Published online Oct 7, 2018. doi: 10.3748/wjg.v24.i37.4291
Peer-review started: July 16, 2018
First decision: August 1, 2018
Revised: August 4, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: October 7, 2018
Processing time: 77 Days and 20.2 Hours
A male patient underwent conventional transcatheter chemoembolization for advanced recurrent hepatocellular carcinoma (HCC). Even after the injection of 7 mL of lipiodol followed by gelatin sponge particles, the flow of feeding arteries did not slow down. A repeat angiography revealed a newly developed vascular lake draining into systemic veins; however, embolization was continued without taking noticing of the vascular lake. The patient’s level of consciousness deteriorated immediately after the procedure, and non-contrast computed tomography revealed pulmonary and cerebral lipiodol embolisms. The patient’s level of consciousness gradually improved after 8 wk in intensive care. In this case, a vascular lake emerged during chemoembolization and drained into systemic veins, offering a pathway carrying lipiodol to pulmonary vessels, the most likely cause of this serious complication. We should be aware that vascular lakes in HCC may drain into systemic veins and can cause intratumoral arteriovenous shunts.
Core tip: Vascular lakes that resemble extravasation within hepatocellular carcinomas occasionally emerge during chemoembolization. To date, the drainage routes from vascular lakes are not well understood. We present a patient with a recurrent large hepatocellular carcinoma in which a vascular lake emerged during conventional chemoembolization, draining into systemic veins and causing pulmonary and cerebral lipiodol embolism.
- Citation: Ishimaru H, Morikawa M, Sakugawa T, Sakamoto I, Motoyoshi Y, Ikebe Y, Uetani M. Cerebral lipiodol embolism related to a vascular lake during chemoembolization in hepatocellular carcinoma: A case report and review of the literature. World J Gastroenterol 2018; 24(37): 4291-4296
- URL: https://www.wjgnet.com/1007-9327/full/v24/i37/4291.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i37.4291
Transcatheter arterial chemoembolization (TACE) is utilized worldwide for the treatment of patients with unresectable hepatocellular carcinoma (HCC). Although various complications of TACE have been reported[1], cerebral lipiodol embolism (CLE) after TACE is very rare. To our knowledge, 27 cases have been reported in the English literature, and possible pathways for carrying lipiodol from HCCs to systemic arteries have been hypothesized[2-18]. This is the first report of CLE, in which the vascular lake phenomenon emerged during the TACE procedure and caused an intratumoral arteriovenous shunt, playing the most important role in its occurrence. We also discussed the mechanism of CLE and technical considerations to avoid this serious complication. We also provided a review of the literature.
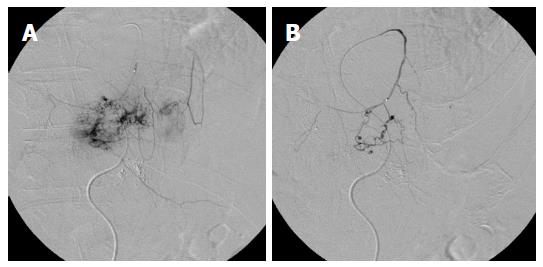
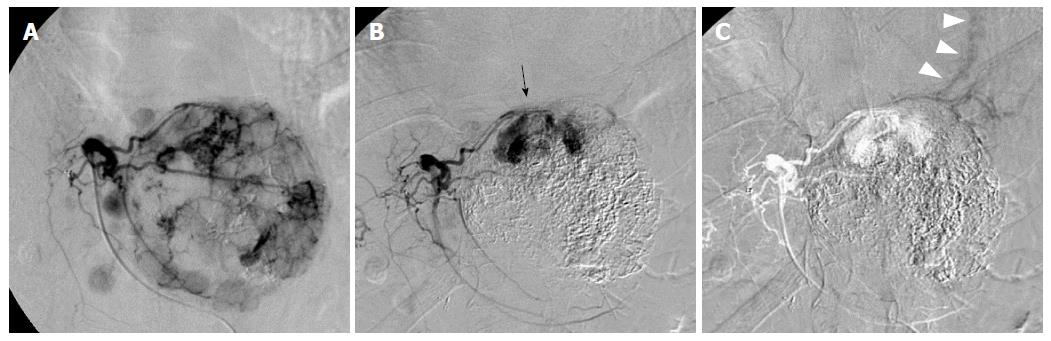
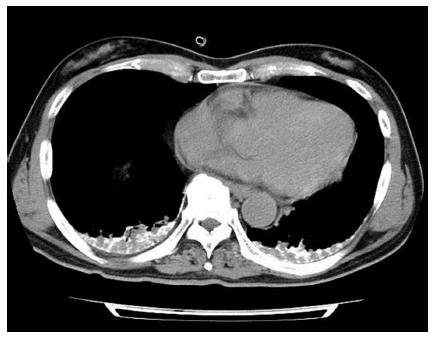
A 63-year-old man with a large recurrent HCC replacing most of the lateral segment of the liver and expanding to the left diaphragm was admitted to our hospital for a second TACE. He had a history of type-B cirrhosis for 3 years. Eight months prior to admission, he had undergone TACE for the same lesion via the left hepatic artery (LHA), and the postprocedural course was uneventful. Laboratory tests before second TACE revealed a serum total bilirubin level of 1.4 mg/dL, serum albumin level of 3.0 mg/dL, and prothrombin activity level of 81%. Neither ascites nor hepatic encephalopathy were found, which corresponded to Child-Pugh class A. The α-fetoprotein level was 900 ng/mL. At angiography, the large HCC was supplied by the LHA and the left inferior phrenic artery (LIPA) (Figure 1A) without an apparent arteriovenous (AV) shunt. For arterial redistribution to convert multiple feeding arteries into a single arterial supply, distal branches of the LIPA supplying HCC were embolized initially. To avoid migration of embolic materials into pulmonary vessels, the LIPA was embolized with 0.6 mL of a 33% mixture of n-butyl cyanoacrylate (Histoacryl; B. Braun, Melsungen, Germany) and lipiodol (Lipiodol; Terumo Corporation, Tokyo, Japan) (Figure 1B). Subsequently, we infused 140 mg of miriplatin (Miripla; Dainippon Sumitomo Pharma, Osaka, Japan) suspended in 7 mL of lipiodol via the LHA (Figure 2A). This was followed by the injection of 1-mm-diameter gelatin sponge particles (Gelpart; Nippon Kayaku Co. Ltd., Tokyo, Japan). However, the flow of the LHA did not slow down. A repeated left hepatic angiography showed contrast material pooling associated with an aberrant intratumoral space newly developed during embolization, called the “vascular lake phenomenon” (Figure 2B). Late-phase imaging revealed that the pericardiacophrenic vein was the draining vein (Figure 2C), which was not recognized at the time of the procedure but rather retrospectively after remasking and pixel shifting were performed. Epirubicin (10 mg) (Farmorubicin; Kyowa Hakko, Tokyo, Japan) emulsified in 9 mL of lipiodol was additionally infused into the LHA, followed by the injection of a 2-mm block gelatin sponge (Spongel; Astellas, Tokyo, Japan) until the flow slowed down. Immediately after TACE, the patient became drowsy and disoriented. Unenhanced CT of the brain obtained 30 minutes after the procedure revealed multiple lesions of increased attenuation in the cerebral cortex, basal ganglia, thalami and cerebellum (Figure 3). Simultaneously, a CT of the chest revealed hyperattenuation at both lung bases (Figure 4). The patient’s oxygen saturation was 88%, indicating hypoxia. The patient was monitored in our intensive care unit on mechanical ventilator support. Echocardiography revealed no atrial septal defect or other intracardiac shunts. The patient’s level of consciousness gradually improved, but his limb weakness persisted. He was discharged 8 wk later requiring the use of a wheelchair and assistance to eat meals.
TACE has been widely accepted as an effective therapy for HCC. Lipiodol is the most common embolic material used in TACE; it is usually mixed with anticancer drugs dissolved in non-ionic contrast medium. Lipiodol can enter the microcirculation of the tumor and flow into the surrounding portal vein, which is the main drainage route from the hypervascular HCC[19]. However, lipiodol can reportedly flow into systemic circulation, causing pulmonary embolism and, rarely, cerebral embolism. We found 27 reports of CLE following TACE in 17 English studies (Table 1). To reach the cerebral arteries, lipiodol must pass through two pathways, one from the tumor to the pulmonary artery (PA) and the other from the pulmonary artery to the left atrium (LA). Lipiodol accumulation in the lung was identified simultaneously in 17 of 27 previously reported cases[2-4,6,8-10,14,16,18].
| Age | Sex | Tumor characteristics | Pulmonary involvement | Embolization via extrahepatic artery supply | Dose of lipiodol | AV shunt | RL shunt | Ref. |
| 52 | M | Advanced | + | - | 35 | ND | [2] | |
| 58 | M | + | 8 | ND | [2] | |||
| 56 | M | + | ND | [2] | ||||
| 76 | M | Large | + | IPA | [3] | |||
| 81 | F | Large | + | IPA | 20 | ND | [4] | |
| 70 | F | Large | 12 | ND | [5] | |||
| 62 | F | 15.0 cm | + | IPA | 30 | ND | ND | [6] |
| 67 | M | IPA | 5 | PFO | [7] | |||
| 63 | F | IPA | 10 | + | [7] | |||
| 36 | M | Huge | + | 40 | ND | [8] | ||
| 51 | F | Huge | + | 40 | ND | [9] | ||
| 41 | M | Multiple and PVTT | + | 30 | + | ND | [10] | |
| 71 | M | ND | [11] | |||||
| 44 | M | Large | BA | [12] | ||||
| 54 | M | 13.0 cm | IPA | 30 | [13] | |||
| 66 | M | 11.5 cn | + | - | 4 | ND | [14] | |
| 62 | M | 16.0 cm | ND | [15] | ||||
| 52 | M | 18.0 cm | + | IPA | 50 | ND | Pulmonary AV shunt | [16] |
| 66 | F | IPA | 20 | [17] | ||||
| 39 | M | 8.0 cm | IPA | 13 | PV | [18] | ||
| 51 | M | 13.0 cm | + | - | 30 | PV | [18] | |
| 73 | F | 19.0 cm | + | IPA | 15 | PV | [18] | |
| 67 | F | 6.0 cm | + | LGA | 10 | [18] | ||
| 54 | F | 3.0 cm | IPA | 90 | [18] | |||
| 63 | M | 14.0 cm | + | RSGA | 50 | [18] | ||
| 52 | M | 17.0 cm | + | - | 30 | [18] | ||
| 72 | M | 10.0 cm | + | - | 20 | [18] | ||
| 63 | M | 9.0 cm | + | IPA | 16 | + | ND | Present case |
Localized pooling of the contrast medium emerges occasionally during chemoembolization using drug eluting beads (DEB-TACE), resembling extravasation within the tumor. This angiographic finding is known as the vascular lake phenomenon (VLP). As we reported herein, this phenomenon may also appear during chemoembolization using lipiodol, although it is difficult to distinguish a vascular lake from lipiodol accumulating in the tumor by fluoroscopy. Concerning the etiology of VLP during chemoembolization, Seki et al[20] speculated that rapid occlusion of most of the tumor vessels increased the pressure inside the fragile tumor microvasculature, causing disruption of the tumor architecture and a new blood space. VLP is thought to indicate a good local response in HCC patients undergoing DEB-TACE[20,21]. Nevertheless, the drainage routes from vascular lakes are unknown. To the best of our knowledge, this is the first report observing a vascular lake draining into systemic veins, resulting in an intratumoral AV shunt and affording lipiodol pathways to pulmonary vessels.
Intratumoral AV shunts can be the pathway to pulmonary vessels[5,7,10], and this was confirmed by angiography in only one CLE case[10]. On hepatic arteriography, the incidence of intratumoral AV shunts was reported to be 2.4%[22]. According to one study using technetium-99-m-labeled macroaggregated albumin[23], lung shunt fractions exceeding 10% were identified in 50 of 125 HCC patients (40%). The study also reported a direct correlation between angiographic tumor vascularity and lung shunt fractions[23]. The particles of lipiodol emulsion were less than 30 μm in size[24], within the range of the size of technetium-99-m-labeled macroaggregated albumin (10-60 μm in size). Lipiodol redistribution in the lungs may be more frequent than that previously thought.
TACE via IPA frequently results in pulmonary complications due to the existence of an AV shunt between the IPA and PA[25]. Some authors speculated that communication between the IPA and pulmonary vessels via adhesive pleurae or tumor invasion is the most likely pathway from the tumor to the PA[4,6]. In the present case, we performed IPA embolization using glue under DSA guidance, and the glue fragment never flowed into the pulmonary vessels.
In most previously reported cases of CLE, including the case described herein, a large dose of lipiodol was infused (Table 1). Cerebral and pulmonary lipiodol embolisms have been reported in patients receiving more than 20 mL of iodized oil after TACE of HCC[4,6,16,18]. According to Kishi et al[26], when lipiodol was infused into the dog’s hepatic artery, the amount of lipiodol oil deposition in the lungs was proportional to the lipiodol dose infused. They also found lipiodol deposits in the brain and pancreas[26]. These findings suggested a dose-dependent circulation of oil droplets via hepatic sinusoids to pulmonary capillaries and then into the systemic circulation. It was suggested that the lipiodol dose should not exceed 15-20 mL to prevent the risk of an extrahepatic embolism[4,6]. Nevertheless, in 7 previous cases, CLE occurred when the lipiodol dose was < 15 mL[2,5,7,14,18]. The required lipiodol dose was determined by multiple factors, including the blood supply to the tumor, tumor size, catheter position and liver function reserve. When lipiodol goes through an intratumoral AV shunt, the dose of lipiodol required to accomplish HCC flow stasis increases. If there had not been an intratumoral AV shunt in the presented case, we could have accomplished flow stoppage with a lower dose of lipiodol.
CLE is thought to be associated with intrapulmonary or intracardiac shunts[4,7,10,16]. Evidence of an underlying intracardiac right-to-left shunt was proven by transesophageal echocardiogram in one previous case[7]; however, the pathway from the PA to the LA was not verified in most previously reported cases of CLE, including the present case. Wu et al[9] speculated that an intra-pulmonary arteriovenous shunt might appear during pulmonary lipiodol embolization due to increasing pulmonary artery pressure or hypoxia. Wu et al[10] added that communication between the systemic and pulmonary vessels might develop via adhesive pleurae or tumor invasion of the diaphragm, leading to a right-to-left shunt. In a case report of delayed CLE, Wu et al[8] concluded that the rapid flow of the feeding artery washed out the lipiodol, and the lipiodol deposited in the lungs was washed out again upon entering the systemic circulation. Since it has been verified that fat globules < 7 μm in diameter can pass directly through the pulmonary arteriolar network[2], lipiodol can enter the systemic circulation in the absence of a right-to-left shunt to cause cerebrovascular complications.
In summary, we presented a case in which a vascular lake draining into systemic veins caused a lipiodol cerebral embolism. As intratumoral AV shunts via vascular lakes may develop during chemoembolization, we recommend performing repeated angiography during TACE procedures, especially when obtaining a decreased blood flow is difficult. When an intratumoral AV shunt is identified by angiography, embolization of the feeding artery using coils or glue should be considered instead of an additional injection of lipiodol or embolic particles. We should try to prevent lipiodol accumulation in the lungs because the pathways from the PA to the LA cannot be blocked regardless of the mechanism by which they are developed.
A 63-year-old man with a large hepatocellular carcinoma underwent transcatheter arterial chemoembolization (TACE), but his level of consciousness deteriorated immediately after the procedure.
CT scan of the brain revealed multiple lesions of increased attenuation, and cerebral lipiodol embolism (CLE) was confirmed.
There is no differential diagnosis.
No specific finding was obtained by laboratory testing.
An angiography during TACE procedure revealed a newly developed vascular lake draining into systemic veins, which offered a pathway carrying lipiodol to pulmonary vessels and was the most likely cause of this serious complication.
No pathological examination was performed.
The patient was treated in our intensive care unit.
This is the first report of CLE, in which vascular lake phenomenon emerging during the procedure caused intratumoral arteriovenous shunt and played the most important role for its occurrence.
The term CLE describes cerebral lipiodol embolism.
Arteriovenous shunt via vascular lake may develop during chemoembolization, repeated angiography during TACE procedures should be performed to prevent CLE, especially when it is difficult to obtain a decrease of blood flow.
| 1. | Sakamoto I, Aso N, Nagaoki K, Matsuoka Y, Uetani M, Ashizawa K, Iwanaga S, Mori M, Morikawa M, Fukuda T. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Yoo KM, Yoo BG, Kim KS, Lee SU, Han BH. Cerebral lipiodol embolism during transcatheter arterial chemoembolization. Neurology. 2004;63:181-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Takao H, Makita K, Doi I, Watanabe T. Cerebral lipiodol embolism after transcatheter arterial chemoembolization of hepatocellular carcinoma. J Comput Assist Tomogr. 2005;29:680-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Wu RH, Tzeng WS, Chang CM. Iodized oil embolization to brain following transcatheter arterial embolization of liver. J Gastroenterol Hepatol. 2005;20:1465-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Matsumoto K, Nojiri J, Takase Y, Egashira Y, Azama S, Kato A, Kitahara K, Miyazaki K, Kudo S. Cerebral lipiodol embolism: a complication of transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2007;30:512-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Choi CS, Kim KH, Seo GS, Cho EY, Oh HJ, Choi SC, Kim TH, Kim HC, Roh BS. Cerebral and pulmonary embolisms after transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2008;14:4834-4837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Kim JT, Heo SH, Choi SM, Lee SH, Park MS, Kim BC, Kim Y, Kim MK, Cho KH. Cerebral embolism of iodized oil (lipiodol) after transcatheter arterial chemoembolization for hepatocellular carcinoma. J Neuroimaging. 2009;19:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Wu JJ, Chao M, Zhang GQ, Li B. Delayed cerebral lipiodol embolism after transcatheter arterial chemoembolization of hepatocellular carcinoma. Chin Med J (Engl). 2009;122:878-880. [PubMed] |
| 9. | Wu JJ, Chao M, Zhang GQ, Li B, Dong F. Pulmonary and cerebral lipiodol embolism after transcatheter arterial chemoembolization [corrected] in hepatocellular carcinoma. World J Gastroenterol. 2009;15:633-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Wu L, Yang YF, Liang J, Shen SQ, Ge NJ, Wu MC. Cerebral lipiodol embolism following transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2010;16:398-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Karapanayiotides T, Goulis J, Theodorou A, Anastasiou A, Georgiadis G, Ilonidis G. Lipiodol brain embolism during hepatic transcatheter arterial chemoembolization. J Neurol. 2009;256:1171-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Lee CS, Kim SJ, Choi JW, Choi CG, Lee DH. Cerebral lipiodol embolism proven by dual-energy computed tomography: a case report. J Comput Assist Tomogr. 2010;34:105-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Jia ZZ, Tian F, Jiang GM. Cerebral lipiodol embolism after transarterial chemoembolization for hepatic carcinoma: a case report. World J Gastroenterol. 2012;18:4069-4070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Zach V, Rapaport B, Yoo JY, Goldfeder L, Weinberger J. Multiple ischemic strokes after transcatheter arterial chemoembolization for hepatocellular carcinoma with a radiographic and pathological correlate. J Stroke Cerebrovasc Dis. 2012;21:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Ishikawa T, Kubota T, Abe H, Toduka Y, Horigome R, Watanabe Y, Kimura N, Honda H, Iwanaga A, Seki K. Case of cerebral lipiodol embolism after repeated transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatol Res. 2013;43:1251-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Bánsághi Z, Kaposi PN, Lovas G, Szentmártoni G, Várallyay G, Bata P, Kalina I, Futácsi B, Bérczi V. Cerebral iodized lipid embolization via a pulmonary arteriovenous shunt: rare complication of transcatheter arterial embolization for hepatocellular carcinoma. World J Surg Oncol. 2013;11:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Wan CC, Liu KL. Cerebral Lipiodol embolism. Liver Int. 2015;35:673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Chu HJ, Lee CW, Yeh SJ, Tsai LK, Tang SC, Jeng JS. Cerebral Lipiodol Embolism in Hepatocellular Carcinoma Patients Treated with Transarterial Embolization/Chemoembolization. PLoS One. 2015;10:e0129367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Ueda K, Matsui O, Kawamori Y, Nakanuma Y, Kadoya M, Yoshikawa J, Gabata T, Nonomura A, Takashima T. Hypervascular hepatocellular carcinoma: evaluation of hemodynamics with dynamic CT during hepatic arteriography. Radiology. 1998;206:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 196] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Seki A, Hori S, Shimono C. Management of vascular lake phenomenon on angiography during chemoembolization with superabsorbent polymer microspheres. Jpn J Radiol. 2015;33:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Cavalcante RN, Nasser F, Motta-Leal-Filho JM, Affonso BB, Galastri FL, De Fina B, Garcia RG, Wolosker N. Occurrence of Vascular Lake Phenomenon as a Predictor of Improved Tumor Response in HCC Patients That Underwent DEB-TACE. Cardiovasc Intervent Radiol. 2017;40:1044-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Ngan H, Peh WC. Arteriovenous shunting in hepatocellular carcinoma: its prevalence and clinical significance. Clin Radiol. 1997;52:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Leung WT, Lau WY, Ho SK, Chan M, Leung NW, Lin J, Metreweli C, Johnson PJ, Li AK. Measuring lung shunting in hepatocellular carcinoma with intrahepatic-arterial technetium-99m macroaggregated albumin. J Nucl Med. 1994;35:70-73. [PubMed] |
| 24. | Kanematsu M. Transcatheter arterial chemoembolization therapy with epirubicin hydrochloride, mitomycin C-iohexol-Lipiodol emulsion (EMILE) for hepatocellular carcinoma. J Gastroenterol. 1995;30:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Tajima T, Honda H, Kuroiwa T, Yabuuchi H, Okafuji T, Yosimitsu K, Irie H, Aibe H, Masuda K. Pulmonary complications after hepatic artery chemoembolization or infusion via the inferior phrenic artery for primary liver cancer. J Vasc Interv Radiol. 2002;13:893-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Kishi K, Sonomura T, Satoh M, Nishida N, Terada M, Shioyama Y, Yamada R. Acute toxicity of lipiodol infusion into the hepatic arteries of dogs. Invest Radiol. 1994;29:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
CARE Checklist (2013): The guidelines of the CARE Checklist (2013) were adopted.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Pompili M, Xiao E, Yao DF S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY