Published online Sep 28, 2018. doi: 10.3748/wjg.v24.i36.4119
Peer-review started: May 30, 2018
First decision: July 6, 2018
Revised: July 11, 2018
Accepted: August 1, 2018
Article in press: August 1, 2018
Published online: September 28, 2018
Processing time: 118 Days and 9.9 Hours
The extracellular calcium-sensing receptor (CaSR) is best known for its action in the parathyroid gland and kidneys where it controls body calcium homeostasis. However, the CaSR has different roles in the gastrointestinal tract, where it is ubiquitously expressed. In the colon, the CaSR is involved in controlling multiple mechanisms, including fluid transport, inflammation, cell proliferation and differentiation. Although the expression pattern and functions of the CaSR in the colonic microenvironment are far from being completely understood, evidence has been accumulating that the CaSR might play a protective role against both colonic inflammation and colorectal cancer. For example, CaSR agonists such as dipeptides have been suggested to reduce colonic inflammation, while dietary calcium was shown to reduce the risk of colorectal cancer. CaSR expression is lost in colonic malignancies, indicating that the CaSR is a biomarker for colonic cancer progression. This dual anti-inflammatory and anti-tumourigenic role of the CaSR makes it especially interesting in colitis-associated colorectal cancer. In this review, we describe the clinical and experimental evidence for the role of the CaSR in colonic inflammation and colorectal cancer, the intracellular signalling pathways which are putatively involved in these actions, and the possibilities to exploit these actions of the CaSR for future therapies of colonic inflammation and cancer.
Core tip: The extracellular calcium-sensing receptor (CaSR) is best known for its roles in maintaining body calcium homeostasis, but it is also expressed in the intestines where it is assumed to be involved in pathologies such as inflammatory bowel disease and colorectal cancer. It has been suggested to act as a tumour suppressor in colorectal tumourigenesis. In this review we highlight the evidence for the anti-inflammatory and anti-tumourigenic roles of the CaSR, its signalling pathways, and its potential for future use as a drug target in the context of inflammatory bowel disease and colorectal cancer.
- Citation: Iamartino L, Elajnaf T, Kallay E, Schepelmann M. Calcium-sensing receptor in colorectal inflammation and cancer: Current insights and future perspectives. World J Gastroenterol 2018; 24(36): 4119-4131
- URL: https://www.wjgnet.com/1007-9327/full/v24/i36/4119.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i36.4119
The extracellular calcium-sensing receptor (CaSR) was first identified in bovine parathyroid cells. It is a G protein-coupled receptor (GPCR) that is activated by extracellular calcium (Ca2+), which acts as a first messenger of the CaSR signalling cascade[1]. The main physiological role of the CaSR is to control serum Ca2+ levels through regulating the synthesis and secretion of parathyroid hormone (PTH), which acts directly on the kidneys, bones and indirectly on the intestines to maintain normocalcaemia[2]. Therefore, the CaSR acts as a “calciostat” which maintains serum Ca2+ concentration within a tight range (1.1-1.3 mmol/L free ionised Ca2+) and is expressed in calcitropic tissues, such as parathyroid glands, kidneys and bone. In addition to its pivotal role in maintaining serum Ca2+ homeostasis, the CaSR also regulates non-calcitropic functions, such as gene expression, smooth muscle contraction, differentiation, proliferation, inflammation, and ion channel activity in other tissues, such as the colon, liver, vasculature, lung, pancreas, brain and the placenta[3-6]. The CaSR is also expressed along the entire gastrointestinal (GI) tract and regulates various functions in the intestines. These include dual regulation of fluid transport, where it stimulates Cl- and short chain fatty acid-dependent HCO3- secretion, but inhibits cyclic adenosine monophosphate (cAMP)-dependent HCO3- secretion[7]. In addition, the CaSR is expressed in the myenteric plexi where it regulates gut motility[8]. It also acts as a nutrient sensor for digestion products[9], such as amino acids. Additionally, it plays a role in intestinal inflammation and in the maintenance of gut microbiota and immune homeostasis[10].
The CaSR is a multifaceted GPCR that couples to several heterotrimeric G proteins. It modulates signalling pathways downstream of Gq/11, Gi/o, G12/13[11] and in specific cell contexts Gs[12]. Signalling output by the CaSR is also ligand-dependent as well as cell-type specific, thus adding to the diversity of the CaSR-mediated signalling pathways. Table 1[5,16-26] shows examples of both naturally occurring and synthetic CaSR ligands and their reported direct effects on inflammation and cancer in vivo.
| Ligand type | Class and examples | Reported effects on inflammation | Reported effects on cancer | Ref. |
| Orthosteric agonists | Inorganic divalent and trivalent cations: Zn2+ 1Ca2+; Mg2+; Gd3+ | Reduces inflammation in mouse models of colitis | High Ca2+ intake: Associated low risk for CRC | [16-18] |
| Intake is correlated with reduced inflammation | ||||
| Polyamines: Spermine spermidine, putrescine | Increase airway inflammation and hyperresponsiveness | Reduce pancreatic cancer growth in mice | [5,19] | |
| Aminoglycoside antibiotics: Neomycin, gentamycin, tobramycin | - | - | ||
| Basic polypeptides: poly-l-arginine, 1poly-l-lysine, and amyloid β-peptides | Induces airway inflammation | - | [5,20] | |
| Reduces inflammation in mouse models of colitis | ||||
| Combined orthosteric and allosteric modulators | D-amino-acid polypeptides: Etelcalcetide | - | - | |
| L-amino acids: Phenylalanine, tryptophan | - | - | ||
| Glutamyl dipeptides: 1γ-Glu-Val, 1γ-Glu-Cys | Reduces inflammation in mouse models of colitis | - | [21] | |
| Allosteric modulators (calcimimetics and calcilytics) | Small molecule calcimimetics: Sensipar (1Cinacalcet HCl), NPS-R568, GSK3004774 | Increases airway hyperresponsiveness | Treatment of parathyroid tumours | [5,22-24] |
| Inhibits neuroblastoma tumour growth | ||||
| Reduces hypercalcaemia of malignancy | ||||
| Small molecule calcilytics: 1NPS-2143, Calhex, Ronacalaret, AXT-914 | Reduces pulmonary inflammation and airway hyperresponsiveness in rodents | - | [5,25,26] |
Mutations in the CASR gene result in Ca2+ homeostasis-related diseases, including familial hypocalciuric hypercalcaemia (FHH1) and neonatal severe hyperparathyroidism (NSHPT), both of which are caused by inactivating mutations, as well as autosomal dominant hypocalcaemia (ADH1), which is caused by activating mutations (for review see[27]). Such disease causing mutations result in altered signalling output by the receptor and/or reduced cell surface expression[28]. In the intestines, more focus is directed towards the CaSR as a therapeutic target for intestinal diseases including diarrhoea, inflammatory bowel disease and colorectal cancer. In the colon, loss of CaSR expression is associated with colonic tumourigenesis[29]. In addition, clinical trials show that Ca2+ intake can favourably modulate normal colon tissue and circulating inflammation biomarkers for risk of colorectal neoplasms in sporadic colorectal adenoma patients[17]. This has led to the hypothesis that the CaSR plays a role in cancer prevention. In the following sections, we highlight the role of the CaSR in intestinal inflammation and colorectal cancer.
The CaSR is expressed in a wide range of inflammation-associated cell types where it regulates various functions. It is expressed in immune cells including macrophages, eosinophils and monocytes[5,30,31]. In these CaSR-expressing human and murine circulating monocytes, extracellular Ca2+ induces a chemokinetic effect[32]. The CaSR is also implicated in immune regulation where it plays a dual role: as a responder to inflammatory cytokine release on the one hand, and as a promoter of inflammation on the other. The link between the CaSR and inflammation has been explored in several studies. In vitro, inflammatory cytokines upregulate the CaSR expression in various cell types through defined response elements on the CASR gene[33,34]. In vivo studies also suggest a link between inflammatory cytokines and the CaSR, as intraperitoneal injection of IL-1β and IL-6 reduced PTH and 1,25(OH)2D3 levels followed by a decrease in serum Ca2+[33,35]. Furthermore, clinical studies show that hypocalcaemia occurs in critically ill patients where plasma inflammatory cytokines levels are increased[36]. In addition, the expression of the CaSR is increased in monocytes from rheumatoid arthritis patients with severe coronary artery calcification[37].
The CaSR regulates diverse and intricate signalling networks and this regulation is tissue-, and ligand-dependent. In murine macrophages, the CaSR activates the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome through a mechanism that involves increased intracellular Ca2+ and decreased cAMP levels[38]. Moreover, the CaSR regulates polymorphonuclear neutrophil function through a mechanism that likely involves the NFκB pathway[39]. The mechanism by which L-tryptophan, L-valine and glutamyl dipeptides mediate the CaSR-dependent inhibition of pro-inflammatory cytokine secretion in colonocytes appears to require β-arrestin 2[20,21]. Moreover, in the thick ascending limb of the kidneys, the CaSR has been shown to induce TNF-α-dependent cyclooxygenase 2 expression and prostaglandin E2 synthesis via a Gi-dependent mechanism[40]. However, the exact mechanism by which the CaSR regulates inflammation is still unclear and needs further investigation.
Interestingly, regulation of inflammation by the CaSR appears to be tissue-dependent. One example is the pivotal role of the CaSR in airway hyperresponsiveness and inflammation in allergic asthma. Studies on mice show that the calcilytic NPS-2143 ameliorates the severity of allergen-induced airway hyperresponsiveness[5]. In agreement with that, NPS-2143 was also shown by an independent group to be protective against lipopolysaccharide-induced pulmonary inflammation[26] and against inflammation caused in cigarette smoke extract-stimulated airway epithelial cells[25]. The CaSR plays a pro-inflammatory role also in human adipose cells and adipose tissue, where it induced the expression of inflammatory cytokines[41]. Paradoxically, in the intestines the CaSR has been suggested by several studies to play an anti-inflammatory role. Below, we highlight the evidence for the anti-inflammatory effects of the CaSR in the intestines and the potential to exploit it for nutraceutical and pharmaceutical intervention.
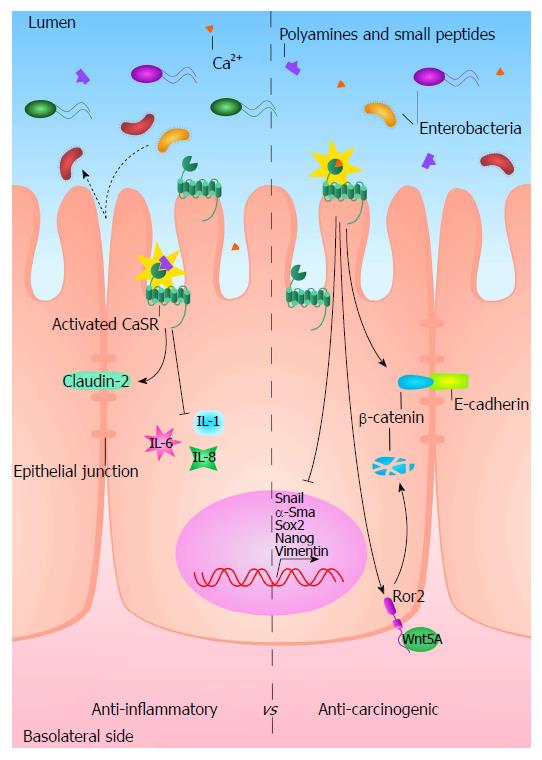
Evidence supporting the role of the CaSR in intestinal inflammation comes from a study in an intestinal epithelial cell-specific CaSR knock-out mouse model. This study showed that deletion of the CaSR from the intestinal epithelial cells diminished intestinal barrier integrity, altered the composition of the gut microbiota and induced stimulatory inflammatory responses[42]. These intestine specific CaSR knock-out mice were more susceptible to dextran sulphate sodium (DSS)-induced inflammation leading to colitis, which is a model for chemically induced inflammation in rodents. Ex vivo assessment of intestinal permeability revealed that in the knock-out mice the paracellular transport pathway was impaired. Consistent with that observation, the colonic expression of tight junction proteins, particularly claudin-2, was reduced in knock-out mice, while the expression of myosin light-chain kinase-1, an enzyme that controls contractility of the perijunctional actomyosin rings and epithelial permeability, was significantly increased[42]. No significant differences were seen between the overall richness and diversity of the gut microbiota of knock-out and wild type littermates, yet the bacterial composition was significantly changed. Moreover, intestine specific CaSR knock-out mice had significantly lower epithelial expression of Reg3β and Reg3γ that encode secreted C-type lectins which bind and protect against translocation and dissemination of bacteria. Furthermore, gene array analysis revealed increased expression of inflammatory cytokines including IL-1R in the distal colons of the intestinal epithelium-specific CaSR knock-out mice, as well as in their colonic CD4+ and CD8+ T lymphocytes. In addition, a marked increase in NFκB-dependent genes was observed in the knock-out mice. The expression of programmed cell death protein 1 (PD-1) was significantly enhanced in colonic CD4+ and CD8+ T cells[42].
Similarly, recent studies support the anti-inflammatory role of the CaSR in a DSS-colitis mouse model, where poly-L-lysine and glutamyl dipeptides, orthosteric agonists of the CaSR, reduced inflammation. These anti-inflammatory effects were suggested to be dependent on the CaSR, as their effect was reduced by the intravenous administration of the calcilytic NPS-2143[20,21]. Whether this inhibition of the anti-inflammatory effects was due to the systemic actions of the calcilytic or due to a direct action of the drug at the inflamed tissue is yet unknown. Studies assessing whether the expression level of the CaSR is affected by the chronic inflammation of the intestine in human patients suffering from inflammatory bowel disease are still outstanding.
Studies on colon cancer cell lines using CaSR agonists and allosteric modulators suggested that the CaSR influences the production of inflammatory cytokines induced by tumour necrosis factor α (TNF-α). L-tryptophan and L-valine inhibited interleukin 8 (IL-8) secretion in both Caco-2 and HT-29 colon cancer cell lines. This effect was reversed by the calcilytic NPS-2143[20]. In addition, glutamyl dipeptides inhibited pro-inflammatory cytokines and chemokines including IL-8, IL-6, and IL-1β, while increasing the expression of the anti-inflammatory IL-10 in Caco-2 cells[21]. However, it was reported that the CaSR is not detectable in colon cancer cell lines, such as HT-29, which is also supported by evidence from independent studies indicating the scarcity of the CaSR in colon cancer tissue and cell lines[43]. Therefore, further validation is needed to confirm whether these anti-inflammatory effects are actually mediated via the CaSR. Of note, inflammatory cytokines, such as TNF-α, IL-1β and IL-6, increased the expression of the CaSR at the mRNA and protein level in some colon cancer cell lines[34]. This was suggested to be a defence mechanism against inflammation in the intestines. However, this explanation will have to be carefully validated, as e.g., in lung epithelium, CaSR expression is also increased in the inflamed tissue. There however, the increase (and indeed the CaSR itself) represent a rather pro-inflammatory mechanism, as inhibition of the CaSR markedly reduced airway inflammation and hyperresponsiveness[5].
Given that inflammation is a high risk factor for colorectal cancer, it is imperative to ask the following question: is there a causal relationship between activation of the CaSR, reduced inflammation and the prevention of colorectal cancer? As of yet, this question remains unanswered. It is unclear whether dietary or pharmacological activation of the CaSR in the GI tract prevents inflammation in humans. It is also still unclear whether loss of the CaSR in colorectal tumours correlates with loss of its proposed anti-inflammatory effects. Moreover, it is noteworthy that the presence of inflammatory cytokines in the GI tract and their effect on the expression and/or function of the CaSR add to the complexity of the scenario in vivo. Nonetheless, inflammation is a key risk factor for colorectal cancer[44,45], thus targeting the CaSR for mitigating inflammation may very well contribute to colorectal cancer prevention in one fell swoop. Below, we summarise the evidence for the involvement of the CaSR in cancer and specifically colorectal cancer as well as its potential as a therapeutic target.
The CaSR plays a ying-yang role in tumours: while it is suggested to be an oncogene in breast and prostate tumours, in parathyroid, neuroblastoma and colorectal cancers it acts as a tumour-suppressor[46-48]. The CaSR signals via multiple signalling pathways and is sensitive to many ligands, the bioavailability of which varies among tissues. The different ligands and different signalling pathways can generate a tissue-specific CaSR response, justifying this dual behaviour during cancer development. Table 2[3,19,29,49-72] summarises the different roles of the CaSR in various types of cancer.
| Cancer type | CaSR | Expression of the CaSR | Detection | Proposed signalling pathway | Ref. |
| Gastric | Oncogene | Increased | mRNA, protein | TRPV4 | [49] |
| Prostate | Oncogene | Increased | mRNA, protein | PTHrP via trans-activation of the EGFR and ERK1/2 phosphorylation | [50-52] |
| AKT phosphorylation | |||||
| Breast | Oncogene | Increased in breast primary tumours and in bone metastases | mRNA, protein | PTHrP via cAMP | [53-57] |
| ERK1/2 and TRPC1 | |||||
| Inhibition of OPG via epiregulin | |||||
| Renal carcinoma | Oncogene | Increased in bone metastasising tumours | mRNA, protein | AKT phosphorylation | [58] |
| Colorectal | Tumour suppressor | Reduced | mRNA, protein | Canonical and non-canonical Wnt/β-catenin pathway and EMT | [3,29,59-62] |
| Endometrial | Tumour suppressor | Reduced | Protein | Apoptosis | [63] |
| Wnt/β-catenin | |||||
| VEGFR3 | |||||
| Parathyroid | Tumour suppressor | Reduced | mRNA, protein | Caveolin-1 and Gαq | [64-69] |
| Cyclin D1 and RGS5 | |||||
| Neuro-blastoma | Tumour suppressor | Reduced | mRNA, protein | Apoptosis via ERK1/2 | [22,70,71] |
| Cancer testis antigens (CTAs) | |||||
| Pancreatic | Unknown | Reduced | mRNA, protein | NCX1/Ca2+/ β-catenin | [19,72] |
The CaSR was implicated in the promotion of metastases from breast, prostate, and kidney tumours, thus acting as an oncogene in these tissues. Its oncogenic role is often mediated by parathyroid hormone related peptide (PTHrP).
Breast cancer has a tendency to form metastases in particular in the bones[73]. Metastases originated from breast tumours promote bone resorption which, in turn, causes the release of trophic factors (e.g., TGF-β and IGF1) that stimulate tumour cell growth, thus forming a vicious cycle. Osteolysis is driven by osteoclasts that are activated by PTHrP, which is synthesised and released from breast cancer cells[3,74,75]. The CaSR, highly expressed in metastatic breast cancer cells[53], stimulates PTHrP release, contributing thereby to bone degradation[54]. A recent study revealed that cancer cells overexpressing the CaSR had a higher osteolytic potential compared with untransfected cells[57]. Therefore, the CaSR could be a predictive marker for bone metastasis and for the patient’s poor prognosis.
Like breast cancers, prostate neoplastic lesions have a high capacity to form metastasis in the bone. Highly aggressive prostate cancer cells, such as PC-3, express the CaSR[50] while there is no evidence of CaSR expression in normal prostate tissue[3]. A cohort study, analysing 1241 prostate cancer patients, found that expression of the CaSR correlated positively with tumour lethality[76].
Although dietary calcium has been suggested to have beneficial effects on the digestive tract as being preventative against colorectal cancer, a recent study pointed out a controversial effect of calcium on gastric cancer development. Xie et al[49], have shown that calcium-activated CaSR promoted gastric cancer cell proliferation and metastasis. Thus, CaSR is suggested to act as an oncogene in the upper part of the gastro-intestinal tract, whereas it seems to act as a tumour suppressor in the lower gastro-intestinal tract (see below) although further studies are required to confirm this hypothesis.
In other cancers like parathyroid cancers, neuroblastoma and colorectal cancer the receptor acts as a tumour suppressor. In parathyroid tumours CaSR expression is inversely correlated with tumour development. CaSR mRNA expression is reduced in parathyroid adenomas and hyperplasias as compared with normal parathyroid tissue and it is lost in parathyroid carcinoma[65]. In the nervous system, the CaSR is expressed during the differentiation of neurons and glial cells[77,78]. In neuroblastoma, CaSR expression is positively correlated with neuroblast differentiation and low clinical risk, while undifferentiated and malignant neuroblastomas are CaSR-negative[79]. Indeed, ectopic re-expression of the CaSR in MYCN-amplified neuroblastoma cells, which are normally CaSR negative, reduced xenograft growth[71]. In addition, treatment with cinacalcet, a positive allosteric modulator of the CaSR, was able to induce the expression of differentiation markers, to inhibit cell proliferation in vitro and the growth of mouse tumour xenografts in vivo[22].
Another organ in which the CaSR acts as tumour suppressor is the colon.
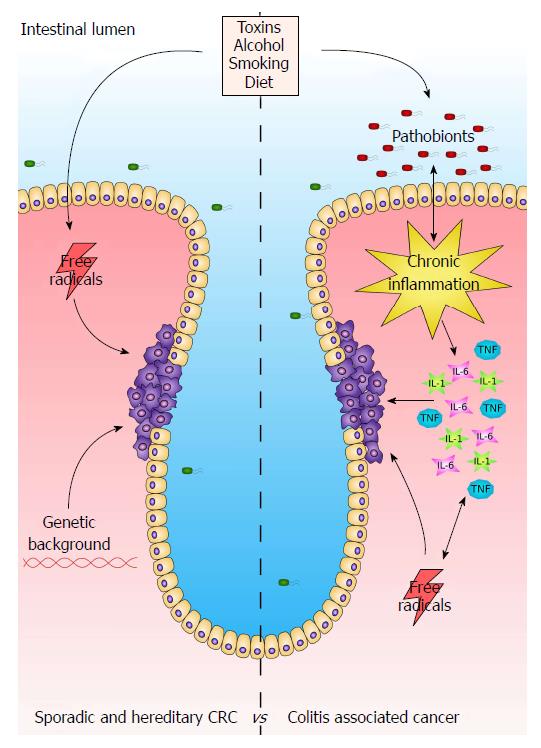
The physiological role of the colon is to process and absorb undigested nutrients, absorb electrolytes and water, and to excrete waste products via the rectum. As it is a highly renewable tissue, it is prone to malignant transformation. Colorectal cancer (CRC) is one of the most recurrent types of malignancies in the western countries and accounts for over 1,2 million of new cases per year[80]. Colorectal tumourigenesis is a complex mechanism developing from the alteration of different molecular processes that control gene expression, cell cycle and apoptosis, which are affected by genetic (e.g., APC mutation), environmental (e.g., diet, alcohol abuse, cigarette smoking, etc.), microbial and inflammatory cues that either activate oncogenes or repress tumour suppressors leading then to tumour development (Figure 1[81,82]).
As mentioned above, colonic inflammation is a risk factor for developing colorectal cancer. Chronic intestinal inflammatory diseases such as Crohn’s disease and ulcerative colitis often lead to colorectal cancer through a process called colitis-associated carcinogenesis (CAC). Similarly to (spontaneous) CRC, CAC leads to genome instability, targeting tumour suppressors and DNA repair mechanisms. However, CRC and CAC differ for prevalence and sequential-timing of the changes in biomarkers during their pathogenesis[83].
CAC is often accompanied by the alteration of the gut microbiota (dysbiosis). Commensal bacteria (eubionts) help to metabolise undigested food, modulating also the immune system of the digestive tract. On the other hand, pathogenic bacteria can trigger an immune response that, in the worst case, can lead to chronic colitis and other inflammatory bowel diseases[84]. As detailed above, the CaSR has been implicated in affecting gut microbiota, and the expression of inflammatory cytokines and thus might play a protective role against the development of CAC by protecting from the deleterious effects of inflammation.
In 1985, a small trial demonstrated for the first time that calcium regulates colonocyte proliferation[85]. In the same year, Garland et al[16] published a retrospective study showing that diets with high calcium content lower the risk of developing colorectal tumours. In the following years, several cohort studies and animal experiments supported the theory that diets rich in calcium and vitamin D prevent the development of colon hyperplasia and cancer - in contrast to western diets with high fat and low calcium and fibre content[86-88]. Meta-analyses have since reported that high Ca2+ intake (more than 1400 mg/d), independent of its source, lowers the risk of CRC, in particular in the distal colon[89,90]. Indeed, the evidence for the protective actions of high levels of dietary calcium intake (dairy products) or calcium supplements was rated to be “probably strong” by the World Cancer Research Fund in its most recent update of 2017[91].
Numerous studies have suggested that there is a close interaction between the CaSR and calcium and its protective action against CRC. A randomised clinical trial found that dietary calcium supplementation increased the expression of the CaSR in the colonic mucosa[92]. In a meta-analysis, Yang et al[93] showed that while dietary calcium reduced the risk of developing CaSR positive tumours, the risk for CaSR negative ones remained unchanged, suggesting that dietary calcium exerts its anti-tumourigenic properties via CaSR. A recent study demonstrated that CaSR expression in the tumours correlated with a reduced risk of mortality, indicating that CaSR expression might be a biomarker for positive prognosis[94].
A common agreement on the pattern of CaSR localization in the intestine is still missing. Whitfield suggested that the Ca2+ concentration is unevenly distributed along the colonic crypts, with low levels found at the bottom of the crypts and higher levels at the top. In this way, Ca2+ could exert its pro-differentiating and anti-proliferative effects only in the upper part of the crypts where the post-differentiated mature colonocytes are localised. CaSR activation would follow this concentration gradient along the crypts. Stronger activation of the CaSR at the top and weaker activation at the bottom could thus provide a physiological rationale for why the CaSR would inhibit proliferation on the differentiated top but allow proliferation at the rapidly dividing bottom of the crypts[95]. It was also suggested that this Ca2+ gradient influences CaSR expression itself, in addition to the receptor’s activation[46]. This theory is supported by the studies of Chakrabarty et al[96], who have found CaSR protein to be expressed only in the upper half of the crypts of human colon cancer biopsies. However, the actual expression pattern of the CaSR in the colon is still under debate. Contrary to the findings by Chakrabarty et al[96], Sheinin et al[97] have found CaSR expression only in the enteroendocrine cells of human colonic mucosa[97], whereas Cheng et al[8] have found the CaSR in the enteric nervous system and in the apical and basolateral side of the crypts of rat colons[8,98]. Further studies are therefore required to determine accurately the location of the CaSR in the colon and whether this expression pattern is dependent on factors like diet, age, etc.
We know that CaSR expression is lost in tumour cells. While it is still found in pre-neoplastic lesions, expression of the CaSR is lost in poorly differentiated tumours[29,96,99,100]. However, whether this loss is cause or effect of the tumourigenesis is still unknown.
Epigenetic aberrancies play a major role in tumour malignancy in general and thereby also in colorectal cancer[101]. CaSR expression is affected by repressive epigenetic marks in malignant colorectal lesions. The promoter region of the CaSR contains a large CpG island which is highly methylated in colorectal tumours. CaSR expression could be partially restored in colorectal cancer cell lines by the administration of 5-aza-2’-deoxycytidine, an inhibitor of DNA methylation. This effect was further enhanced with the addition of histone deacetylase inhibitors, suggesting that in the CaSR promoter regions the acetylation of histones is reduced and, therefore, the chromatin has a less permissive structure that hinders the recruitment of the transcription machinery[29,102]. The level of CaSR methylation increases from hyperplastic polyps and adenomas to lymph node metastases in parallel with the reduction of the receptor’s expression[102]. However, this is not a general mechanism, as in parathyroid tumours no hypermethylation of the CaSR locus was found[64,103].
Non-coding RNAs, such as miRNAs, also regulate CaSR expression in colorectal tumours. Different studies found that miR-21, miR-135a, miR-135b, miR-145, miR-146b and miR-503 inhibited CaSR expression in CRC cell lines and therefore constitute potential targets for restoring CaSR mRNA level[61,104,105].
So far, no CaSR mutations have been found that would promote tumour development in the intestine[48], although several SNPs (e.g., Q1011E, A986S, R990G) might increase colorectal cancer susceptibility although their contribution is controversial[106-109].
It is important to fully understand the molecular mechanisms that drive CaSR loss during colorectal carcinogenesis and whether this loss could be reverted or prevented and whether such an action would be beneficial for patient prognosis, pointing towards the CaSR as potential therapeutic target for a novel anti-CRC therapy or prevention.
Mouse models of systemic CaSR knock-out are not viable or die shortly after birth due to severe hyperparathyroidism and hypercalcemia[110]. However knocking out PTH rescues the lethal CaSR-/- phenotype in the PTH double knock-out (PTH-/- CaSR-/-) mouse model[111]. The colonic mucosa of the PTH-/- CaSR-/- mice as well as that of the intestinal epithelium-specific CaSR knock-out mouse model show signs of hyperproliferation. These mice develop pre-malignant intestinal lesions and are highly susceptible to the carcinogen azoxymethane (AOM)[112].The intestines of these mice are often inflamed and express pro-inflammatory markers. Furthermore, PTH-/- CaSR-/- mice are highly sensitive to DSS induced inflammation as well, suggesting a possible role of the CaSR as an anti-inflammatory factor[42,112].
Overexpression of the exogenous CaSR in colon cancer cell lines induced cellular differentiation and apoptosis, and inhibited proliferation and invasion capacity in these transfected cells. Presence of the CaSR repressed expression of stem cells markers, re-established the expression of E-cadherin and inhibited epithelial to mesenchymal transition, a process exploited by cancer cells to form metastases[47,59].
Ca2+ exerts its anti-tumourigenic function not only by binding and precipitating toxic agents such as secondary bile acids and fatty acids but also by modulating different cellular mechanisms such as proliferation, differentiation and apoptosis, potentially via the CaSR[3,100,113,114]. The mechanism involves inhibition of c-myc, upregulation of E-cadherin and inhibition of the canonical wnt-signalling pathway[99,100,115]. A recent study reported that Ca2+ inhibited the expression of replication-licensing factors in a CaSR dependent manner[60].
Both colorectal cancer cell lines and CaSR-deficient mice show that loss of the CaSR causes a higher recruitment of β-catenin into the nucleus, thus sustaining a proliferative Wnt pathway[59,112,116]. However, some studies have discovered that the CaSR is able to activate the non-canonical Wnt pathway involving the interaction between Wnt5a and its receptor, Ror2 (receptor tyrosine kinase-like orphan receptor 2). Wnt5a/Ror2 counteracts the proliferative signalling of Wnt/β-catenin, recruiting the ubiquitin ligase Siah2, which, in turn, degrades β-catenin. In myofibroblasts, CaSR activation induces the secretion of Wnt5a, while, in colonic epithelia CaSR increases the expression of Ror2[62]. Thus, the CaSR might stimulate the Wnt5a/Ror2 paracrine pathway which inhibits colonic proliferation, interfering with Wnt/β-catenin, and seems to promote the expression of colonic differentiation markers such as sucrase-isomaltase, caudal type homeobox 2 and villin[62,117,118].
CaSR pathways could potentially interact with many cellular processes in preventing or counteracting tumour development and progression. In this context, the existence of a cross talk between the CaSR and the vitamin D system has been suggested. It seems that both pathways converge in the modulation of the Wnt signalling to control colonocyte proliferation. Moreover, vitamin D seems to regulate CaSR transcription[119,120] through regulatory elements present in the CaSR promoter, which are recognised by the transcription factor vitamin D receptor. Indeed, a high vitamin D (2500 IU/kg) diet over 5 weeks more than doubled the expression of the CaSR in the colon mucosa of mice[121,122].
As of yet, a detailed description of CaSR signalling in the intestine is still missing. Given the fact that the CaSR is able to sense not only Ca2+, but also polyamines and amino acids, which are highly abundant in the intestinal lumen through the food, and that ligand biased signalling is a known feature of the CaSR[3], it is possible that the CaSR could activate different down-stream signals depending on these specific ligands also in the colon. Potential mechanisms by which the CaSR could affect inflammation and CRC are summed up in Figure 2 but a detailed map of the molecular pathways that the CaSR activates in the gut is still missing. This would allow researchers to discover potential therapeutic targets for counteracting intestinal tumourigenesis.
The CaSR is considered to prevent or counteract intestinal carcinogenesis and inflammation. Thus, the CaSR might constitute a promising therapeutic target for the treatment of colorectal cancer and of inflammatory bowel diseases. Dietary Ca2+ supplementation reduces the risk for developing colorectal cancer and studies have shown the beneficial effects of CaSR agonists, such as dipeptides, polyamines for preventing colonic inflammation and cancer. As chronic inflammation is a risk factor for colorectal cancer, the CaSR might actually be a link that connects the beneficial effect of Ca2+ in preventing both inflammation and cancer in the colon. Indeed, these roles of the CaSR indicate that activating the CaSR, or in the case of CRC also restoring CaSR expression - or preventing its loss - might be an important way for treating or preventing colonic inflammation, CRC, and, especially, CAC. However, a direct pharmacological intervention study targeting the CaSR in colonic inflammation or colorectal cancer is still missing.
Further research will be required for finding and evaluating means to restore or prevent the loss of the expression of the CaSR during carcinogenesis. One such mean could be the use of pharmacological CaSR activators, the calcimimetics. In addition to their action as allosteric agonists of the CaSR, calcimimetics also act as so called “pharmacochaperones” for the CaSR. They stabilise the expression of the CaSR, preventing the receptor’s degradation. At the same time, they increase trafficking of the CaSR from its intracellular reservoirs into the cell membrane[123,124]. As of yet, there are no data for the efficacy of calcimimetics for the prevention / treatment of CRC or CAC.
As chronic inflammation is posing a high risk for developing CAC, preventative measures should be administrable over long periods of time and should therefore ideally elicit few or no systemic side effects. Recently a novel calcimimetic, GSK3004774, which is non-resorbable and thus has gut restricted effects, has been published[125]. This compound could be useful for testing whether locally acting calcimimetics can elicit a preventive effect against intestinal inflammation, CRC and CAC without affecting systemic calcium homeostasis.
Known side effects of the FDA-approved calcimimetic cinacalcet treatment include hypocalcaemia and, notably, nausea[126]. Whether these gastrointestinal tract-related side effects are elicited via the systemic actions of the drug or a direct effect of the drug on the gastrointestinal organs is unclear. In addition, calcimimetics have been shown to actually enhance inflammation in other epithelial tissues, e.g., the lung, while calcilytics, antagonists of the CaSR, ameliorated the inflammation. In this context, the CaSR also promoted the activation of the immune system and showed a general pro-inflammatory action[5]. Whether these in vivo effects - in the complex context of immune-cells, inflamed tissue and cytokines - are tissue specific or related to a ubiquitous activation of CaSR-bearing lymphocytes is unclear. Taken together, these considerations do not allow a definite conclusion for a potential treatment of colonic inflammation or cancer with pharmacological CaSR modulators alone or in combination with conventional or targeted chemotherapies. Extensive future studies will be required to satisfactorily answer all these questions.
The CaSR emerges as a direct player in colonic inflammation and cancer development. Current evidence suggests that activation of the CaSR reduces the risk for both diseases, the strongest evidence being that dietary Ca2+ reduces the risk for CRC and that this effect is apparently mediated by the CaSR while expression of the CaSR is lost during tumourigenesis and progression of CRC. Making direct use of the CaSR as a drug target to reduce or prevent colonic inflammation and at the same time prevent colonic tumourigenesis seems a promising strategy, especially for CAC, where a dietary or pharmacological intervention could hit two birds with one stone, as it were. Future studies will be needed to address where exactly the receptor is expressed in the colonic microenvironment, which signalling pathways are mediated by the CaSR in the settings of inflammation and cancer in vivo, and whether these actions of the CaSR can be exploited for therapy and prevention.
| 1. | Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 1780] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 2. | Conigrave AD. The Calcium-Sensing Receptor and the Parathyroid: Past, Present, Future. Front Physiol. 2016;7:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 3. | Brennan SC, Thiem U, Roth S, Aggarwal A, Fetahu ISh, Tennakoon S, Gomes AR, Brandi ML, Bruggeman F, Mentaverri R, Riccardi D, Kallay E. Calcium sensing receptor signalling in physiology and cancer. Biochim Biophys Acta. 2013;1833:1732-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 4. | Schepelmann M, Yarova PL, Lopez-Fernandez I, Davies TS, Brennan SC, Edwards PJ, Aggarwal A, Graça J, Rietdorf K, Matchkov V. The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am J Physiol Cell Physiol. 2016;310:C193-C204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Yarova PL, Stewart AL, Sathish V, Britt RD Jr, Thompson MA, P Lowe AP, Freeman M, Aravamudan B, Kita H, Brennan SC, Schepelmann M, Davies T, Yung S, Cholisoh Z, Kidd EJ, Ford WR, Broadley KJ, Rietdorf K, Chang W, Bin Khayat ME, Ward DT, Corrigan CJ, T Ward JP, Kemp PJ, Pabelick CM, Prakash YS, Riccardi D. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci Transl Med. 2015;7:284ra60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 6. | Lopez-Fernandez I, Schepelmann M, Brennan SC, Yarova PL, Riccardi D. The calcium-sensing receptor: one of a kind. Exp Physiol. 2015;100:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Tang L, Peng M, Liu L, Chang W, Binder HJ, Cheng SX. Calcium-sensing receptor stimulates Cl(-)- and SCFA-dependent but inhibits cAMP-dependent HCO3(-) secretion in colon. Am J Physiol Gastrointest Liver Physiol. 2015;308:G874-G883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Cheng SX. Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2012;303:G60-G70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Brennan SC, Davies TS, Schepelmann M, Riccardi D. Emerging roles of the extracellular calcium-sensing receptor in nutrient sensing: control of taste modulation and intestinal hormone secretion. Br J Nutr. 2014;111 Suppl 1:S16-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Owen JL, Cheng SX, Ge Y, Sahay B, Mohamadzadeh M. The role of the calcium-sensing receptor in gastrointestinal inflammation. Semin Cell Dev Biol. 2016;49:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Conigrave AD, Ward DT. Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab. 2013;27:315-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 168] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J Biol Chem. 2008;283:24435-24447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Widler L. Calcilytics: antagonists of the calcium-sensing receptor for the treatment of osteoporosis. Future Med Chem. 2011;3:535-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Colella M, Gerbino A, Hofer AM, Curci S. Recent advances in understanding the extracellular calcium-sensing receptor. F1000Res. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Ward DT, Riccardi D. New concepts in calcium-sensing receptor pharmacology and signalling. Br J Pharmacol. 2012;165:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Heilbrun LK, Nomura A, Hankin JH, Stemmermann GN. Dietary vitamin D and calcium and risk of colorectal cancer. Lancet. 1985;925-925. [PubMed] |
| 17. | Bostick RM. Effects of supplemental vitamin D and calcium on normal colon tissue and circulating biomarkers of risk for colorectal neoplasms. J Steroid Biochem Mol Biol. 2015;148:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Schepens MA, Schonewille AJ, Vink C, van Schothorst EM, Kramer E, Hendriks T, Brummer RJ, Keijer J, van der Meer R, Bovee-Oudenhoven IM. Supplemental calcium attenuates the colitis-related increase in diarrhea, intestinal permeability, and extracellular matrix breakdown in HLA-B27 transgenic rats. J Nutr. 2009;139:1525-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Tang B, Chow JY, Dong TX, Yang SM, Lu DS, Carethers JM, Dong H. Calcium sensing receptor suppresses human pancreatic tumorigenesis through a novel NCX1/Ca(2+)/β-catenin signaling pathway. Cancer Lett. 2016;377:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Mine Y, Zhang H. Anti-inflammatory Effects of Poly-L-lysine in Intestinal Mucosal System Mediated by Calcium-Sensing Receptor Activation. J Agric Food Chem. 2015;63:10437-10447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Zhang H, Kovacs-Nolan J, Kodera T, Eto Y, Mine Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim Biophys Acta. 2015;1852:792-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Rodríguez-Hernández CJ, Mateo-Lozano S, García M, Casalà C, Briansó F, Castrejón N, Rodríguez E, Suñol M, Carcaboso AM, Lavarino C. Cinacalcet inhibits neuroblastoma tumor growth and upregulates cancer-testis antigens. Oncotarget. 2016;7:16112-16129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Asonitis N, Kassi E, Kokkinos M, Giovanopoulos I, Petychaki F, Gogas H. Hypercalcemia of malignancy treated with cinacalcet. Endocrinol Diabetes Metab Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Rothe HM, Liangos O, Biggar P, Petermann A, Ketteler M. Cinacalcet treatment of primary hyperparathyroidism. Int J Endocrinol. 2011;2011:415719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Lee JW, Park JW, Kwon OK, Lee HJ, Jeong HG, Kim JH, Oh SR, Ahn KS. NPS2143 Inhibits MUC5AC and Proinflammatory Mediators in Cigarette Smoke Extract (CSE)-Stimulated Human Airway Epithelial Cells. Inflammation. 2017;40:184-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Lee JW, Park HA, Kwon OK, Park JW, Lee G, Lee HJ, Lee SJ, Oh SR, Ahn KS. NPS 2143, a selective calcium-sensing receptor antagonist inhibits lipopolysaccharide-induced pulmonary inflammation. Mol Immunol. 2017;90:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 978] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 28. | Leach K, Sexton PM, Christopoulos A, Conigrave AD. Engendering biased signalling from the calcium-sensing receptor for the pharmacotherapy of diverse disorders. Br J Pharmacol. 2014;171:1142-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Fetahu IS, Höbaus J, Aggarwal A, Hummel DM, Tennakoon S, Mesteri I, Baumgartner-Parzer S, Kállay E. Calcium-sensing receptor silencing in colorectal cancer is associated with promoter hypermethylation and loss of acetylation on histone 3. Int J Cancer. 2014;135:2014-2023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Yamaguchi T, Kifor O, Chattopadhyay N, Bai M, Brown EM. Extracellular calcium (Ca2+o)-sensing receptor in a mouse monocyte-macrophage cell line (J774): potential mediator of the actions of Ca2+o on the function of J774 cells. J Bone Miner Res. 1998;13:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Yamaguchi T, Olozak I, Chattopadhyay N, Butters RR, Kifor O, Scadden DT, Brown EM. Expression of extracellular calcium (Ca2+o)-sensing receptor in human peripheral blood monocytes. Biochem Biophys Res Commun. 1998;246:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Olszak IT, Poznansky MC, Evans RH, Olson D, Kos C, Pollak MR, Brown EM, Scadden DT. Extracellular calcium elicits a chemokinetic response from monocytes in vitro and in vivo. J Clin Invest. 2000;105:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Canaff L, Hendy GN. Calcium-sensing receptor gene transcription is up-regulated by the proinflammatory cytokine, interleukin-1beta. Role of the NF-kappaB PATHWAY and kappaB elements. J Biol Chem. 2005;280:14177-14188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Fetahu IS, Hummel DM, Manhardt T, Aggarwal A, Baumgartner-Parzer S, Kállay E. Regulation of the calcium-sensing receptor expression by 1,25-dihydroxyvitamin D3, interleukin-6, and tumor necrosis factor alpha in colon cancer cells. J Steroid Biochem Mol Biol. 2014;144 Pt A:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Canaff L, Zhou X, Hendy GN. The proinflammatory cytokine, interleukin-6, up-regulates calcium-sensing receptor gene transcription via Stat1/3 and Sp1/3. J Biol Chem. 2008;283:13586-13600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Zaloga GP. Hypocalcemia in critically ill patients. Crit Care Med. 1992;20:251-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 124] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Paccou J, Boudot C, Renard C, Liabeuf S, Kamel S, Fardellone P, Massy Z, Brazier M, Mentaverri R. Total calcium-sensing receptor expression in circulating monocytes is increased in rheumatoid arthritis patients with severe coronary artery calcification. Arthritis Res Ther. 2014;16:412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 860] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 39. | Zhai TY, Cui BH, Zou L, Zeng JY, Gao S, Zhao Q, Wang Y, Xie WL, Sun YH. Expression and Role of the Calcium-Sensing Receptor in Rat Peripheral Blood Polymorphonuclear Neutrophils. Oxid Med Cell Longev. 2017;2017:3869561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Ferreri NR, Hao S, Pedraza PL, Escalante B, Vio CP. Eicosanoids and tumor necrosis factor-alpha in the kidney. Prostaglandins Other Lipid Mediat. 2012;98:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Cifuentes M, Fuentes C, Tobar N, Acevedo I, Villalobos E, Hugo E, Ben-Jonathan N, Reyes M. Calcium sensing receptor activation elevates proinflammatory factor expression in human adipose cells and adipose tissue. Mol Cell Endocrinol. 2012;361:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Cheng SX, Lightfoot YL, Yang T, Zadeh M, Tang L, Sahay B, Wang GP, Owen JL, Mohamadzadeh M. Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses. FEBS Lett. 2014;588:4158-4166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Rey O, Young SH, Jacamo R, Moyer MP, Rozengurt E. Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J Cell Physiol. 2010;225:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 968] [Article Influence: 107.6] [Reference Citation Analysis (2)] |
| 45. | Lukas M. Inflammatory bowel disease as a risk factor for colorectal cancer. Dig Dis. 2010;28:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Saidak Z, Mentaverri R, Brown EM. The role of the calcium-sensing receptor in the development and progression of cancer. Endocr Rev. 2009;30:178-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Aggarwal A, Prinz-Wohlgenannt M, Tennakoon S, Höbaus J, Boudot C, Mentaverri R, Brown EM, Baumgartner-Parzer S, Kállay E. The calcium-sensing receptor: A promising target for prevention of colorectal cancer. Biochim Biophys Acta. 2015;1853:2158-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Tennakoon S, Aggarwal A, Kállay E. The calcium-sensing receptor and the hallmarks of cancer. Biochim Biophys Acta. 2016;1863:1398-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 49. | Xie R, Xu J, Xiao Y, Wu J, Wan H, Tang B, Liu J, Fan Y, Wang S, Wu Y. Calcium Promotes Human Gastric Cancer via a Novel Coupling of Calcium-Sensing Receptor and TRPV4 Channel. Cancer Res. 2017;77:6499-6512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 50. | Sanders JL, Chattopadhyay N, Kifor O, Yamaguchi T, Brown EM. Ca(2+)-sensing receptor expression and PTHrP secretion in PC-3 human prostate cancer cells. Am J Physiol Endocrinol Metab. 2001;281:E1267-E1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Liao J, Schneider A, Datta NS, McCauley LK. Extracellular calcium as a candidate mediator of prostate cancer skeletal metastasis. Cancer Res. 2006;66:9065-9073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Bernichtein S, Pigat N, Barry Delongchamps N, Boutillon F, Verkarre V, Camparo P, Reyes-Gomez E, Méjean A, Oudard SM, Lepicard EM. Vitamin D3 Prevents Calcium-Induced Progression of Early-Stage Prostate Tumors by Counteracting TRPC6 and Calcium Sensing Receptor Upregulation. Cancer Res. 2017;77:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Mihai R, Stevens J, McKinney C, Ibrahim NB. Expression of the calcium receptor in human breast cancer--a potential new marker predicting the risk of bone metastases. Eur J Surg Oncol. 2006;32:511-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Lines C, Sanders JL, Chattopadhyay N, Kifor O, Yamaguchi T, Butters RR, Brown EM. Extracellular Calcium-Sensing Receptor Expression and Its Potential Role in Regulating Parathyroid Hormone-Related Peptide Secretion in Human Breast Cancer. 2015;0-7. |
| 55. | Saidak Z, Boudot C, Abdoune R, Petit L, Brazier M, Mentaverri R, Kamel S. Extracellular calcium promotes the migration of breast cancer cells through the activation of the calcium sensing receptor. Exp Cell Res. 2009;315:2072-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | El Hiani Y, Lehen’kyi V, Ouadid-Ahidouch H, Ahidouch A. Activation of the calcium-sensing receptor by high calcium induced breast cancer cell proliferation and TRPC1 cation channel over-expression potentially through EGFR pathways. Arch Biochem Biophys. 2009;486:58-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Boudot C, Hénaut L, Thiem U, Geraci S, Galante M, Saldanha P, Saidak Z, Six I, Clézardin P, Kamel S. Overexpression of a functional calcium-sensing receptor dramatically increases osteolytic potential of MDA-MB-231 cells in a mouse model of bone metastasis through epiregulin-mediated osteoprotegerin downregulation. Oncotarget. 2017;8:56460-56472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Joeckel E, Haber T, Prawitt D, Junker K, Hampel C, Thüroff JW, Roos FC, Brenner W. High calcium concentration in bones promotes bone metastasis in renal cell carcinomas expressing calcium-sensing receptor. Mol Cancer. 2014;13:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 59. | Aggarwal A, Prinz-Wohlgenannt M, Gröschel C, Tennakoon S, Meshcheryakova A, Chang W, Brown EM, Mechtcheriakova D, Kállay E. The calcium-sensing receptor suppresses epithelial-to-mesenchymal transition and stem cell- like phenotype in the colon. Mol Cancer. 2015;14:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Aggarwal A, Schulz H, Manhardt T, Bilban M, Thakker RV, Kallay E. Expression profiling of colorectal cancer cells reveals inhibition of DNA replication licensing by extracellular calcium. Biochim Biophys Acta. 2017;1864:987-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Fetahu IS, Tennakoon S, Lines KE, Gröschel C, Aggarwal A, Mesteri I, Baumgartner-Parzer S, Mader RM, Thakker RV, Kállay E. miR-135b- and miR-146b-dependent silencing of calcium-sensing receptor expression in colorectal tumors. Int J Cancer. 2016;138:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | MacLeod RJ, Hayes M, Pacheco I. Wnt5a secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G403-G411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 63. | Xin X, Zeng X, Feng D, Hua T, Liu S, Chi S, Hu Q, Wang H. The suppressive role of calcium sensing receptor in endometrial cancer. Sci Rep. 2018;8:1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Farnebo F, Enberg U, Grimelius L, Bäckdahl M, Schalling M, Larsson C, Farnebo LO. Tumor-specific decreased expression of calcium sensing receptor messenger ribonucleic acid in sporadic primary hyperparathyroidism. J Clin Endocrinol Metab. 1997;82:3481-3486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 65. | Haven CJ, van Puijenbroek M, Karperien M, Fleuren GJ, Morreau H. Differential expression of the calcium sensing receptor and combined loss of chromosomes 1q and 11q in parathyroid carcinoma. J Pathol. 2004;202:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Corbetta S, Eller-Vainicher C, Vicentini L, Lania A, Mantovani G, Beck-Peccoz P, Spada A. Modulation of cyclin D1 expression in human tumoral parathyroid cells: effects of growth factors and calcium sensing receptor activation. Cancer Lett. 2007;255:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Kifor O, Kifor I, Moore FD Jr, Butters RR Jr, Cantor T, Gao P, Brown EM. Decreased expression of caveolin-1 and altered regulation of mitogen-activated protein kinase in cultured bovine parathyroid cells and human parathyroid adenomas. J Clin Endocrinol Metab. 2003;88:4455-4464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Koh J, Dar M, Untch BR, Dixit D, Shi Y, Yang Z, Adam MA, Dressman H, Wang X, Gesty-Palmer D. Regulator of G protein signaling 5 is highly expressed in parathyroid tumors and inhibits signaling by the calcium-sensing receptor. Mol Endocrinol. 2011;25:867-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Corbetta S, Mantovani G, Lania A, Borgato S, Vicentini L, Beretta E, Faglia G, Di Blasio AM, Spada A. Calcium-sensing receptor expression and signalling in human parathyroid adenomas and primary hyperplasia. Clin Endocrinol (Oxf). 2000;52:339-348. [PubMed] |
| 70. | de Torres C, Beleta H, Díaz R, Toran N, Rodríguez E, Lavarino C, García I, Acosta S, Suñol M, Mora J. The calcium-sensing receptor and parathyroid hormone-related protein are expressed in differentiated, favorable neuroblastic tumors. Cancer. 2009;115:2792-2803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Casalà C, Gil-Guiñón E, Ordóñez JL, Miguel-Queralt S, Rodríguez E, Galván P, Lavarino C, Munell F, de Alava E, Mora J. The calcium-sensing receptor is silenced by genetic and epigenetic mechanisms in unfavorable neuroblastomas and its reactivation induces ERK1/2-dependent apoptosis. Carcinogenesis. 2013;34:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Rácz GZ, Kittel A, Riccardi D, Case RM, Elliott AC, Varga G. Extracellular calcium sensing receptor in human pancreatic cells. Gut. 2002;51:705-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Suva LJ, Griffin RJ, Makhoul I. Mechanisms of bone metastases of breast cancer. Endocr Relat Cancer. 2009;16:703-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 74. | Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, Gillespie MT. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451-4458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 331] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 75. | Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2060] [Cited by in RCA: 2068] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 76. | Ahearn TU, Tchrakian N, Wilson KM, Lis R, Nuttall E, Sesso HD, Loda M, Giovannucci E, Mucci LA, Finn S. Calcium-Sensing Receptor Tumor Expression and Lethal Prostate Cancer Progression. J Clin Endocrinol Metab. 2016;101:2520-2527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 77. | Chattopadhyay N, Ye CP, Yamaguchi T, Kifor O, Vassilev PM, Nishimura R, Brown EM. Extracellular calcium-sensing receptor in rat oligodendrocytes: expression and potential role in regulation of cellular proliferation and an outward K+ channel. Glia. 1998;24:449-458. [PubMed] |
| 78. | Chattopadhyay N, Espinosa-Jeffrey A, Tfelt-Hansen J, Yano S, Bandyopadhyay S, Brown EM, de Vellis J. Calcium receptor expression and function in oligodendrocyte commitment and lineage progression: potential impact on reduced myelin basic protein in CaR-null mice. J Neurosci Res. 2008;86:2159-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 79. | Mateo-Lozano S, García M, Rodríguez-Hernández CJ, de Torres C. Regulation of Differentiation by Calcium-Sensing Receptor in Normal and Tumoral Developing Nervous System. Front Physiol. 2016;7:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2351] [Article Influence: 195.9] [Reference Citation Analysis (2)] |
| 81. | Raskov H, Pommergaard HC, Burcharth J, Rosenberg J. Colorectal carcinogenesis--update and perspectives. World J Gastroenterol. 2014;20:18151-18164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 82. | Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101-2114.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1539] [Article Influence: 96.2] [Reference Citation Analysis (0)] |
| 83. | Robles AI, Traverso G, Zhang M, Roberts NJ, Khan MA, Joseph C, Lauwers GY, Selaru FM, Popoli M, Pittman ME. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology. 2016;150:931-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 84. | Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1303] [Cited by in RCA: 1690] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 85. | Lipkin M, Newmark H. Effect of added dietary calcium on colonic epithelial-cell proliferation in subjects at high risk for familial colonic cancer. N Engl J Med. 1985;313:1381-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 318] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 86. | Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, Corner G, Livote E, Lesser M, Edelmann W. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008;68:7803-7810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 87. | Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 88. | Lipkin M. Preclinical and early human studies of calcium and colon cancer prevention. Ann N Y Acad Sci. 1999;889:120-127. [PubMed] |
| 89. | Zhang X, Keum N, Wu K, Smith-Warner SA, Ogino S, Chan AT, Fuchs CS, Giovannucci EL. Calcium intake and colorectal cancer risk: Results from the nurses’ health study and health professionals follow-up study. Int J Cancer. 2016;139:2232-2242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 90. | Keum N, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. Int J Cancer. 2014;135:1940-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 91. | Colorectal cancer. World Cancer Research Fund International. Available from: https://www.wcrf.org/int/continuous-update-project/cup-findings-reports/colorectal-cancer. |
| 92. | Ahearn TU, McCullough ML, Flanders WD, Long Q, Sidelnikov E, Fedirko V, Daniel CR, Rutherford RE, Shaukat A, Bostick RM. A randomized clinical trial of the effects of supplemental calcium and vitamin D3 on markers of their metabolism in normal mucosa of colorectal adenoma patients. Cancer Res. 2011;71:413-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Yang W, Liu L, Masugi Y, Qian ZR, Nishihara R, Keum N, Wu K, Smith-Warner S, Ma Y, Nowak JA. Calcium intake and risk of colorectal cancer according to expression status of calcium-sensing receptor (CASR). Gut. 2018;67:1475-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 94. | Momen-Heravi F, Masugi Y, Qian ZR, Nishihara R, Liu L, Smith-Warner SA, Keum N, Zhang L, Tchrakian N, Nowak JA. Tumor expression of calcium sensing receptor and colorectal cancer survival: Results from the nurses’ health study and health professionals follow-up study. Int J Cancer. 2017;141:2471-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 95. | Whitfield JF. Calcium, calcium-sensing receptor and colon cancer. Cancer Lett. 2009;275:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium sensing receptor in human colon carcinoma: interaction with Ca(2+) and 1,25-dihydroxyvitamin D(3). Cancer Res. 2005;65:493-498. [PubMed] |
| 97. | Sheinin Y, Kállay E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa. J Histochem Cytochem. 2000;48:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 98. | Cheng SX, Okuda M, Hall AE, Geibel JP, Hebert SC. Expression of calcium-sensing receptor in rat colonic epithelium: evidence for modulation of fluid secretion. Am J Physiol Gastrointest Liver Physiol. 2002;283:G240-G250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 99. | Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63:67-71. [PubMed] |
| 100. | Kállay E, Kifor O, Chattopadhyay N, Brown EM, Bischof MG, Peterlik M, Cross HS. Calcium-dependent c-myc proto-oncogene expression and proliferation of Caco-2 cells: a role for a luminal extracellular calcium-sensing receptor. Biochem Biophys Res Commun. 1997;232:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3397] [Cited by in RCA: 3761] [Article Influence: 156.7] [Reference Citation Analysis (0)] |
| 102. | Hizaki K, Yamamoto H, Taniguchi H, Adachi Y, Nakazawa M, Tanuma T, Kato N, Sukawa Y, Sanchez JV, Suzuki H. Epigenetic inactivation of calcium-sensing receptor in colorectal carcinogenesis. Mod Pathol. 2011;24:876-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 103. | Cetani F, Pinchera A, Pardi E, Cianferotti L, Vignali E, Picone A, Miccoli P, Viacava P, Marcocci C. No evidence for mutations in the calcium-sensing receptor gene in sporadic parathyroid adenomas. J Bone Miner Res. 1999;14:878-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Singh N, Chakrabarty S. Induction of CaSR expression circumvents the molecular features of malignant CaSR null colon cancer cells. Int J Cancer. 2013;133:2307-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Noguchi T, Toiyama Y, Kitajima T, Imaoka H, Hiro J, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Toden S. miRNA-503 Promotes Tumor Progression and Is Associated with Early Recurrence and Poor Prognosis in Human Colorectal Cancer. Oncology. 2016;90:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 106. | Peters U, Chatterjee N, Yeager M, Chanock SJ, Schoen RE, McGlynn KA, Church TR, Weissfeld JL, Schatzkin A, Hayes RB. Association of genetic variants in the calcium-sensing receptor with risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2004;13:2181-2186. [PubMed] |
| 107. | Zhu Y, Wang PP, Zhai G, Bapat B, Savas S, Woodrow JR, Sharma I, Li Y, Zhou X, Yang N. Vitamin D receptor and calcium-sensing receptor polymorphisms and colorectal cancer survival in the Newfoundland population. Br J Cancer. 2017;117:898-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 108. | Mahmoudi T, Karimi K, Arkani M, Farahani H, Nobakht H, Dabiri R, Asadi A, Zali MR. Parathyroid hormone gene rs6256 and calcium sensing receptor gene rs1801725 variants are not associated with susceptibility to colorectal cancer in Iran. Asian Pac J Cancer Prev. 2014;15:6035-6039. [PubMed] |
| 109. | Jenab M, McKay J, Bueno-de-Mesquita HB, van Duijnhoven FJ, Ferrari P, Slimani N, Jansen EH, Pischon T, Rinaldi S, Tjønneland A. Vitamin D receptor and calcium sensing receptor polymorphisms and the risk of colorectal cancer in European populations. Cancer Epidemiol Biomarkers Prev. 2009;18:2485-2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 110. | Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, Brown EM, Seidman JG, Seidman CE. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 394] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 111. | Kos CH, Karaplis AC, Peng JB, Hediger MA, Goltzman D, Mohammad KS, Guise TA, Pollak MR. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J Clin Invest. 2003;111:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 112. | MacLeod RJ. Extracellular calcium-sensing receptor/PTH knockout mice colons have increased Wnt/β-catenin signaling, reduced non-canonical Wnt signaling, and increased susceptibility to azoxymethane-induced aberrant crypt foci. Lab Invest. 2013;93:520-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 113. | Fedirko V, Bostick RM, Flanders WD, Long Q, Sidelnikov E, Shaukat A, Daniel CR, Rutherford RE, Woodard JJ. Effects of vitamin d and calcium on proliferation and differentiation in normal colon mucosa: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2009;18:2933-2941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 114. | Fedirko V, Bostick RM, Flanders WD, Long Q, Shaukat A, Rutherford RE, Daniel CR, Cohen V, Dash C. Effects of vitamin D and calcium supplementation on markers of apoptosis in normal colon mucosa: a randomized, double-blind, placebo-controlled clinical trial. Cancer Prev Res (Phila). 2009;2:213-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 115. | Bhagavathula N, Kelley EA, Reddy M, Nerusu KC, Leonard C, Fay K, Chakrabarty S, Varani J. Upregulation of calcium-sensing receptor and mitogen-activated protein kinase signalling in the regulation of growth and differentiation in colon carcinoma. Br J Cancer. 2005;93:1364-1371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 116. | Rey O, Chang W, Bikle D, Rozengurt N, Young SH, Rozengurt E. Negative cross-talk between calcium-sensing receptor and β-catenin signaling systems in colonic epithelium. J Biol Chem. 2012;287:1158-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 117. | Pacheco II, Macleod RJ. CaSR stimulates secretion of Wnt5a from colonic myofibroblasts to stimulate CDX2 and sucrase-isomaltase using Ror2 on intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G748-G759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 118. | Cheung R, Kelly J, Macleod RJ. Regulation of villin by wnt5a/ror2 signaling in human intestinal cells. Front Physiol. 2011;2:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 119. | Canaff L, Hendy GN. Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J Biol Chem. 2002;277:30337-30350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 230] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 120. | Singh N, Aslam MN, Varani J, Chakrabarty S. Induction of calcium sensing receptor in human colon cancer cells by calcium, vitamin D and aquamin: Promotion of a more differentiated, less malignant and indolent phenotype. Mol Carcinog. 2015;54:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 121. | Aggarwal A, Höbaus J, Tennakoon S, Prinz-Wohlgenannt M, Graça J, Price SA, Heffeter P, Berger W, Baumgartner-Parzer S, Kállay E. Active vitamin D potentiates the anti-neoplastic effects of calcium in the colon: A cross talk through the calcium-sensing receptor. J Steroid Biochem Mol Biol. 2016;155:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Aggarwal A, Kállay E. Cross Talk between the Calcium-Sensing Receptor and the Vitamin D System in Prevention of Cancer. Front Physiol. 2016;7:451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 123. | Huang Y, Cavanaugh A, Breitwieser GE. Regulation of stability and trafficking of calcium-sensing receptors by pharmacologic chaperones. Adv Pharmacol. 2011;62:143-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 124. | Cook AE, Mistry SN, Gregory KJ, Furness SG, Sexton PM, Scammells PJ, Conigrave AD, Christopoulos A, Leach K. Biased allosteric modulation at the CaS receptor engendered by structurally diverse calcimimetics. Br J Pharmacol. 2015;172:185-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 125. | Sparks SM, Spearing PK, Diaz CJ, Cowan DJ, Jayawickreme C, Chen G, Rimele TJ, Generaux C, Harston LT, Roller SG. Identification of potent, nonabsorbable agonists of the calcium-sensing receptor for GI-specific administration. Bioorg Med Chem Lett. 2017;27:4673-4677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 126. | Palmer SC, Nistor I, Craig JC, Pellegrini F, Messa P, Tonelli M, Covic A, Strippoli GF. Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials. PLoS Med. 2013;10:e1001436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Austria
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Shi YJ, Camara NOS, Gupta R S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y