells. The adaptive immune system comprises the T lymphocyte subsets. Failure of HCC antigen presentation by dendritic cells is one defect in the immune system seen in HCC. Activated dendritic cells in HCC are not able to infiltrate cancer tissue effectively[53] and tumour associated macrophages express cytokines that favour tumour growth, invasion and suppress the anti-tumour immune response[54]. MDSCs possess strong immunosuppressive activities and expand in cancer and regulate T cell responses, increased quantities of these cells are seen in the tumour environment of a HCC[55]. NK cells are cytotoxic lymphocytes and they can modulate the activity of other immune cells, including dendritic cells and macrophages, via cytokine release. They are critical to the innate immune system and are capable of rapid responses and can destroy tumour cells without prior priming. In HCC a reduction in NK cell subsets has been reported with reduced cytotoxic ability[56].
The adaptive immune system has a significant role in thwarting the development and advancement of cancer. CD8+ cytotoxic T cells play a salient part in anti-tumour mechanisms and CD4+ helper cells have a role in generating CD8+memory T cells[57] which assist in the destruction of tumour cells. In the setting of cirrhosis there is a reduction in CD4+ cells[58]. Tregs expressing CD4+, CD25+ and forkhead box P3 (Foxp3) have an inhibitory role and they can suppress effective anti-tumour responses[59]. There are increased Tregs seen in patients with HCC and depletion can increase anti-tumour responses[60] and lead to a reduction in tumour growth[61]. In advanced HCC there are increased numbers of CD8+FoxP3+ regulatory T cells perhaps helping the tumour evade the immune system[62]. NK T cells accumulate in the tumour environment and they appear to be able to function either as anti-tumour cells or can promote tumour tolerance depending on the subset[63]. We can therefore conclude that a complex, partially understood, dysregulated immune environment has a key role in the development and evolution of liver tumours. An overview of the key responses to hepatic injury leading to fibrosis and HCC development together with therapeutic strategies are summarised in Figure 1.
The fact that the majority of HCC arise in the cirrhotic liver, which affects liver function, can severely impact on therapeutics. A detailed review of the management of HCC has been covered elsewhere[64]. There are several algorithms for the management of HCC including TNM stage, the Japanese integrated system, Cancer of the Liver Italian group and the Hong Kong Liver Cancer staging system. The most well recognised being the Barcelona Liver Cancer (BCLC) criteria which is recommended by several international guidelines[65,66]. Considering the underlying liver disease is vital in HCC, the BCLC guidelines include both tumour stage and the severity of underlying liver cirrhosis (Child Pugh score) and helps guide treatment and to predict overall prognosis. The seminal study by Hoshida et al[67] highlighted the importance of underlying liver disease to overall prognosis in HCC. In this study the gene expression of the tumour was not associated with overall survival but the gene expression in adjacent non-tumour liver tissue correlated strongly with survival. More recently the severity of underlying liver disease as a prognostic marker has been highlighted with a study focusing on a scoring system based on bilirubin levels and albumin values. Johnson et al[68] developed a model incorporating just bilirubin and albumin levels called the ALBI score (Figure 2) which was an accurate discriminatory method for assessing liver function in HCC. Within the Child-Pugh class A patients, the ALBI score was able to differentiate patient groups with different prognoses. The model across a database of 3887 patients identified a median ten month difference in survival between ALBI grade 1 and ALBI grade 2 within the Child-Pugh class A group for European and US patients[64].
Patients who present with early HCC are amenable to curative treatments including surgery (resection or transplant) and local ablative therapies. Those with more intermediate stage HCC have non curative options such as transarterial chemoembolisaton which has been shown to improve survival in randomised control trials[69]. A significant proportion of patients present with advanced incurable disease; these patients have a poor prognosis and the only licensed medical treatment has been the multi-kinase inhibitor Sorafenib[70]. The SHARP trial demonstrated an improved survival in patients with advanced HCC who took Sorafenib but this was by only a median of three months. Several other agents have been studied in randomised trials but none have successfully demonstrated superiority to Sorafenib but most recently a phase 3 trial demonstrated Lenvatinib, an inhibitor of VEGF receptors 1-3, FGF receptors 1-4, PDGF receptor alpha, RET and KIT, had similar efficacy to Sorafenib[71]. Trials in which Sorafenib has been combined with other treatment such as TACE have not been successful in improving efficacy of these treatments[72]. Until recently no second line agents in randomised trials had demonstrated clinical benefit after Sorafenib therapy, but a recent trial with Regorafinib, another multikinase inhibitor, has finally demonstrated improved survival with a second line agent[73]. This has led to FDA approval for patients who have failed therapy with Sorafenib but overall survival for advanced HCC remains poor. These experiences have provided impetus to explore immunotherapy in HCC. We have already described that progressive HCC is associated with an immunosuppressive microenvironment. Recent early stage trials with checkpoint inhibitors which activate T cells have shown promising results. Checkpoint inhibitors currently include cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) blockers and inhibitors of programmed cell death protein-1 (PD-1)/programmed cell death protein ligand-1 (PDL-1) interaction with three studies being reported in the context of HCC. One study with 30 patients involved the administration of Tremelimumab, a CTLA-4 blocker, combined with ablative therapy, showed some patients did demonstrate immune responses and led to the accumulation of CD8+ T cells in tumours. A further study with the same drug was performed with 20 patients and demonstrated both anti-tumour and antiviral activity[74]. Recently, a large phase II trial (CheckMate-040 trial) with the agent Nivolumab (anti-PD-1 drug) has led to significant attention because of strong anti-tumour responses, leading to improved survival and led to accelerated approval by the FDA in 2017 for the treatment of patients after failure with Sorafenib[75].
Novel therapeutics which alter T-cell regulation are now being pursued for HCC. A study by Sia et al[76] looked at over 900 HCC samples and identified that around 25% exhibited high expression levels of programmed death ligand-1 (PD-L1) and programmed cell death protein 1 (PD-1) and a subgroup expressed many genes that are regulated by TGF β1, a cytokine which is linked to aggressive cancers and suppresses the immune response. TGF-β is involved in cell proliferation, angiogenesis, migration, immune infiltration, metastases dissemination, and drug resistance[77]. There are ongoing trials in HCC using the TGFβ1 inhibitor Galunisertib. The phase 2 trial using Galunisertib as monotherapy has shown promise with improved overall survival in AFP responders[78]. We are entering an era where we may be able to identify which tumours are most likely to respond to immunotherapy, tailoring treatment to the tumour biology.
With these advances in treatment of advanced HCC, clinicians will need to consider how best to manage the underlying chronic liver disease that is associated with HCC. Attempts to improve liver function will have the aim of (1) increasing the number of patients eligible for these novel therapies; (2) to minimise the potential liver related side effects of these novel agents and (3) to prolong the overall survival in patients whose tumours respond to these agents.
Targeting the initiating factors of chronic liver disease can significantly improve liver function in patients even when they have established cirrhosis. Animal models have demonstrated that severe fibrosis can undergo resolution and healing through cell-mediated mechanisms including reducing the number of activated HSCs and the contribution of macrophages[79]. Patients with identifiable factors such as excess alcohol intake and metabolic syndrome benefit significantly from becoming abstinent and improving their metabolic risk factors respectively. Those with active chronic hepatitis B undergo significant improvement of liver function by suppressing viral replication. The treatment of HCV has seen dramatic improvements with the advent of direct acting antivirals[80]. Significant improvement of fibrosis has also been demonstrated in patients with HBV and HCV antiviral medication[81]. One assumption would be that the clearance of Hepatitis C would be beneficial in patients to improve liver function and reduction of future HCC recurrence. This is countered by recent reports suggesting that the viral clearance of HCV could alter immunological surveillance of tumour cells. Case series have described aggressive HCC in patients with cirrhosis after completing successful treatment of hepatitis C[82]. It is not possible to draw conclusions from these findings currently and further studies are required to clarify the situation.
In addition to treating the cause of chronic liver disease, there may also be benefit in preventing the complications of cirrhosis. Variceal bleeding is a complication of cirrhosis associated with a very high mortality rate (20%)[83]. It is well established that patients with HCC have a higher mortality rate compared to matched cirrhosis groups[84,85]. Ripoll et al[86] in their study confirmed this and furthermore demonstrated that less than half of HCC patients who were eligible for primary prophylaxis for bleeding were actually prescribed this medication. They also suggested that secondary prophylaxis improved survival in HCC patients.
Loss of muscle mass and function is provided the term sarcopenia and is frequently seen in advanced liver disease. The prevalence is estimated to be between 40%-70% in patients with cirrhosis alone[87]. The underlying mechanism is complex and not fully understood but includes inadequate intake, malabsorption and abnormal metabolism favouring proteolysis for gluconeogenesis. The addition of anti-cancer therapies can further compound the situation. A study looking at whether sarcopenia predicts the prognosis of patients treated with Sorafenib showed that skeletal muscle depletion is an independent prognostic factor[88]. The same has also been shown to apply to patients who have undergone transarterial chemoembolisation treatment for their HCC[89]. Although there is no evidence that sarcopenia is impacting on HCC progression, there is an impact on outcomes and therefore it should be recognised and addressed as part of the patient management pathway.
With the aim to reduce the complications of cirrhosis including decompensation there has been progress in recent years to try to prevent or reverse liver fibrosis and some evidence that this reduces HCC risk[90]. The WHO recognise that the viral hepatitis pandemic contributes to an estimated 1.4 million deaths per year including HCC and cirrhosis[91]. The published strategy outlines aims of reducing transmission of HCV and creating global access to treatment by 2030.
Identification and addressing of specific aetiologies, such as viral hepatitis and addressing metabolic risk factors in those with NAFLD is clearly essential but there is also a drive to develop specific anti-fibrotic agents. Whether we can reduce carcinogenesis by interrupting or reversing fibrogenic process with specific anti-fibrotic agents remains to be seen but certainly improvement in liver function is desirable to facilitate management and improve outcomes when cancer occurs.
With a better understanding of the processes that lead to fibrogenesis and fibrolysis it is now possible to target the relevant cytokines and effector cells including HSCs, myofibroblasts, profibrogenic immune cells and other ECM targets including collagens and matrix metalloproteinases (MMP) inhibitors like tissue metallopeptidase inhibitor 1. We know that there are collagen types which are increased in liver fibrosis and are potentially targetable by small interfering RNA (siRNA) or antisense oligonucleotides. The therapeutic advantage of these nucleic acid based therapies is that they can now be delivered in vehicles which are preferentially taken up by the cell being targeted achieving knockdown effects only at the site of interest[92,93]
The identification of novel targets in the fibrotic environment has led to the development of therapeutic agents with efficacy in preclinical models and we have seen the first clinical trials take place. We summarise in Table 2[94-104] some of the therapeutic targets being actively pursued for their anti-fibrotic effects. Unfortunately trial design for direct anti-fibrotic agents are not at present designed to assess if they have an effect on HCC prevention.
| Drug | Mechanism | Comment | Ref. |
| BMS-986263/ND-LO2-s0201 | siRNA that inhibits HSP47, reducing type 1 collagen synthesis | A lipid nanoparticle containing siRNA that inhibits HSP47. Vitamin A conjugated to the nanoparticle surface target facilitating targeted delivery to HSC and preclinical studies suggest disruption of collagen synthesis which may reverse fibrosis. A phase 1 study has demonstrated tolerability | [94] |
| Simtuzumab | Inhibition of Lysyl oxidases (LOX) mediated collagen cross linking reduces the breakdown of collagen by proteases such as MMPs | Simtuzumab is a humanized monoclonal antibody. It binds to LOXL2 and acts as an immunomodulator. However in a large phase 2 clinical trial in patients with NASH fibrosis the results were disappointing and focus has been diverted to LOXL1 inhibition where expression appears constant in carbon tetrachloride induced fibrosis in mouse models | [95,96] |
| Selonsertib | Inhibits apoptosis signal-regulating kinase 1 which in the setting of oxidative stress activates pathways which lead to fibrosis | In patients with NASH has shown promise that it may lead to a reduction in fibrosis in a phase 2 trial where it was given with and without Simtuzumab and compared to Simtuzumab alone | [97,98] |
| Cenicriviroc | Dual antagonist of C-C motif chemokine receptor (CCR) types 2 and 5 | Demonstrated anti-fibrotic activity in animal models of liver fibrosis. In a phase 2 study improvements were seen in noninvasive markers of hepatic fibrosis. Antagonism of CCR2 reduces pro-inflammatory monocytes and macrophages. CCR5 antagonism impairs the activation of HSCs | |
| [99-101] | |||
| Emricasan | Inhibitor of apoptotic and inflammatory caspases | Emricasan in the murine NASH model attenuated HSC activation. In phase 2 clinical trials for the regression of hepatic fibrosis caused by HCV infection after liver transplantation, the study didn’t reach its primary endpoint but the results from a phase 2 trial in NASH are awaited | [102] |
| GR-MD-02 | Targets galectin-3 | Phase 3 studies are already planned for GR-MD-02. Phase 1 and 2 have been completed in the NASH cohort. Preclinical data showed some reversal of fibrosis and a reduction in portal pressures in cirrhosis | [103] |
| Erlotinib | Epidermal growth factor (EGF) receptor inhibitor | In preclinical models the FDA approved inhibitor regressed fibrosis in some animals and blocked the development of HCC. A pilot phase1/2 trial is underway | [104] |
Pre-clinical experiments have demonstrated inhibiting the pathways for pro-fibrogenic cytokines including TGF-β and PDGF are also potential anti-fibrotic strategies to be explored further[105]. Integrins are transmembrane receptors that promote ECM adhesion. Some integrins can facilitate MMP activation and activate fibrogenic mediators such as TGF-β1. The identification of integrins which are important in fibrosis has led to mouse studies involving their direct and indirect inhibition[106]. Other preclinical studies are looking at targeting transmembrane collagen receptors, proteins expressed on myofibroblasts and manipulating the inflammatory environment to deactivate matrix producing myofibroblasts and hepatic stellate cells.
Hepatocellular cancer is a deadly complication of cirrhosis and remains difficult to diagnose at an early curative stage. The prognosis in advanced cases remains extremely poor despite significant changes in epidemiology and causes of chronic liver disease, with the rising epidemic of obesity. Nevertheless, new studies with novel agents are demonstrating increasing rates of tumour response and stability and there are exciting developments with immunotherapies. The challenge in patients who do respond to anti-cancer therapies is to maintain their underlying liver function in order that intolerable liver related side effects are minimised and patients have the best chance for a prolonged overall survival. Furthermore, standard of care for cirrhosis may not be met in patients with hepatocellular cancer because of assumptions of lack of benefit, but with new treatments leading to improved survival this will need to be reconsidered. There have been significant improvements in elucidating the underlying cellular mechanisms which drive fibrosis and cancer within the liver. Hopefully, new therapies will take advantage of these findings leading to personalised therapeutic combinations for these patients which have the dual effect of promoting fibrosis regression and anti-cancer effects.
The NIHR Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20722] [Article Influence: 1883.8] [Reference Citation Analysis (23)] |
| 2. | Forman D, Bray F, Brewster DH, Gombe Mbalawa C, Kohler B, Piñeros M, Steliarova-Foucher E, Swaminathan R, Ferlay J, editors . Cancer Incidence in Five Continents, Vol. X. IARC Scientific Publication. 2014;International Agency for Research on Cancer. |
| 3. | McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 678] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 4. | Konfortion J, Coupland VH, Kocher HM, Allum W, Grocock MJ, Jack RH. Time and deprivation trends in incidence of primary liver cancer subtypes in England. J Eval Clin Pract. 2014;20:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | White DL, Thrift AP, Kanwal F, Davila J, El-Serag HB. Incidence of Hepatocellular Carcinoma in All 50 United States, From 2000 Through 2012. Gastroenterology. 2017;152:812-820.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 339] [Article Influence: 37.7] [Reference Citation Analysis (1)] |
| 6. | Evert M, Dombrowski F. [Hepatocellular carcinoma in the non-cirrhotic liver]. Pathologe. 2008;29:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 8. | Xiang X, You XM, Zhong JH, Li LQ. Hepatocellular carcinoma in the absence of cirrhosis in patients with chronic hepatitis B virus infection. J Hepatol. 2017;67:885-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 988] [Article Influence: 54.9] [Reference Citation Analysis (1)] |
| 10. | Gaddikeri S, McNeeley MF, Wang CL, Bhargava P, Dighe MK, Yeh MM, Dubinsky TJ, Kolokythas O, Lalwani N. Hepatocellular carcinoma in the noncirrhotic liver. AJR Am J Roentgenol. 2014;203:W34-W47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Mittal S, Sada YH, El-Serag HB, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594-601.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 211] [Article Influence: 19.2] [Reference Citation Analysis (2)] |
| 12. | Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 428] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 13. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4301] [Article Influence: 226.4] [Reference Citation Analysis (2)] |
| 14. | Ascione A, Fontanella L, Imparato M, Rinaldi L, De Luca M. Mortality from cirrhosis and hepatocellular carcinoma in Western Europe over the last 40 years. Liver Int. 2017;37:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology. 2012;55:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 16. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1833] [Article Influence: 83.3] [Reference Citation Analysis (3)] |
| 17. | Werner M, Driftmann S, Kleinehr K, Kaiser GM, Mathé Z, Treckmann JW, Paul A, Skibbe K, Timm J, Canbay A. All-In-One: Advanced preparation of Human Parenchymal and Non-Parenchymal Liver Cells. PLoS One. 2015;10:e0138655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 18. | Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 335] [Cited by in RCA: 436] [Article Influence: 36.3] [Reference Citation Analysis (10)] |
| 19. | Li JT, Liao ZX, Ping J, Xu D, Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J Gastroenterol. 2008;43:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 20. | Casini A, Ceni E, Salzano R, Biondi P, Parola M, Galli A, Foschi M, Caligiuri A, Pinzani M, Surrenti C. Neutrophil-derived superoxide anion induces lipid peroxidation and stimulates collagen synthesis in human hepatic stellate cells: role of nitric oxide. Hepatology. 1997;25:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Borkham-Kamphorst E, Herrmann J, Stoll D, Treptau J, Gressner AM, Weiskirchen R. Dominant-negative soluble PDGF-beta receptor inhibits hepatic stellate cell activation and attenuates liver fibrosis. Lab Invest. 2004;84:766-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Borkham-Kamphorst E, van Roeyen CR, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Pro-fibrogenic potential of PDGF-D in liver fibrosis. J Hepatol. 2007;46:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | Liu X, Hu H, Yin JQ. Therapeutic strategies against TGF-beta signaling pathway in hepatic fibrosis. Liver Int. 2006;26:8-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 24. | Kirmaz C, Terzioglu E, Topalak O, Bayrak P, Yilmaz O, Ersoz G, Sebik F. Serum transforming growth factor-beta1(TGF-beta1) in patients with cirrhosis, chronic hepatitis B and chronic hepatitis C. Eur Cytokine Netw. 2004;15:112-116. [PubMed] |
| 25. | Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, Pachter HL, Bar-Sagi D, Frey AB, Miller G. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119:3213-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Varela-Rey M, Fontán-Gabás L, Blanco P, López-Zabalza MJ, Iraburu MJ. Glutathione depletion is involved in the inhibition of procollagen alpha1(I) mRNA levels caused by TNF-alpha on hepatic stellate cells. Cytokine. 2007;37:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Gieling RG, Wallace K, Han YP. Interleukin-1 participates in the progression from liver injury to fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1324-G1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 227] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 28. | Hara M, Kono H, Furuya S, Hirayama K, Tsuchiya M, Fujii H. Interleukin-17A plays a pivotal role in cholestatic liver fibrosis in mice. J Surg Res. 2013;183:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Chou WY, Lu CN, Lee TH, Wu CL, Hung KS, Concejero AM, Jawan B, Wang CH. Electroporative interleukin-10 gene transfer ameliorates carbon tetrachloride-induced murine liver fibrosis by MMP and TIMP modulation. Acta Pharmacol Sin. 2006;27:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 366] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 31. | Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 602] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 32. | Frank A, Seitz HK, Bartsch H, Frank N, Nair J. Immunohistochemical detection of 1,N6-ethenodeoxyadenosine in nuclei of human liver affected by diseases predisposing to hepato-carcinogenesis. Carcinogenesis. 2004;25:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Plentz RR, Caselitz M, Bleck JS, Gebel M, Flemming P, Kubicka S, Manns MP, Rudolph KL. Hepatocellular telomere shortening correlates with chromosomal instability and the development of human hepatoma. Hepatology. 2004;40:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1933] [Cited by in RCA: 1956] [Article Influence: 195.6] [Reference Citation Analysis (4)] |
| 35. | Mohammed NA, Abd El-Aleem S, Appleton I, Maklouf MM, Said M, McMahon RF. Expression of nitric oxide synthase isoforms in human liver cirrhosis. J Pathol. 2003;200:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Yu DC, Chen J, Ding YT. Hypoxic and highly angiogenic non-tumor tissues surrounding hepatocellular carcinoma: the ‘niche’ of endothelial progenitor cells. Int J Mol Sci. 2010;11:2901-2909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Girón-González JA, Martínez-Sierra C, Rodriguez-Ramos C, Macías MA, Rendón P, Díaz F, Fernández-Gutiérrez C, Martín-Herrera L. Implication of inflammation-related cytokines in the natural history of liver cirrhosis. Liver Int. 2004;24:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1403] [Cited by in RCA: 1409] [Article Influence: 88.1] [Reference Citation Analysis (2)] |
| 39. | Maeda S, Hikiba Y, Sakamoto K, Nakagawa H, Hirata Y, Hayakawa Y, Yanai A, Ogura K, Karin M, Omata M. Ikappa B kinasebeta/nuclear factor-kappaB activation controls the development of liver metastasis by way of interleukin-6 expression. Hepatology. 2009;50:1851-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Lanaya H, Natarajan A, Komposch K, Li L, Amberg N, Chen L, Wculek SK, Hammer M, Zenz R, Peck-Radosavljevic M. EGFR has a tumour-promoting role in liver macrophages during hepatocellular carcinoma formation. Nat Cell Biol. 2014;16:972-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 41. | Ji J, Eggert T, Budhu A, Forgues M, Takai A, Dang H, Ye Q, Lee JS, Kim JH, Greten TF. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology. 2015;62:481-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 42. | Tang Y, Kitisin K, Jogunoori W, Li C, Deng CX, Mueller SC, Ressom HW, Rashid A, He AR, Mendelson JS. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci USA. 2008;105:2445-2450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 268] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 43. | Marquardt JU, Andersen JB, Thorgeirsson SS. Functional and genetic deconstruction of the cellular origin in liver cancer. Nat Rev Cancer. 2015;15:653-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 44. | Tummala KS, Brandt M, Teijeiro A, Graña O, Schwabe RF, Perna C, Djouder N. Hepatocellular Carcinomas Originate Predominantly from Hepatocytes and Benign Lesions from Hepatic Progenitor Cells. Cell Rep. 2017;19:584-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 45. | Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2330] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 46. | Yu LX, Yan HX, Liu Q, Yang W, Wu HP, Dong W, Tang L, Lin Y, He YQ, Zou SS. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 47. | Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 48. | Zeuzem S. Gut-liver axis. Int J Colorectal Dis. 2000;15:59-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Zhang HL, Yu LX, Yang W, Tang L, Lin Y, Wu H, Zhai B, Tan YX, Shan L, Liu Q. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J Hepatol. 2012;57:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 50. | Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 1078] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 51. | Yu LX, Schwabe RF. The gut microbiome and liver cancer: mechanisms and clinical translation. Nat Rev Gastroenterol Hepatol. 2017;14:527-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 446] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 52. | Sachdeva M, Chawla YK, Arora SK. Immunology of hepatocellular carcinoma. World J Hepatol. 2015;7:2080-2090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 53. | Chen S, Akbar SM, Tanimoto K, Ninomiya T, Iuchi H, Michitaka K, Horiike N, Onji M. Absence of CD83-positive mature and activated dendritic cells at cancer nodules from patients with hepatocellular carcinoma: relevance to hepatocarcinogenesis. Cancer Lett. 2000;148:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Capece D, Fischietti M, Verzella D, Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F, Alesse E. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 55. | Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 649] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 56. | Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008;129:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 57. | Wang JC, Livingstone AM. Cutting edge: CD4+ T cell help can be essential for primary CD8+ T cell responses in vivo. J Immunol. 2003;171:6339-6343. [PubMed] |
| 58. | McGovern BH, Golan Y, Lopez M, Pratt D, Lawton A, Moore G, Epstein M, Knox TA. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 59. | Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 488] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 60. | Greten TF, Ormandy LA, Fikuart A, Höchst B, Henschen S, Hörning M, Manns MP, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 61. | Cany J, Tran L, Gauttier V, Judor JP, Vassaux G, Ferry N, Conchon S. Immunotherapy of hepatocellular carcinoma: is there a place for regulatory T-lymphocyte depletion? Immunotherapy. 2011;3:32-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 716] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 63. | Bricard G, Cesson V, Devevre E, Bouzourene H, Barbey C, Rufer N, Im JS, Alves PM, Martinet O, Halkic N. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. J Immunol. 2009;182:5140-5151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4365] [Article Influence: 545.6] [Reference Citation Analysis (6)] |
| 65. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (5)] |
| 66. | Heimbach JK. Overview of the Updated AASLD Guidelines for the Management of HCC. Gastroenterol Hepatol (NY. ). 2017;13:751-753. [PubMed] |
| 67. | Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1044] [Cited by in RCA: 1017] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 68. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O’Beirne J, Fox R, Skowronska A, Palmer D. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 2173] [Article Influence: 197.5] [Reference Citation Analysis (0)] |
| 69. | Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2207] [Cited by in RCA: 2303] [Article Influence: 100.1] [Reference Citation Analysis (1)] |
| 70. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10531] [Article Influence: 585.1] [Reference Citation Analysis (9)] |
| 71. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4112] [Article Influence: 514.0] [Reference Citation Analysis (5)] |
| 72. | Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, Stubbs C, Stocken DD, Wall L, Watkinson A. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol. 2017;2:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 387] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 73. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2843] [Article Influence: 315.9] [Reference Citation Analysis (1)] |
| 74. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 774] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 75. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3454] [Article Influence: 383.8] [Reference Citation Analysis (2)] |
| 76. | Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, Putra J, Camprecios G, Bassaganyas L, Akers N. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology. 2017;153:812-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 690] [Cited by in RCA: 708] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 77. | Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, de Gramont A. Targeting the TGFβ pathway for cancer therapy. Pharmacol Ther. 2015;147:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 503] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 78. | Faivre SJ, Santoro A, Kelley RK, Merle P, Gane E, Douillard J, Waldschmidt D, Mulcahy MF, Costentin C, Minguez B. A phase 2 study of a novel transforming growth factor-beta (TGF-β1) receptor I kinase inhibitor, LY2157299 monohydrate (LY), in patients with advanced hepatocellular carcinoma (HCC). Journal of Clinical Oncology. 2014;32:LBA173. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Campana L, Iredale JP. Regression of Liver Fibrosis. Semin Liver Dis. 2017;37:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 296] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 80. | Chung RT, Baumert TF. Curing chronic hepatitis C--the arc of a medical triumph. N Engl J Med. 2014;370:1576-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 81. | Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 722] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 82. | Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65:719-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 818] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 83. | El-Serag HB, Everhart JE. Improved survival after variceal hemorrhage over an 11-year period in the Department of Veterans Affairs. Am J Gastroenterol. 2000;95:3566-3573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 84. | Lang BH, Poon RT, Fan ST, Wong J. Outcomes of patients with hepatocellular carcinoma presenting with variceal bleeding. Am J Gastroenterol. 2004;99:2158-2165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Singal AK, Jampana SC, Singal V, Kuo YF. Hepatocellular carcinoma predicts in-hospital mortality from acute variceal hemorrhage among patients with cirrhosis. J Clin Gastroenterol. 2012;46:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Ripoll C, Genescà J, Araujo IK, Graupera I, Augustin S, Tejedor M, Cirera I, Aracil C, Sala M, Hernandez-Guerra M. Rebleeding prophylaxis improves outcomes in patients with hepatocellular carcinoma. A multicenter case-control study. Hepatology. 2013;58:2079-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 87. | Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis--aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016;43:765-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 88. | Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, Kochi T, Suetsugu A, Shiraki M, Shimizu M. Skeletal muscle depletion predicts the prognosis of patients with hepatocellular carcinoma treated with sorafenib. Int J Mol Sci. 2015;16:9612-9624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 89. | Parikh ND, Zhang P, Singal AG, Derstine BA, Krishnamurthy V, Barman P, Waljee AK, Su GL. Body Composition Predicts Survival in Patients with Hepatocellular Carcinoma Treated with Transarterial Chemoembolization. Cancer Res Treat. 2018;50:530-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121:2874-2882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 91. | World Health Organisation. Global health sector strategy on viral hepatitis 2016-2021. World Health Organisation. WHO reference No: WHO/HIV/2016.06. June 2016; Available from: http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/. Cited 28 August 2018. |
| 92. | Prakash TP, Graham MJ, Yu J, Carty R, Low A, Chappell A, Schmidt K, Zhao C, Aghajan M, Murray HF. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42:8796-8807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 502] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 93. | Zimmermann TS, Karsten V, Chan A, Chiesa J, Boyce M, Bettencourt BR, Hutabarat R, Nochur S, Vaishnaw A, Gollob J. Clinical Proof of Concept for a Novel Hepatocyte-Targeting GalNAc-siRNA Conjugate. Mol Ther. 2017;25:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 94. | Soule B, Tirucherai G, Kavita U, Kundu S, Christian R. Safety, tolerability, and pharmacokinetics of BMS-986263/ND-L02-s0201, a novel targeted lipid nanoparticle delivering HSP47 siRNA, in healthy participants: A randomised, placebo-controlled, double-blind, phase 1 study. J Hepatology. 2018;68:S112. [DOI] [Full Text] |
| 95. | Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, Lawitz EJ, Rockey DC, Schall RA, Jia C. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology. 2018;155:1140-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 299] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 96. | Perepelyuk M, Terajima M, Wang AY, Georges PC, Janmey PA, Yamauchi M, Wells RG. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. Am J Physiol Gastrointest Liver Physiol. 2013;304:G605-G614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 97. | Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology. 2017;. [PubMed] [DOI] [Full Text] |
| 98. | Budas G, Karnik S, Jonnson T, Shafizadeh T, Watkins S, Breckenridge D. Reduction of liver steatosis and fibrosis with an ASK1 inhibitor in a murine model of NASH is accomplished by improvements in cholesterol, bile acid and lipid metabolism. J Hepatol. 2016;64:S170. [DOI] [Full Text] |
| 99. | Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 548] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 100. | Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR Phase 2b study design. Contemp Clin Trials. 2016;47:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 101. | Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577-594.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 652] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 102. | Barreyro FJ, Holod S, Finocchietto PV, Camino AM, Aquino JB, Avagnina A, Carreras MC, Poderoso JJ, Gores GJ. The pan-caspase inhibitor Emricasan (IDN-6556) decreases liver injury and fibrosis in a murine model of non-alcoholic steatohepatitis. Liver Int. 2015;35:953-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 103. | Harrison SA, Marri SR, Chalasani N, Kohli R, Aronstein W, Thompson GA, Irish W, Miles MV, Xanthakos SA, Lawitz E. Randomised clinical study: GR-MD-02, a galectin-3 inhibitor, vs. placebo in patients having non-alcoholic steatohepatitis with advanced fibrosis. Aliment Pharmacol Ther. 2016;44:1183-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 104. | Fuchs BC, Hoshida Y, Fujii T, Wei L, Yamada S, Lauwers GY, McGinn CM, DePeralta DK, Chen X, Kuroda T. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 294] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 105. | Weiskirchen R, Weiskirchen S, Tacke F. Recent advances in understanding liver fibrosis: bridging basic science and individualized treatment concepts. F1000Res. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 106. | Schuppan D, Ashfaq-Khan M, Yang AT, Kim YO. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018;68-69:435-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 355] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Boscá L, Chen YK, Corrales FJ, Guan YS, Reggiani GM S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y