Published online Sep 28, 2018. doi: 10.3748/wjg.v24.i36.4093
Peer-review started: July 16, 2018
First decision: July 31, 2018
Revised: August 2, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: September 28, 2018
Processing time: 72 Days and 19.2 Hours
Considering that both innate and adaptive immune responses are involved in the pathogenesis of Crohn’s disease (CD), novel therapeutic options have significantly been developed. Biological agents represent an important addition to the conventional treatments for immuno-inflammatory conditions, acting as antagonists of adhesion molecules or various inflammatory cytokines. The interleukin 12 (IL-12)/IL-23 common pathway has been found to play a determinant role in the induction of inflammation in adaptive immune responses. In particular, IL-23 promotes the differentiation of naïve T helper cells into Th17 phenotype with the concomitant secretion of several inflammatory cytokines such as IL-17 and IL-22, whereas IL-12 induces the Th1 polarization and production of critical cytokines such as interferon-γ and tumor necrosis factor. Nowadays, there is increased interest regarding the role of IL-23 as a therapeutic target of CD through the blockage of IL-23 mediated pathways. In this editorial, we focus on the role of IL-12/IL-23 pathway in the regulation of mucosal immunity and in the induction and maintenance of chronic inflammation. In parallel, we critically discuss the available data regarding the therapeutic effect of the IL-12/IL-23 inhibitors and especially of ustekinumab, a human monoclonal antibody which has been recently approved by the United States Food and Drug Administration for the management of moderate-to-severe CD and its potential to be used as first-line therapy in everyday clinical practice.
Core tip: The therapeutic management of Crohn’s disease patients not responding to treatment with anti-tumor necrosis factor agents remains a clinical challenge. Recently, there has been increased evidence regarding the development of new drugs with alternative mechanisms of action. Interleukin (IL)-12 and IL-23 are important cytokines which are involved in the adaptive immune responses and their common pathway has been found to play a determinant role in the induction of inflammation. Clinical trials have assessed the therapeutic effect of an IL-12/IL-23 inhibitor (ustekinumab), demonstrating rapid clinical effect with a safety profile. Further studies are needed to elucidate its potential role as first-line therapy in Crohn’s disease.
- Citation: Aggeletopoulou I, Assimakopoulos SF, Konstantakis C, Triantos C. Interleukin 12/interleukin 23 pathway: Biological basis and therapeutic effect in patients with Crohn's disease. World J Gastroenterol 2018; 24(36): 4093-4103
- URL: https://www.wjgnet.com/1007-9327/full/v24/i36/4093.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i36.4093
Crohn’s disease (CD) is an immune-mediated inflammatory disorder characterized by chronic relapsing inflammation in different segments of the gastrointestinal tract. Although the typical preferential regions of involvement are the distal ileum, the ileocaecal region, the colon and the perianal region, extra-intestinal manifestations are not uncommon[1]. The etiology of this disease in not yet fully understood. However, it is currently considered that genetic and environmental factors, impaired immune regulation, gut barrier dysfunction and changes in the intestinal microbiome are involved in the pathogenesis and development of this condition[2-4]. CD treatment is generally individualized and is associated with several factors including disease phenotype, disease severity, affected region, and related luminal or extraluminal complications. The treatment strategy is mainly classified into two stages: (1) Appropriate treatment of the acute flare aiming to induce remission (2) maintenance of remission[5]. Until recently, the initial choice of treatment has focused on long-term use of corticosteroids and immunosuppressants like thiopurines and methotrexate for the induction and the maintenance of remission, respectively[6-8]. During the last years, therapeutic options have significantly benefited from the introduction of biological agents, which became the mainstay of moderate to severe CD treatment, using monoclonal antibodies targeting tumor necrosis factor (TNF)[9-11] or adhesion molecules (integrins)[12,13]. However, a significant proportion of patients (about 30%) will not respond adequately to induction therapy with TNF inhibitors. Furthermore, another subgroup of patients that achieve initial (short-term) response, run a risk of secondary loss which occurs in approximately 40% of patients[14,15]. The main causes of secondary failure are non-compliance to anti-TNF treatment, drug immunogenicity and non-immune clearance of anti-TNF or the persistence of inflammatory activity in spite of sufficient anti-TNF levels[16]. This latter clinical scenario is usually performed by switching to another class of biological agents[16].Moreover, the humanized anti-α4β7 integrin antibody that has been recently introduced in clinical practice, has displayed efficacy on the induction and maintenance of remission in moderate-to-severe refractory CD patients; however, safety concerns have been raised due to rare but possible adverse events[17].
Current data suggest that the initiation and perpetuation of inflammation in CD are associated with a disruption in the balance among the intestinal epithelium, the commensal microbiota and the innate immune response[18]. This condition is maintained by the presence of defects in the intestinal wall, environmental factors, genetic predisposition and dysfunction in regulatory mechanisms, which in turn lead to the release of an array of cytokines that promote the inflammatory immune response[18].
Taking into consideration the adverse events resulted from previous treatments regiments, target tailored treatment options that aim at specific pathways of inflammation have emerged. CD is characterized by dysfunction in both innate and adaptive immune responses. Disturbances in adaptive immune response are closely related to tissue damage, mainly driven by interleukin (IL)-12 and IL-23[4]. Therefore, inhibitors of IL-12/IL-23 and specific inhibitors of IL-23 have been developed for the management of CD. In this editorial, we focus on the role of IL-12/IL-23 pathway in the modulation of mucosal immunity and in the induction and maintenance of remission of the associated chronic inflammation of the intestinal epithelium. Moreover, we critically discuss the therapeutic effects of the IL-12/IL-23 blocker in patients with CD and its potential position as first-line therapy in everyday clinical practice.
Both innate and adaptive immune responses are involved in the pathogenesis of CD. Adaptive immunity is provided by resident and recruited cells, including mucosal B cells which produce the secretary immunoglobulins A and G, T cells subpopulations and especially T helper 1 (Th1), Th17, or Th2 cells, and T and B regulatory cells[4]. Th1 phenotype is induced by microbes which in turn activate the excretion of interferon (IFN)-γ and IL-12p40 through the signal transducer and activator of transcription 1 (STAT1), T-box factor 21 (TBX21) and STAT4 signaling pathways[19]. CD seems to be driven by a Th1-mediated pathology, with an increased synthesis of IFN-γ[20]. In parallel, inflamed intestinal mucosa is infiltrated by Th17 cells with a concomitant production of IL-17 cytokine[21]. Th17 lineage commitment is directed by transforming growth factor beta (TGF-β) in the presence of a proinflammatory environment, and IL-23 is related to the expansion and maintenance of Th17 cells[22]. Moreover, CD is characterized by an increased production of IL-12, the major Th1-stimulating factor[23,24].
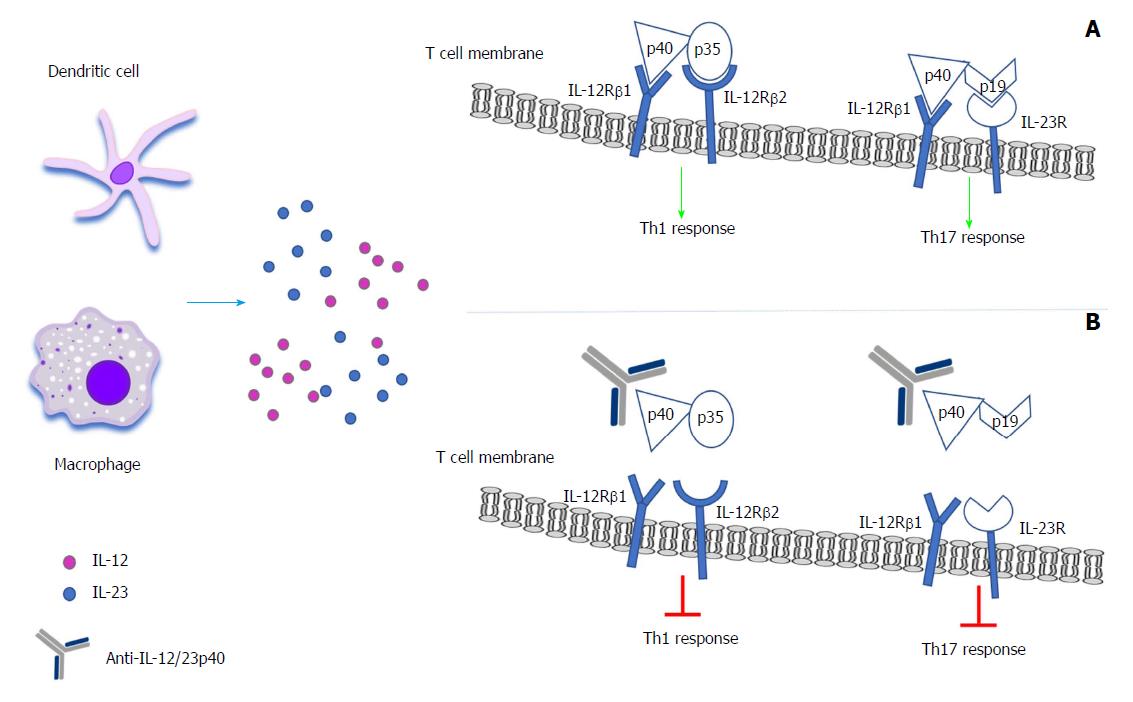
IL-12 family includes IL-12, IL-2, IL-35 and IL-27, key mediators of inflammatory response[25]. IL-12 is a heterodimeric cytokine comprising of two covalently linked subunits (p40 and p35) and is mainly produced by activated phagocytic cells [monocytes/macrophages, neutrophils, dendritic cells (DCs)] in response to bacterial stimulation, intracellular pathogens and intestinal inflammation[26,27]. IL-12 exerts its biological function through the binding to its heterodimeric receptor formed by IL-12R-β1 and IL-12R-β2. β2 receptor subunit plays a major role in IL-12 function, as it controls the Th1 cell lineage commitment. Moreover, IL-12R-β2 is overexpressed by T cells in inflamed mucosa[26] as well as in CD T-lamina propria lymphocytes (T-LPL)[28]. Data has shown that the inhibition of IL-12 resulted in reduced production of IFN-γ and IL-21 in CD mucosal T cells cultures[23], and T cell stimulation in T cell cultures from fetal gut explants, promoting Th1 immune response and causing mucosal degradation[29]. Beyond the role of IL-12 in T cells, a recent study has demonstrated its role in a distinct population, the innate lymphoid cells (ILCs), which are considerably encountered in inflamed tissue of intestinal wall of CD patients. IL-12 stimulates these cells to produce IFN-γ indicating the role of ILCs in the pathogenesis of gut mucosal inflammation[30].
Recent studies have demonstrated the crucial role of IL-23 in the regulation of Th1 cell responses and its potential role in the CD pathogenesis. IL-23 belongs to the IL-12 family, it is composed of one subunit of p40, that is shared with IL12, and one subunit of p19, which is unique[31]. IL-23 is produced by myeloid DCs or conventional DCs and macrophages in response to bacterial stimulation, endogenous signals or CD40L activation[32,33]. Depending on the various environmental signals, macrophages can obtain distinct functional phenotypes through undergoing different polarization[34]. Macrophage M1 phenotype is induced by microbial products or proinflammatory cytokines and is characterized by high production of IL-12 and IL-23 cytokines, in contrast to M2 phenotype which is mainly associated with Th2 immune responses and promotes tissue repair[35]. The binding of IL-23 on its receptor, which is composed of IL-12R β1 and IL-23R results in the specific induction of naïve CD4+ T cells into Th17 cells, with a concomitant activation of numerous proinflammatory cytokines such as IL-17, IL-17F, TNF-α and IL-6[36]. Beyond CD4+ T induction, IL-23 participates in the ILCs[37], CD8+ T[38], natural killer (NK), NKT[39] and γδ T cells[40] activation. The presence of inflammation in the intestinal wall stems from the pathological Th1 immune response against the bacterial microbiota which are closely related to the IL-12 and IL-23 expression[41]. IL-23 expression is highly increased in ILCs in the inflamed intestine in CD patients, indicating the presence of IL-23-responsive ILCs in the human gut and promoting IL-17A and IFN-γ production[37,42]. Moreover, studies have demonstrated the existence of single nucleotide polymorphisms (SNPs) in the IL-23R gene, which are highly protective in CD patients, suggesting that the blockade of IL-23 signaling could decrease the risk of CD development[43-45].
The above data highlighted the determinant role of IL-12 and IL-23 in intestinal inflammation, since they are able to trigger signals in different cell populations and lead to the introduction of monoclonal antibodies as therapeutic agents for CD, targeting the common p40 subunit of IL-12 and IL-23.
Ustekinumab is an IgG1 humanized monoclonal antibody directed against the common p40 subunit of the IL-12 and IL-23. It binds to the p40 subunit and impedes the interaction with the IL-12Rβ1 on the cell surface of NK, T cells, or antigen-presenting cells[46]. This process results in the blockade of IL-12 and IL-23 mediated downstream cell signaling, gene activation and cytokine production[46]. Ustekinumab binding to the IL-12 and IL-23, equally neutralizes IL-12 mediated responses, including the intracellular phosphorylation of STAT4, the expression of cell surface markers and the production of IFN-γ and IL-23 mediated responses including the intracellular phosphorylation of STAT3 and IL-17A, IL-17F, and the production of IL-22[46] (Figure 1).
Briakinumab is an IgG1 monoclonal antibody with similar mechanism of action as ustekinumab, which was tested for the induction and maintenance of remission in patients with moderately to severely active CD. The results of a placebo-controlled phase 2b trial showed that although briakinumab presented a similar safety and tolerability profile to placebo in the induction and maintenance phases, it did not achieve the primary end point of clinical remission at week 6[47].
MEDI2070 (or AMG 139) is a humanized monoclonal IgG2 antibody that selectively binds the p19 subunit, specifically blocking the binding of IL-23 to its receptor. In a phase 2a trial of patients with moderate to severe CD who had failed to anti-TNF treatment, the use of MEDI2070 showed induction of clinical response in CD patients compared to placebo[48].
Risankizumab, a humanized monoclonal antibody targeting the p19 subunit of IL-23, resembles the mechanism of action of MEDI2070. The efficacy and safety of this antibody were assessed in a randomized, double-blind, placebo-controlled phase 2 study and the results showed that it was significantly better than placebo in inducing clinical remission[49].
Ustekinumab was approved in 2017 by the United States Food and Drug Administration (FDA) for the treatment of moderate to severe active CD in patients who have failed or were intolerant to therapy with corticosteroids or other immunomodulators but have never failed anti-TNF treatment, or in patients who have failed or were intolerant to therapy with one or more anti-TNF agents[50]. In parallel, in the same year, the use of ustekinumab was approved by the European Medicines Agency (EMA) for adults with moderate to severe active CD with inadequate response, or loss of response, or intolerance to either conventional treatment, or anti-TNF agents, or with medical contraindications to such therapies[51].
The clinical efficacy of ustekinumab in humans was first evaluated in immune-mediated diseases, such as psoriasis[52,53], psoriatric arthritis[54-56] and multiple sclerosis[57]. The current use of ustekinumab in patients with CD has been assessed by multiple clinical trials (Tables 1 and 2).
| Study (reference) | Publication year | Type of publication | Study design | Study phase |
| Sandborn et al[58] | 2008 | Full paper | Multicenter, double-blind, placebo-controlled, parallel cross-over | IIa |
| Sandborn et al[59] (CERTIFI) | 2012 | Full paper | Randomized, multicenter, double-blind, placebo-controlled | IIb induction |
| IIb maintenance | ||||
| Feagan et al[60] (UNITI-1) | 2016 | Full paper | Randomized, multicenter, double-blind, placebo-controlled | III |
| Feagan et al[60] (UNITI-2) | 2016 | Full paper | Randomized, multicenter, double-blind, placebo-controlled | III |
| Feagan et al[60] (IM-UNITI) | 2016 | Full paper | Randomized, multicenter, double-blind, placebo-controlled | Maintenance phase of UNITI 1 and 2 responders |
| Sandborn et al[63] (IM-UNITI long term extension) | 2017 | Abstract | Randomized, multicenter, double-blind, placebo-controlled | Maintenance phase of UNITI 1 and 2 responders |
| Sands et al[62] (UNITI-IM) | 2018 | Abstract | Randomized, multicenter, double-blind, placebo-controlled | Maintenance phase of UNITI 1 and 2 responders |
| Rutgeerts et al[61] | 2018 | Full paper | Randomized, multicenter, double-blind, placebo-controlled | Induction and maintenance of endoscopic healing |
| Study (reference) | Patients | Endpoints | Intervention parameters | Outcomes |
| Sandbornetal[58] | 25 | Clinical response at week 4/week 8 | 90 mg SC week 0-3→Placebo SC week 8-11 | Ustekinumab: 53%/49% Placebo: 30%/40% |
| 26 | Placebo SC week 0-3→90mg SC week 8-11 | |||
| 26 | 4.5 mg/Kg IV week 0→Placebo IV week 8 | |||
| 27 | Placebo IV week 0→4.5 mg/Kg IV week 8 | |||
| 27 (primary or secondary non-responders to infliximab) | Clinical response at week 8 | 90 mg SC | 43% | |
| 4.5 mg/kg IV | 54% | |||
| Sandborn et al[59] (CERTIFI) Induction | 131 | Clinical response at week 6 | 1 mg/kg IV | 36.60% |
| 132 | 3 mg/kg IV | 34.10% | ||
| 131 | 6 mg/kg IV | 39.70% | ||
| 132 | Placebo IV | 23.50% | ||
| Sandborn et al[59] (CERTIFI) Maintenance | 145 Responders | Clinical response at week 22 | 90 mg SC | 69.40% |
| 72 Ustekinumab | Placebo SC | 42.50% | ||
| 73 Placebo | Clinical remission at week 22 | 90 mg SC | 41.70% | |
| Placebo SC | 27.40% | |||
| Feagan et al[60] UNITI-1 induction | 245 | Clinical response at week 6 | 130 mg IV | 34.30% |
| 249 | 6 mg/kg IV | 33.70% | ||
| 247 | Placebo IV | 21.50% | ||
| Feagan et al[60] UNITI-2 induction | 209 | Clinical response at week 6 | 130 mg IV | 51.70% |
| 209 | 6 mg/kg IV | 55.50% | ||
| 210 | Placebo IV | 28.70% | ||
| Feagan et al[60] IM- UNITI maintenance | 132 | Clinical remission at week 44 | 90 mg SC every 8 wk | 53.10% |
| 132 | 90 mg SC every 12 wk | 48.80% | ||
| 133 | Placebo SC | 35.90% | ||
| Sandborn et al[63] (IM- UNITI long term extension) | 1281 | Clinical remission at week 92 | 90 mg SC every 8 wk | 74.40% |
| 90 mg SC every 12 wk | 72.60% | |||
| Subjects with prior dose adjustment | 53.50% | |||
| All ustekinumab treated | 67.50% | |||
| Sands et al[62] (IM-UNITI patients with dose adjustment following loss of response) | 51 | Clinical response [CR-100]1 | Placebo to 90 mg SC ustekinumab every 8 wk | 71% |
| 29 | Ustekinumab 90 mg SC every 12 wk to ustekinumab 90 mg SC every 8 wk | 55% | ||
| 28 | No dose adjustment | 46% | ||
| Sands et al[62] (IM-UNITI non-responders during induction phase having an additional SC dose) | 467 | Clinical response 8 wk after one additional dose | Additional dose of 90 mg SC | 50.50% |
| Clinical remission 8 wk after one additional dose | 28.90% | |||
| Rutgeerts et al[61] Induction week 8 | 155 | SES-CD Change from baseline, mean (SD) | 130 mg IV/6 mg/kg | -2.8 (8.10)a |
| 97 | Placebo IV | -0.7 (4.97) | ||
| Rutgeerts et al[61] Maintenance week 44 | 47 | SES-CD Change from baseline, mean (SD) | 90mg SC every 12 wk | -1.5 (4.22) |
| 74 | 90mg SC every 8 wk | -3.8 (6.02) | ||
| 51 | Placebo SC | -2.0 (5.35) |
The use of ustekinumab in the treatment of moderate to severe CD was first investigated in 2008 in a randomized, placebo-controlled, phase 2a induction trial[58]. The study comprised of two patient groups. Population 1 (the double-blind, cross-over phase IIa arm of the study) included 104 patients who had previously received conventional therapy or anti-TNF regimens. The second group, population 2 - open-label arm, consisted of 27 non-responders (primary or secondary) to infliximab. The results showed that ustekinumab could induce clinical response in patients with moderate-to-severe active CD, especially in those who were previously treated with infliximab[58]. Regarding the development of serious adverse events, there was no difference in patients receiving ustekinumab compared to placebo[58]. The above results led to the conduct of a 36-wk, randomized, double-blind, placebo-controlled phase IIb trial (CERTIFI) on the role of ustekinumab in the induction and maintenance of remission in patients with moderate-to-severe CD who were resistant to anti-TNF treatment[59]. The study enlisted 526 patients in the induction arm and 145 responders in the maintenance phase. The results demonstrated that patients who were resistant to anti-TNF therapy showed an increased response rate to induction with ustekinumab compared to placebo, although remission rates were comparable[59]. However, ustekinumab induction responders showed significantly increased rates of response and remission during the maintenance phase[59]. No difference was reported in the incidence of adverse events between examined groups during the maintenance phase[59]. Basal-cell carcinoma developed in 1 patient receiving ustekinumab.
Phase III, multicentre, double-blind, placebo-controlled, trials for the evaluation of ustekinumab in patients with moderate to severe CD have been recently completed. The first trial (UNITI-1) included 741 patients who were primary or secondary non-responders to anti-TNF treatment or had severe side effects[60]. In the second trial (UNITI-2) 628 patients who had failed the conventional therapy or had experienced severe side effects were enrolled[60]. The results showed that intravenous ustekinumab induced clinical response and remission in patients from both trials (UNITI 1-2)[60]. No difference in adverse and serious adverse events was reported between the groups. Moreover, there was no report of death, malignancy, opportunistic infections or tuberculosis in ustekinumab treated patients[60]. The 397 patients who completed the induction trials (UNITI 1 and 2) and were responders to ustekinumab, were enrolled in the IM-UNITI trial[60]. Primary endpoint for this trial was the maintenance of remission at week 44 and the results showed that treatment with ustekinumab was more effective than placebo for maintaining remission[60]. Between the placebo and the ustekinumab groups, the rates of adverse events development and severity were similar[60].
A sub-study of the UNITI trial enrolled 334 patients with moderate to severe CD and assessed the clinical effect of ustekinumab in the simplified endoscopic activity score for CD (SES-CD) and the efficacy of maintenance therapy[61]. Patients treated with ustekinumab had higher reduction in SES-CD compared to placebo during the induction phase[61]. The results were similar in patients from different induction trials (UNITI 1 or 2) and in those receiving different ustekinumab doses. Greater reduction in the SES-CD at week 44 was also observed in the ustekinumab group compared to placebo[61].
Another sub-study of the UNITI-IM maintenance programme addressed important points of clinical application of ustekinumab. This trial evaluated the clinical effect of dose adjustment of ustekinumab in patients who (1) entered the maintenance trial in response and subsequently lost response (LOR) (2) were non-responders to intravenous ustekinumab during induction phase[62]. The results showed that in patients with LOR, the dose adjustment of ustekinumab (12-wk interval to 8-wk interval) provided clinical benefits compared to patients who remained to the 8-wk interval. Moreover, patients who were initial non-responders to induction treatment benefited from continued treatment (at least 1 additional subcutaneous dose) following the initial intravenous dose (rescue therapy - late responders)[62].
The long-term efficacy and safety of ustekinumab were evaluated in an ongoing IM-UNITI study with a duration of approximately 5 years[63]. The preliminary results through week 96 showed that the clinical response and remission were maintained in patients who were under treatment with subcutaneous ustekinumab. There was no difference in adverse events and infection rates between patients treated with ustekinumab or placebo from week 44 through week 96[63].
The introduction of monoclonal antibodies in the last decades has changed the therapeutic strategy of CD. The biological factors that have been approved to date for the management of CD include anti-TNF agents (adalimumab, certolizumab and infliximab), anti-integrins (natalizumab and vedolizumab) and the anti-IL-12/IL-23p40 agent (ustekinumab). The clinical benefits of monoclonal antibodies are the efficacy and safety during the induction and maintenance of clinical response as well as a decreased risk of hospitalization and surgery. Anti-TNF agents are currently positioned as first line biologic treatment for the management of moderate to severe CD and have been proven to be effective for both induction and maintenance of CD patients[64]. However, these agents do not fully cover the needs of all patients as there is significant percentage who was not respond to treatment, or even if they achieved an initial short-term response, they could undergo secondary failure to anti-TNF agents or develop unacceptable adverse events, which lead to treatment discontinuation. Currently, for patients with primary failure to anti-TNF treatment, the use of a second TNF agonist is not indicated and a switch to another agent with a different mechanism of action is suggested[65,66]. The anti-α4β7 integrin antibody was approved for induction and maintenance of CD patients, but due to its fairly slow-action, it is considered better for the maintenance phase.
Clinical evidence suggests that ustekinumab may be preferred over the anti-integrin treatment given its rapid onset of action[60]. In particular, the clinical benefits of ustekinumab over vedolizumab in inducing clinical response and remission have been shown in patients who were non-responders or were intolerant to anti-TNF treatment, since ustekinumab treated patients responded as early as week 3[60] compared to patients treated with vedolizumab who responded at week 10[67]. The results of the study IM-UNITI LTE have shown that the rapid onset of ustekinumab action is accompanied by a long duration of action, as 75% of patients in remission at year 1, were still in remission at year 2, indicating one more advantage over the anti-TNF or anti-integrins agents[63].
The route of ustekinumab administration could be considered as an important benefit over the other treatment options. During induction phase, only one intravenous dose of ustekinumab is required for the development of clinical response and during maintenance phase, a single subcutaneous dose is able to induce clinical response up to 44 wk in one-third of the patients[60]. These results highlight the usefulness of ustekinumab as a more convenient option with its potential for a home-based therapy. Moreover, a sub-study of the IM-UNITI programme has shown that ustekinumab dose adjustment can provide additional clinical advantage in patients with loss of response. The results demonstrated that initial non-responders to the induction therapy could benefit more from continued treatment with at least one additional dose[62].
The study by Fegan et al[60], has shown that the rapid onset of clinical efficacy was accompanied by a reduction in CRP and fecal calprotectin levels which persisted during the maintenance phase up to week 44. The improvement in CRP and fecal calprotectin levels following ustekinumab treatment suggests that decrease of inflammation occurred along with the clinical improvement[60]. In parallel, recent data has demonstrated the ability of ustekinumab to induce endoscopic healing during the induction phase (at week 8) in patients with moderate to severe CD[61].
Beyond the obvious benefits of ustekinumab in the management of moderate to severe CD, patient safety is an important factor of determining the risk/benefit ratio of each treatment option. Although the data from studies evaluating the long-term safety of ustekinumab in patients with moderate to severe psoriasis and multiple sclerosis suggests an increased risk of serious infections, in CD patients the drug seems to have a rather favorable safety profile[58-60,63], which is very important considering the role of IL-12/IL-23 in maintaining immune homeostasis[68-71].
Taking into consideration the above advantages, we can speculate the use of ustekinumab as a first-line treatment or its use in conjunction with or before other biological agents, following corticosteroid failure. Ustekinumab may be the ideal option for frail patients, considering its safety profile and the mode of administration. In addition, CD patients with other immune mediated diseases such as previous history of multiple sclerosis and psoriasis or patients with TNF agonist induced psoriasis represent promising candidates for ustekinumab treatment considering its systemic anti-inflammatory action[52-56,72,73]. On the other hand, the use of ustekinumab may not be favored in patients with perianal fistulizing CD in whom the use of infliximab is proposed[74]. Furthermore, there is limited evidence concerning the use of ustekinumab in pregnancy and breast feeding. Studies in animals have shown no developmental toxicity due to ustekinumab exposure[75]. In humans, there are two case reports of abortions in ustekinumab-exposed pregnancies[76,77] and two successful pregnancies after prolonged ustekinumab treatment[78,79].
Ustekinumab has exhibited considerable potential in the management of intestinal inflammation by downregulating the immune system through its binding in the common subunit of IL-12 and IL-23 and by blocking their action. In light of current evidence, ustekinumab is considered to be an effective drug with a favorable safety profile for the management of patients with moderate-to-severe active CD. Its use appears to be very promising in patients with an inadequate response, loss of response, intolerance or contraindications to treatment with traditional anti-TNF agents. Moreover, ustekinumab could be considered as first line biological treatment in patients who failed conventional therapy, although the high treatment cost poses severe limitations to this alternative. Large scale-multicentre trials with long term follow up and high-quality evidence are required to further explore the spot that this novel agent should hold in the CD treatment algorithms, and its role in specific disease phenotypes (such as fistulizing disease, early-onset CD or postoperative setting). Lastly, conduct of head-to-head comparison studies of ustekinumab with other biological agents, evaluation of drug to drug interactions and pharmacoeconomic (cost effective) analysis of ustekinumab are the next steps towards thoroughly delineating the place of ustekinumab in clinical practice.
| 1. | Freeman HJ. Natural history and long-term clinical course of Crohn’s disease. World J Gastroenterol. 2014;20:31-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (5)] |
| 2. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1601] [Article Influence: 106.7] [Reference Citation Analysis (3)] |
| 3. | Liu TC, Stappenbeck TS. Genetics and Pathogenesis of Inflammatory Bowel Disease. Annu Rev Pathol. 2016;11:127-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 4. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3415] [Article Influence: 179.7] [Reference Citation Analysis (12)] |
| 5. | Raad MA, Chams NH, Sharara AI. New and Evolving Immunotherapy in Inflammatory Bowel Disease. Inflamm Intest Dis. 2016;1:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1580] [Article Influence: 112.9] [Reference Citation Analysis (0)] |
| 7. | Feagan BG, Rochon J, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Gillies R, Hopkins M. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med. 1995;332:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 615] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 8. | Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 802] [Cited by in RCA: 710] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 9. | Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1598] [Cited by in RCA: 1654] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 10. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3104] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 11. | Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 815] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 12. | Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1416] [Cited by in RCA: 1635] [Article Influence: 125.8] [Reference Citation Analysis (1)] |
| 13. | Vermeire S, Loftus EV Jr, Colombel JF, Feagan BG, Sandborn WJ, Sands BE, Danese S, D’Haens GR, Kaser A, Panaccione R, Rubin DT, Shafran I, McAuliffe M, Kaviya A, Sankoh S, Mody R, Abhyankar B, Smyth M. Long-term Efficacy of Vedolizumab for Crohn’s Disease. J Crohns Colitis. 2017;11:412-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Hazlewood GS, Rezaie A, Borman M, Panaccione R, Ghosh S, Seow CH, Kuenzig E, Tomlinson G, Siegel CA, Melmed GY. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology. 2015;148:344-354.e5; quiz e14-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 202] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Danese S, Fiorino G, Reinisch W. Review article: Causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-α therapy. Aliment Pharmacol Ther. 2011;34:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn’s disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 255] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 17. | Pagnini C, Arseneau KO, Cominelli F. Natalizumab in the treatment of Crohn’s disease patients. Expert Opin Biol Ther. 2017;17:1433-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1587] [Cited by in RCA: 1572] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 19. | Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1427] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 20. | Neurath MF, Weigmann B, Finotto S, Glickman J, Nieuwenhuis E, Iijima H, Mizoguchi A, Mizoguchi E, Mudter J, Galle PR. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J Exp Med. 2002;195:1129-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 495] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 21. | Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. [PubMed] |
| 22. | Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 23. | Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, Pallone F. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 398] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 24. | Parronchi P, Romagnani P, Annunziato F, Sampognaro S, Becchio A, Giannarini L, Maggi E, Pupilli C, Tonelli F, Romagnani S. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn’s disease. Am J Pathol. 1997;150:823-832. [PubMed] |
| 25. | Gee K, Guzzo C, Che Mat NF, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 26. | Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008-4027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7651] [Cited by in RCA: 3158] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 27. | Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2933] [Article Influence: 127.5] [Reference Citation Analysis (0)] |
| 28. | Parrello T, Monteleone G, Cucchiara S, Monteleone I, Sebkova L, Doldo P, Luzza F, Pallone F. Up-regulation of the IL-12 receptor beta 2 chain in Crohn’s disease. J Immunol. 2000;165:7234-7239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 90] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Monteleone G, MacDonald TT, Wathen NC, Pallone F, Pender SL. Enhancing Lamina propria Th1 cell responses with interleukin 12 produces severe tissue injury. Gastroenterology. 1999;117:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14:221-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 835] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 31. | Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 657] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 32. | Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 1020] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 33. | Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol Rev. 2008;226:112-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098-1102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1047] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 35. | Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2202] [Cited by in RCA: 2115] [Article Influence: 124.4] [Reference Citation Analysis (1)] |
| 36. | Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 803] [Article Influence: 40.2] [Reference Citation Analysis (1)] |
| 37. | Geremia A, Arancibia-Cárcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, Travis SP, Powrie F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 38. | Vanden Eijnden S, Goriely S, De Wit D, Willems F, Goldman M. IL-23 up-regulates IL-10 and induces IL-17 synthesis by polyclonally activated naive T cells in human. Eur J Immunol. 2005;35:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | van de Wetering D, de Paus RA, van Dissel JT, van de Vosse E. IL-23 modulates CD56+/CD3- NK cell and CD56+/CD3+ NK-like T cell function differentially from IL-12. Int Immunol. 2009;21:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol. 2010;184:7268-7280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473-2483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 667] [Cited by in RCA: 653] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 42. | Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 924] [Cited by in RCA: 922] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 43. | Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2428] [Cited by in RCA: 2345] [Article Influence: 117.3] [Reference Citation Analysis (1)] |
| 44. | Pidasheva S, Trifari S, Phillips A, Hackney JA, Ma Y, Smith A, Sohn SJ, Spits H, Little RD, Behrens TW. Functional studies on the IBD susceptibility gene IL23R implicate reduced receptor function in the protective genetic variant R381Q. PLoS One. 2011;6:e25038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Van Limbergen J, Wilson DC, Satsangi J. The genetics of Crohn’s disease. Annu Rev Genomics Hum Genet. 2009;10:89-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 194] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 46. | Benson JM, Peritt D, Scallon BJ, Heavner GA, Shealy DJ, Giles-Komar JM, Mascelli MA. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011;3:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 47. | Panaccione R, Sandborn WJ, Gordon GL, Lee SD, Safdi A, Sedghi S, Feagan BG, Hanauer S, Reinisch W, Valentine JF. Briakinumab for treatment of Crohn’s disease: results of a randomized trial. Inflamm Bowel Dis. 2015;21:1329-1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Sands BE, Chen J, Feagan BG, Penney M, Rees WA, Danese S, Higgins PDR, Newbold P, Faggioni R, Patra K. Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients With Moderate to Severe Crohn’s Disease: A Phase 2a Study. Gastroenterology. 2017;153:77-86.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 49. | Feagan BG, Sandborn WJ, D’Haens G, Panés J, Kaser A, Ferrante M, Louis E, Franchimont D, Dewit O, Seidler U. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 369] [Article Influence: 41.0] [Reference Citation Analysis (1)] |
| 50. | FDA approves STELARA® (Ustekinumab) for treatment of moderate to severe Crohn’s disease. New York, CCFA, 2016. Available from: http://www.ccfa.org/news/Stelara.html. |
| 51. | European Commission approves Stelara® (Ustekinumab) for treatment of adults with moderately to severely active Crohn’s disease. Beerse, Belgium: Johnson Johnson Media Center, 2016. Available from: https://www.jnj.com/media-center/press-releases/european-commission-approves-stelara-ustekinumab-for-treatment-of-adults-with-moderately-to-severely-active-crohns-disease. |
| 52. | Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB; PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1244] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 53. | Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, Guzzo C, Hsu MC, Wang Y, Li S. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1092] [Cited by in RCA: 1104] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 54. | Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, Fretzin S, Kunynetz R, Kavanaugh A. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 434] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 55. | McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 604] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 56. | Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, Wang Y, Shen YK, Doyle MK, Mendelsohn AM. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-mo and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73:990-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 507] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 57. | Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH; Ustekinumab MS Investigators. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 364] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 58. | Sandborn WJ, Feagan BG, Fedorak RN, Scherl E, Fleisher MR, Katz S, Johanns J, Blank M, Rutgeerts P; Ustekinumab Crohn’s Disease Study Group. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 594] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 59. | Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 893] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 60. | Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL, Miao Y. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med. 2016;375:1946-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1445] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 61. | Rutgeerts P, Gasink C, Chan D, Lang Y, Pollack P, Colombel JF, Wolf DC, Jacobstein D, Johanns J, Szapary P. Efficacy of Ustekinumab in Inducing Endoscopic Healing in Patients with Crohn’s Disease. Gastroenterology. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 144] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 62. | Sands B, Gasink C, Jacobstein D, Gao L, Johanns J, Szapary P, Colombel J, Targan S, Ghosh S, Sandborn W. A85 Efficacy and safety of dose adjustment and delayed response to ustekinumab in moderate-severe Crohn’s disease patients: results from the IM-UNIT maintenance study. Can J Gastroenterol Hepatol. 2018;1 Suppl 1:147-148. |
| 63. | Sandborn W, Rutgeerts P, Gasink C, Jacobstein D, Gao L-L, Johanns J, Sands B, Hanauer S, Targan S, Ghosh S. OP010 Long term efficacy and safety of Ustekinumab for Crohn’s disease: results from IM-UNITI long-term extension through 2 years. J Crohns Colitis. 2017;11 Suppl 1:S6-S6. |
| 64. | Terdiman JP, Gruss CB, Heidelbaugh JJ, Sultan S, Falck-Ytter YT; AGA Institute Clinical Practice and Quality Management Committee. American Gastroenterological Association Institute guideline on the use of thiopurines, methotrexate, and anti-TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1459-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 65. | Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41:613-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 258] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 66. | Armuzzi A, Gionchetti P, Daperno M, Danese S, Orlando A, Lia Scribano M, Vecchi M, Rizzello F; GIVI (Gruppo Italiano su Vedolizumab nelle IBD) Group. Expert consensus paper on the use of Vedolizumab for the management of patients with moderate-to-severe Inflammatory Bowel Disease. Dig Liver Dis. 2016;48:360-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, D’Haens G, Ben-Horin S, Xu J, Rosario M. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618-627.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 551] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 68. | Dávila-Seijo P, Dauden E, Descalzo MA, Carretero G, Carrascosa JM, Vanaclocha F, Gómez-García FJ, De la Cueva-Dobao P, Herrera-Ceballos E, Belinchón I. Infections in Moderate to Severe Psoriasis Patients Treated with Biological Drugs Compared to Classic Systemic Drugs: Findings from the BIOBADADERM Registry. J Invest Dermatol. 2017;137:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 69. | Fiorentino D, Ho V, Lebwohl MG, Leite L, Hopkins L, Galindo C, Goyal K, Langholff W, Fakharzadeh S, Srivastava B. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 70. | Papp K, Gottlieb AB, Naldi L, Pariser D, Ho V, Goyal K, Fakharzadeh S, Chevrier M, Calabro S, Langholff W. Safety Surveillance for Ustekinumab and Other Psoriasis Treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Drugs Dermatol. 2015;14:706-714. [PubMed] |
| 71. | Singh S, Fumery M, Sandborn WJ, Murad MH. Systematic review and network meta-analysis: first- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment Pharmacol Ther. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 72. | Tillack C, Ehmann LM, Friedrich M, Laubender RP, Papay P, Vogelsang H, Stallhofer J, Beigel F, Bedynek A, Wetzke M. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014;63:567-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 73. | Wils P, Bouhnik Y, Michetti P, Flourie B, Brixi H, Bourrier A, Allez M, Duclos B, Grimaud JC, Buisson A. Subcutaneous Ustekinumab Provides Clinical Benefit for Two-Thirds of Patients With Crohn’s Disease Refractory to Anti-Tumor Necrosis Factor Agents. Clin Gastroenterol Hepatol. 2016;14:242-50.e1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 74. | Lee MJ, Parker CE, Taylor SR, Guizzetti L, Feagan BG, Lobo AJ, Jairath V. Efficacy of Medical Therapies for Fistulizing Crohn’s Disease: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 75. | Martin PL, Sachs C, Imai N, Tsusaki H, Oneda S, Jiao Q, Treacy G. Development in the cynomolgus macaque following administration of ustekinumab, a human anti-IL-12/23p40 monoclonal antibody, during pregnancy and lactation. Birth Defects Res B Dev Reprod Toxicol. 2010;89:351-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 76. | Fotiadou C, Lazaridou E, Sotiriou E, Ioannides D. Spontaneous abortion during ustekinumab therapy. J Dermatol Case Rep. 2012;6:105-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Venturin C, Nancey S, Danion P, Uzzan M, Chauvenet M, Bergoin C, Roblin X, Flourié B, Boschetti G. Fetal death in utero and miscarriage in a patient with Crohn’s disease under therapy with ustekinumab: case-report and review of the literature. BMC Gastroenterol. 2017;17:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 78. | Cortes X, Borrás-Blasco J, Antequera B, Fernandez-Martinez S, Casterá E, Martin S, Molés JR. Ustekinumab therapy for Crohn’s disease during pregnancy: a case report and review of the literature. J Clin Pharm Ther. 2017;42:234-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 79. | Galli-Novak E, Mook SC, Büning J, Schmidt E, Zillikens D, Thaci D, Ludwig RJ. Successful pregnancy outcome under prolonged ustekinumab treatment in a patient with Crohn’s disease and paradoxical psoriasis. J Eur Acad Dermatol Venereol. 2016;30:e191-e192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gassler N, Naito Y S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y