Published online Sep 21, 2018. doi: 10.3748/wjg.v24.i35.4077
Peer-review started: June 21, 2018
First decision: July 31, 2018
Revised: August 5, 2018
Accepted: August 24, 2018
Article in press: August 24, 2018
Published online: September 21, 2018
Processing time: 90 Days and 22.2 Hours
To prospectively investigate the efficacy and safety of clip-flap assisted endoscopic submucosal dissection (ESD) for gastric tumors.
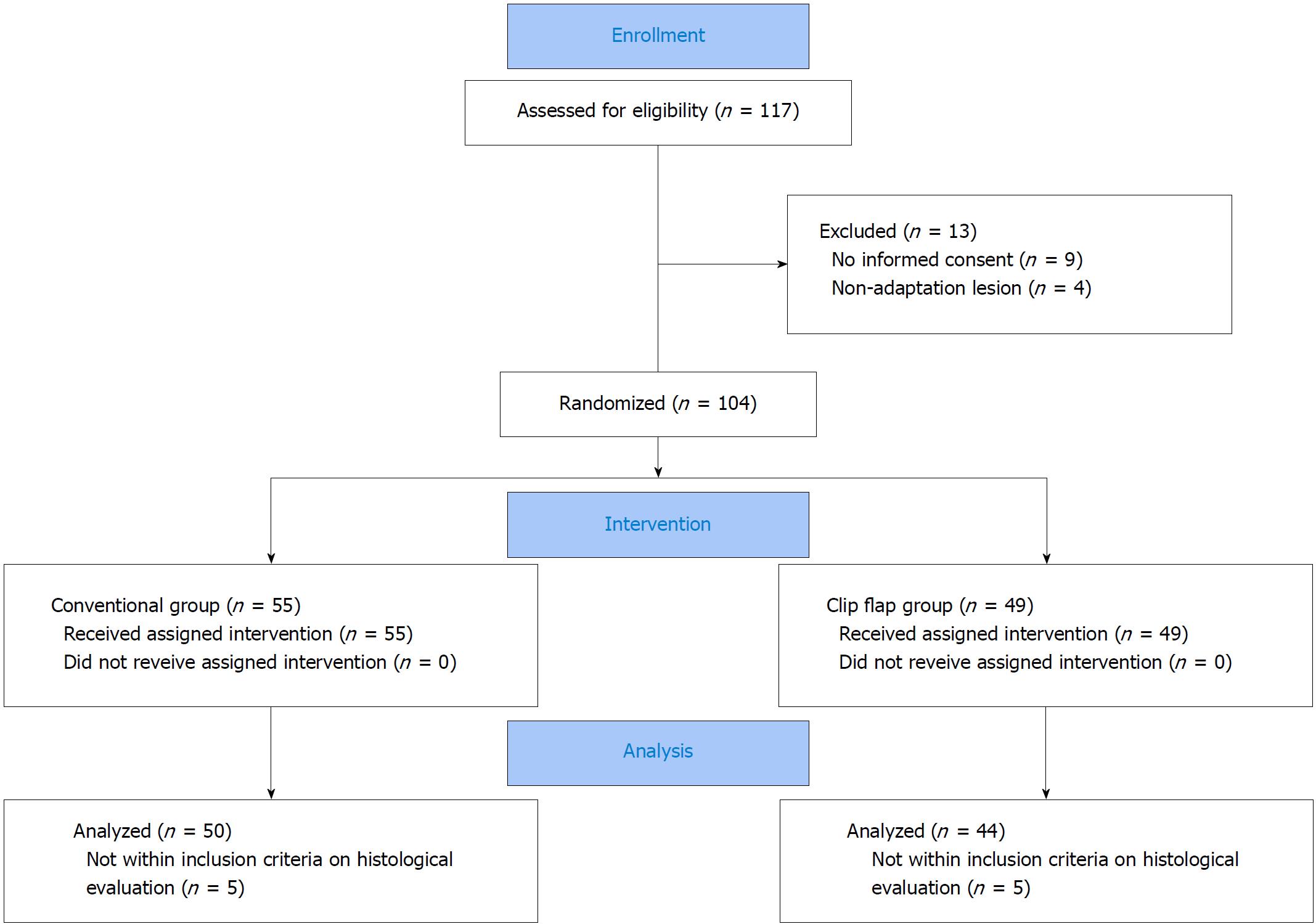
From May 2015 to October 2016, we enrolled 104 patients with gastric cancer or adenoma scheduled for ESD at Shiga University of Medical Science Hospital. We randomized patients into two subgroups using the minimization method based on location of the tumor (upper, middle or lower third of the stomach), tumor size (< 20 mm or > 20 mm) and ulcer status: ESD using an endoclip (the clip-flap group) and ESD without an endoclip (the conventional group). Therapeutic efficacy (procedure time) and safety (complication: Gastrointestinal bleeding and perforation) were assessed.
En bloc resection was performed in all patients. Four patients had delayed bleeding (3.8%) and two had perforation (1.9%). No significant differences in en bloc resection rate (conventional group: 100%, clip flap group: 100%), curative endoscopic resection rate (conventional group: 90.9%, clip flap group: 89.8%, P = 0.85), procedure time (conventional group: 70.8 ± 46.2 min, clip flap group: 74.7 ± 53.3 min, P = 0.69), area of resected specimen (conventional group: 884.6 ± 792.1 mm2, clip flap group: 1006.4 ± 1004.8 mm2, P = 0.49), delayed bleeding rate (conventional group: 5.5%, clip flap group: 2.0%, P = 0.49), or perforation rate (conventional group: 1.8%, clip flap group: 2.0%, P = 0.93) were found between the two groups. Less-experienced endoscopists did not show any differences in procedure time between the two groups.
For patients with early-stage gastric tumors, the clip-flap method has no advantage in efficacy or safety compared with the conventional method.
Core tip: We conducted a prospective study to investigate efficacy of the clip-flap method of endoscopic submucosal dissection (ESD) for early-stage gastric tumor. Recently, although the efficacy of the clip-flap method for ESD of large colorectal tumors is shown, we failed to show advantage of clip-flap method in efficacy or safety compared with the conventional method. Efficacy of clip-flap method-assisted ESD for gastric tumors may be limited, especially in cases with large size of tumor and with difficulty to make mucosal flap.
- Citation: Ban H, Sugimoto M, Otsuka T, Murata M, Nakata T, Hasegawa H, Inatomi O, Bamba S, Andoh A. Usefulness of the clip-flap method of endoscopic submucosal dissection: A randomized controlled trial. World J Gastroenterol 2018; 24(35): 4077-4085
- URL: https://www.wjgnet.com/1007-9327/full/v24/i35/4077.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i35.4077
Endoscopic submucosal dissection (ESD) is a procedure that enables en bloc resection of gastric neoplastic lesions that are difficult to resect via conventional endoscopic mucosal resection (EMR)[1,2]. ESD is first-line treatment for early-stage gastrointestinal cancer[3-5]. Treatment of relatively large lesions and lesions with peptic ulcers, ulcer scars, or fibrosis increases operating time, and increases the risk of adverse events such as perforation and bleeding from the artificial ulcer produced[6-10]. Poor visualization in the resection area also results in longer procedure times and their associated adverse events. Poor visualization may be associated with lesion size, histological type, location, ulcer status, condition of the gastric mucosa, and the degree of operator experience[11,12]. Although precise visualization is important to perform ESD safely, the gold standardized method for resection by ESD for all of patients with early-stage gastrointestinal cancer has not been established.
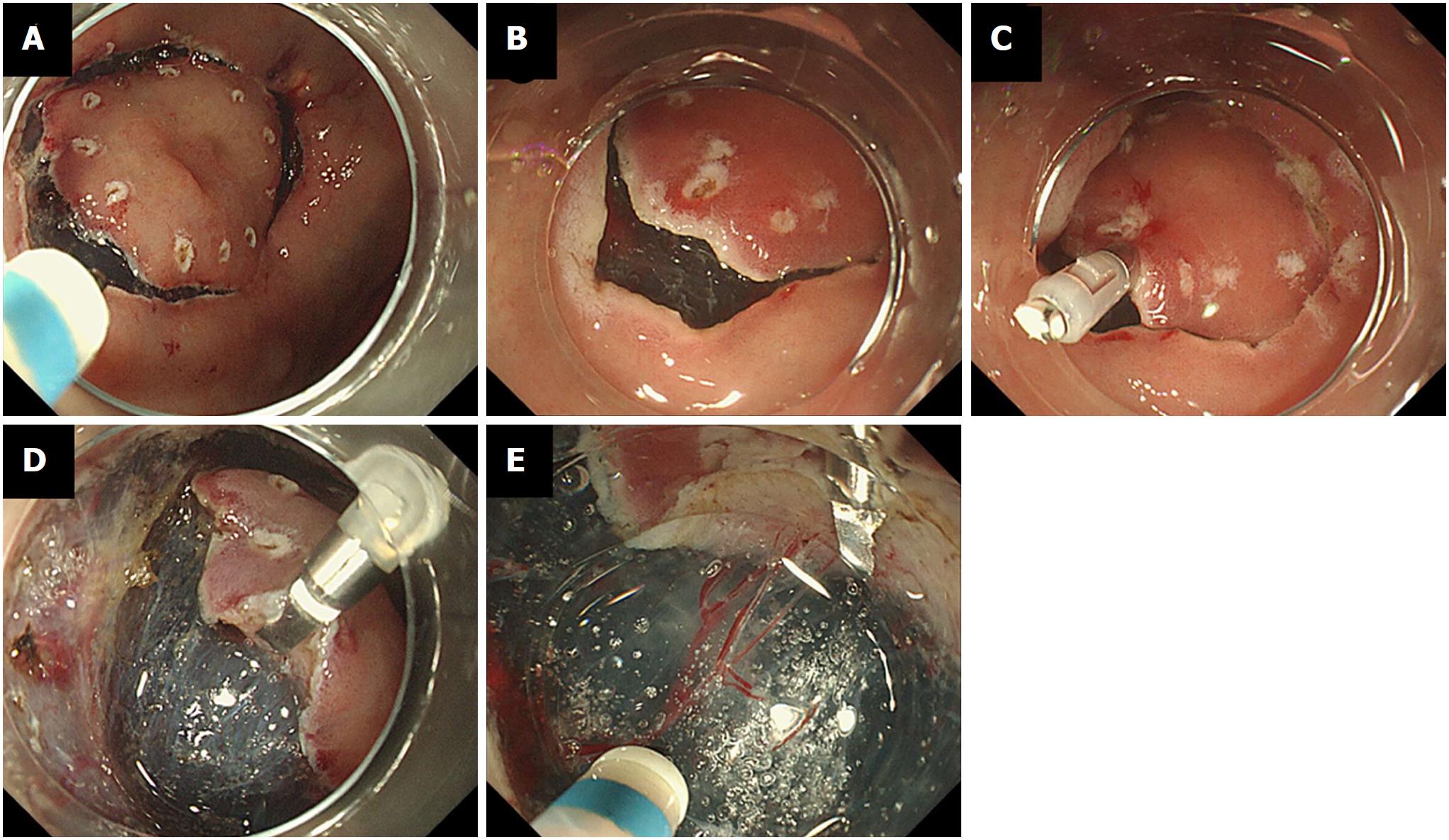
To create a mucosal flap at the early phase after starting ESD procedure is important to prevent complications[13]. The efficacy and safety of several traction systems, such as sinker assistance[14], magnetic anchor guidance[15], use of a clip with a line[16], use of a spring-action clip[17], the clip-band technique[18], and the double-channel scope method[19]. These traction methods are complicated to perform safety and correctly. Recently, Yamamoto et al[20-22] reported on the efficacy of the clip-flap method, in which an endoclip is used to substitute for the mucosal flap until it is formed, for ESD of large colorectal tumors. This method is simple and effective in most cases with colorectal tumors, even in the presence of submucosal fibrosis or with a vertical approach. However, it is unknown whether the clip-flap method is appropriate for patients with early-stage gastric tumors. Because the clip-flap techniques differ between ESD of colorectal tumors and gastric tumors, we wished to assess the efficacy of the clip-flap method for gastric tumor ESD.
We prospectively compared the efficacy (i.e., procedure time) and safety (i.e., incidence of complications) of the clip-flap method in ESD of tumors in different locations (upper, middle or lower third of the stomach), sizes (< 20 mm or > 20 mm), ulcer status (positive or negative), Kyoto classification of gastric mucosa, Helicobacter pylori (H. pylori) infection status, and operator experience.
We enrolled 104 patients who were scheduled to undergo ESD for gastric cancers or gastric adenomas at Shiga University of Medical Science Hospital from May 2015 to October 2016 (Figure 1). Inclusion criteria were age > 20 years and the diagnosis of gastric adenoma or clinical early-stage gastric cancer, irrespective of H. pylori infection. Early-stage gastric cancers were clinically diagnosed using endoscopy, endoscopic ultrasonography, histopathology, and computed tomography. The criteria of early-stage gastric cancer were: (1) An intramucosal intestinal-type cancer without ulcerative lesion, regardless of tumor size; (2) intramucosal intestinal-type cancer with ulcerative lesion, ≤ 3 cm in size; and (3) intramucosal diffuse-type cancer ≤ 2 cm in size without ulcerative lesion. Exclusion criteria were advanced-stage gastric cancer and lack of informed consent. Written informed consent was obtained from all other patients, and approval for the study protocol was given in advance by the Institutional Review Board of the Shiga University of Medicine Science (Number: 26-207). This trial was registered in the University Hospital Medical Information Network, UMIN 000018199.
This study was a prospective randomized trial to assess the efficacy of clip-flap-assisted ESD with regard to operation time and incidence rates of ESD-induced complications in relation to endoscopist experience, characteristics of gastric tumor (i.e., size, differentiation and location), and Kyoto classification of gastric mucosa. Using the minimization method based on location of the tumor (upper, middle or lower third of the stomach), tumor size (< 20 mm or > 20 mm) and ulcer status (positive or negative), we randomized patients with early-stage gastric tumor into two groups: ESD using an endoclip (EZCLIP, HX-610-135; Olympus, Tokyo, Japan) to make the mucosal flap (clip-flap group) (n = 49) and ESD without an endoclip (conventional group) (n = 55) (Figure 1). Procedure time was calculated as the time from the beginning of submucosal injection to the end of submucosal dissection. We performed ESD for patients receiving anti-thrombotic drugs according to the guideline for endoscopic procedures in antithrombotic drug-users from Japan Gastroenterological Endoscopy Society on July 2012.
Curative endoscopic resection rate was decided as lesion within criteria of early-stage gastric cancer. Delayed bleeding was defined as postprocedural bleeding with hematemesis or melena requiring endoscopic hemostasis, decrease in the hemoglobin level by > 2 g/dL.
Primary endpoint of this study was to clarify the reduction effects of procedure time in the clip-flap-assisted ESD for early-stage gastric cancers compared with the conventional ESD. Secondary endpoint were to compare with incident rates of ESD-associated complications, such as bleeding and perforation, between two kinds of treatment methods, and to clarify efficacy of the clip-flap-assisted ESD for en bloc resection rate of gastric neoplastic lesions.
After pathological evaluation of ESD sample, we excluded patients with gastric tumor penetrating > 500 μm from the muscularis mucosa into the submucosa.
ESD was carried out with a single channel endoscope (GIF-H290Z; Olympus, Tokyo, Japan). We used a dual knife (KD-650; Olympus, Tokyo, Japan) as the cutting device, and an electrical current was applied using an electrosurgical generator (VIO300D; ERBE Elektromedizin GmbH, Tubingen, Germany). Visible vessels were heat-coagulated using hemostatic forceps (Coagrasper G; FD-412LR, Olympus, Tokyo, Japan).
In the clip-flap group, after the mucosal circumference of the tumor was incised in the conventional manner, an edge of the exfoliated mucosa was grasped with an endoclip (Figure 2). The endoscope attachment was slipped under the endoclip, and the submucosal layer was then dissected with the endoknife. After creating the mucosal flap, ESD was performed in the conventional manner. We divided endoscopists into two groups; experts (higher that 50 procedure experiences, n = 2) and beginner (less than 50 procedure experiences, n = 3).
H. pylori infection status was determined with an anti-H. pylori IgG serological test (E plate Eiken H. pylori antibody®; Eiken Chemical Co. Ltd., Tochigi, Japan).
The endoscopic severity of H. pylori-associated gastritis was characterized by the Kyoto classification[23,24]. According to the Kyoto classification of gastritis, patients are scored according to atrophy (none: A0, atrophic patterns with a margin between the non-atrophic fundic mucosa and atrophic mucosa located in the lesser curvature of the stomach: A1, and atrophic patterns whose margin does not cross the lesser curvature: A2), intestinal metaplasia (none: IM0, within antrum: IM1, and up to corpus: IM2), hypertrophy of gastric folds (negative: H0, positive: H1), and diffuse erythema (negative: DR0, mild: DR1, severe: DR2)[23,24].
Age, body weight, body mass index, and ESD procedure time are expressed as mean ± standard deviation (SD). Statistically significant differences in these parameters between the clip-flap group and the conventional group were determined using one-way ANOVA and Fisher’s exact tests. All P values were two-sided, and P < 0.05 was considered statistically significant. Calculations were conducted using commercial software (SPSS version 20, IBM Inc; Armonk NY, United States).
The sample size and the study power were calculated by using our previous data of procedure time of conventional ESD method for gastric tumor (80 min). We chose an unmatched case-control study design (assuming 1 conventional ESD per clip-flap-assisted ESD) and hypothesized that clip-flap-assisted ESD reduced 25% of procedure time compared with conventional ESD method. For the desired power of our study of 80% with a significance level of 0.05 in a two-sided test, at least 100 patients by conventional ESD method and 100 patients by clip-flap-assisted ESD were required. At first, we decided to conduct an intermediate analysis when total patients for gastric tumor reached 100, half of required total patients. If there was no significant difference of efficacy between both regimens at an intermediate analysis, we decided to stop the examination.
At an intermediate analysis after enrolling half of required patients, because the reduction effect of procedure time as primary endpoint of this study was similar between two kinds of methods, we decided to stop the examination as initial protocol.
Of 117 patients undergoing ESD from May 2015 to October 2016, 104 patients were randomized into two groups: The conventional group (n = 55) and the clip-flap group (n = 49) (Figure 1). Thirteen patients were excluded due to the withholding of informed consent (n = 9) and non-adaptation lesion (n = 4). There were no significant differences in demographic characteristics (age, sex, body weight, BMI, received drugs, H. pylori infection rate), Kyoto classification, or clinical characteristics of gastric neoplasms (i.e., histological diagnosis, depth, location and size) between the conventional group and the clip-flap group (Table 1).
| Total | Conventional group | Clip-flap group | P value | |
| Number (n) | 104 | 55 | 49 | |
| Age (yr) | 70.1 ± 8.3 | 69.0 ± 9.5 | 71.2 ± 6.5 | 0.17 |
| Sex (male : female) | 80:24 | 42:13 | 38:11 | 0.89 |
| Body weight (kg) | 59.0 ± 11.2 | 58.0 ± 11.0 | 60.2 ± 11.5 | 0.34 |
| BMI (kg/m2) | 22.2 ± 3.1 | 21.7 ± 2.9 | 22.8 ± 3.3 | 0.08 |
| Drugs, n (%) | ||||
| Anticoagulants | 19 (18.3) | 11(20.0) | 8 (16.3) | 0.63 |
| Antihypertensive dugs | 56 (53.8) | 25 (45.5) | 31 (63.3) | 0.07 |
| Oral hypoglycemics | 16 (15.4) | 8 (14.5) | 8 (16.3) | 0.80 |
| Cholesterol-lowering agents | 25 (24.0) | 13 (23.6) | 12 (24.5) | 0.91 |
| H. pylori infection (positive) | 36 (34.6) | 16 (29.1) | 20 (40.8) | 0.21 |
| Hemodialysis, n (%) | 0 (0) | 0 (0) | 0 (0) | - |
| Kyoto classification of gastric mucosa | ||||
| Atrophy (A0:A1:A2) | 3:7:94 | 2:4:49 | 1:3:45 | 0.86 |
| Intestinal metaplasia (IM0:IM1:IM2) | 8:36:60 | 5:15:35 | 3:21:25 | 0.24 |
| Diffuse redness (DR0:DR1 :DR2) | 47:24:33 | 26:13:16 | 21:11:17 | 0.83 |
| Tumor | ||||
| Histological diagnosis (adenoma/cancer) | 9/95 | 6/49 | 3/46 | 0.39 |
| Differentiation (tub1 + tub2/por + sig) | 92/3 | 48/1 | 44/2 | 0.52 |
| Depth (m/sm) | 82/13 | 42/7 | 40/6 | 0.86 |
| Location (upper third/middle/lower) | 10/37/57 | 7/20/28 | 3/17/29 | 0.47 |
| Major axis of tumor (mm) | 18.5 ± 13.4 | 17.5 ± 11.8 | 19.7 ± 15.1 | 0.39 |
| ESD | ||||
| Procedure time (min) | 72.6 ± 49.5 | 70.8 ± 46.2 | 74.7 ± 53.3 | 0.69 |
| Area of resected specimen (mm2) | 962 1 ± 896.2 | 884.6 ± 792.1 | 1006.4 ± 1004.8 | 0.49 |
| En bloc resection rate (n, %) | 104 (100) | 55 (100) | 49 (100) | - |
| Curative endoscopic resection rate (n, %) | 94 (90.4) | 50 (90.9) | 44 (89.8) | 0.85 |
| Coagulation of vessels at 2nd look (n, %) | 28 (26.9) | 16 (29.6) | 12 (25.0) | 0.60 |
| Delayed bleeding (n, %) | 4 (3.8) | 3 (5.5) | 1 (2.0) | 0.37 |
| Perforation (n, %) | 2 (1.9) | 1 (1.8) | 1 (2.0) | 0.93 |
| Operator | ||||
| Procedure times (< 50 cases) | 83 | 43 | 40 | 0.66 |
All patients underwent en bloc resection. The curative endoscopic resection rate within the criteria of early-stage gastric cancer was 90.4% (94/104). In histopathological evaluation after ESD, lesions of nine patients did not meet the inclusion criteria of clinical early-stage gastric cancer. Five patients had tumor > 500 μm from the muscularis mucosa, 2 had tumors > 3 cm in size with submucosal layer invasion, 2 had diffuse-type adenocarcinoma > 2 cm in size, and 1 had a tumor > 3 cm in size with ulceration (Table 1).
The mean area of resected specimens was 962.1 ± 896.2 mm2 and mean procedure time was 72.6 min ± 49.5 min. ESD-related adverse events included delayed bleeding in 4 patients (3.8%) and perforation in 2 (1.9%) (Table 1). There were no significant differences in Kyoto classification, background of tumor, en bloc resection rate, curative endoscopic resection rate, procedure time, area of resected specimens, or complication rate between the two groups.
In analysis of efficacy for treatment methods in patients within inclusion criteria, all parameters, such as H. pylori infection, background of gastric mucosa, characteristics of tumor, and ESD-related factors, procedure time of ESD were similar between two groups (Table 2). In addition, the clip-flap method had no effect on procedure time, regardless of operator experience.
| Conventional group(n = 50) | Clip-flap group(n = 44) | P value | ||
| Procedure time (min) | 67.3 ± 44.9 | 67.6 ± 48.4 | 0.98 | |
| H. pylori infection | Positive | 64.1 ± 27.8 (n = 15) | 65.6 ± 53.1 (n = 19) | 0.92 |
| Negative | 68.6 ± 50.7 (n = 35) | 69.1 ± 45.5 (n = 25) | 0.98 | |
| Kyoto classification of gastric mucosa | ||||
| Atrophy | A2 | 68.7 ± 45.2 (n = 44) | 70.3 ± 49.7 (n = 40) | 0.87 |
| A0 + 1 | 57.2 ± 44.8 (n = 6) | 40.3 ± 17.6 (n = 4) | 0.50 | |
| Intestinal metaplasia | IM2 | 71.6 ± 45.8 (n = 31) | 65.2 ± 45.2 (n = 22) | 0.62 |
| IM0 + IM1 | 60.2 ± 43.6 (n = 19) | 70.0 ± 52.3 (n = 22) | 0.53 | |
| Tumor | ||||
| Histological diagnosis | Adenoma | 36.5 ± 11.1 (n = 6) | 58.0 ± 40.2 (n = 3) | 0.24 |
| Cancer | 71.5 ± 46.1 (n = 44) | 68.3 ± 49.3 (n = 41) | 0.76 | |
| Differentiation | tub1 + tub2 | 71.1 ± 46.6 (n = 43) | 69.0 ± 49.7 (n = 40) | 0.85 |
| Por + sig | 90 (n = 1) | 40 (n = 1) | - | |
| Depth | m | 71.4 ± 47.2 (n = 42) | 62.7 ± 40.1 (n = 38) | 0.38 |
| sm | 74.0 ± 1.4 (n = 2) | 139.6 ± 97.1 (n = 3) | 0.43 | |
| Location | Upper | 119.8 ± 60.9 (n=6) | 158.5 ± 101.1 (n=2) | 0.52 |
| Middle | 74.8 ± 37.3 (n = 19) | 91.4 ± 52.1 (n = 14) | 0.29 | |
| Lower | 49.0 ± 34.7 (n = 25) | 49.2 ± 28.0 (n = 28) | 0.98 | |
| Major axis of tumor (mm) | < 10 | 50.0 ± 35.9 (n = 24) | 46.6 ± 31.0 (n = 20) | 0.74 |
| 10 <, < 20 | 72.3 ± 46.2 (n = 16) | 59.8 ± 23.7 (n = 14) | 0.37 | |
| 20 < | 100.8 ± 44.9 (n = 10) | 120.5 ± 64.5 (n = 10) | 0.44 | |
| ESD | ||||
| Area of specimen (mm2) | < 500 | 33.8 ± 14.0 (n = 22) | 53.2 ± 51.7 (n = 15) | 0.17 |
| 500 <, < 1000 | 72.6 ± 38.4 (n = 16) | 71.1 ± 29.3 (n = 23) | 0.89 | |
| 1000 < | 86.0 ± 32.1 (n = 12) | 138.5 ± 79.3 (n = 9) | 0.06 | |
| Coagulation of vessels at 2nd look | Done | 69.5 ± 38.4 (n = 13) | 67.4 ± 28.8 (n = 12) | 0.89 |
| No | 66.5 ± 47.4 (n = 37) | 67.6 ± 52.6 (n = 35) | 0.92 | |
| Delayed bleeding (n, %) | 105.7 ± 23.7 (n = 3) | 80 (n = 1) | - | |
| Perforation (n, %) | 71 (n = 1) | 234 (n = 1) | - | |
| Operator | ||||
| Procedure times | < 50 times | 61.1 ± 37.1 (n = 39) | 58.8 ± 32.1 (n = 36) | 0.78 |
| > 50 times | 89.3 ± 62.9 (n = 11) | 107.0 ± 83.9 (n = 8) | 0.60 |
As shown in previous reports, there were significance in the difference of procedure time between tumor size (< 20 mm or > 20 mm) and location of tumor (upper and middle or lower), whereas the clip-flap method was selected or not.
Factors associated with prolonged procedure time were the same in both groups (Table 3).
| Parameters | Conventional group | Clip-flap group | |||||
| Odds ratio | 95%CI | P value | Odds ratio | 95%CI | P value | ||
| Age (yr) | > 70 | 0.67 | 0.22-2.10 | 0.49 | 0.79 | 0.22-2.86 | 0.72 |
| Sex | Male | 2.70 | 0.63-11.55 | 0.18 | - | - | - |
| H. pylori infection | Positive | 1.31 | 0.39-4.44 | 0.66 | 0.69 | 0.20-2.43 | 0.57 |
| Kyoto classification of gastric mucosa | |||||||
| Atrophy (vs A0 + A1) | A2 | 1.52 | 0.25-9.19.57 | 0.65 | - | - | - |
| Intestinal metaplasia (vs IM0 + IM1) | IM2 | 2.03 | 0.61-6.72 | 0.25 | 1.00 | 0.29-3.42 | 1.00 |
| Tumor | |||||||
| Depth (vs m) | sm | - | - | - | 3.85 | 0.31-46.49 | 0.29 |
| Location (vs Lower) | Upper/middle | 10.12 | 2.42-42.41 | 0.02 | 4.75 | 1.41-16.05 | < 0.01 |
| Major axis of tumor (mm) (vs < 10) | 10 <, < 20 | 3.00 | 0.78-11.54 | 0.11 | 3.15 | 0.61-16.29 | 0.17 |
| 20 < | 7.00 | 1.36-36.01 | 0.02 | 22.67 | 3.14-163.63 | < 0.01 | |
| Ulceration | UL+ | 1.42 | 0.18-10.99 | 0.74 | 1.80 | 0.104-30.89 | 0.69 |
| ESD | |||||||
| Area of specimen (mm2) (vs < 500) | 500 < | 3.29 | 0.28-39.14 | 0.35 | - | - | - |
| Operator | |||||||
| Procedure experience | < 50 times | 0.32 | 0.08-1.29 | 0.11 | 0.26 | 0.05-1.30 | 0.10 |
We wished to clarify the efficacy and safety of the clip-flap assisted ESD for patients with early-stage gastric tumors, looking at the effect of operator experience as well as other factors. We however failed to show any benefits of the clip-flap method (en bloc resection, procedure time and complication). Although this method is efficacious for patients with colorectal tumors, our observations suggest that it does not improve rate of en bloc resection, procedure time of ESD and safety (bleeding and perforation) in patients with gastric tumors.
To prevent ESD-associated complications, such as perforation and unexpected bleeding, it is crucial to ensure good visualization of the submucosal layer by creating a mucosal flap[13-19]. When the clip-flap method was selected as treatment for patients with early-stage gastrointestinal tumors, it was important that the endoclip securely clipped the edge of the exfoliated mucosa on the oral or anal side of the tumor, excluding the deep layer of the submucosa, after the mucosal circumference incision (Figure 2)[20-22]. Then the endoscope attachment is slipped under the endoclip. If this technique is performed, the endoclip attached to the exfoliated mucosa acts like a surgical hook to widen the cutting area when lifted with the endoscope attachment, with resulting good visualization of the cutting area and countertraction against the submucosal layer until the mucosal flap is created[20-22]. In this study, there was no benefit of the clip-flap method for gastric tumors, irrespective of different background of gastric mucosa (severity of gastric atrophic atrophy and inflammation), histological diagnosis, depth, location, or size. There were no significant differences in the results between the conventional group and the clip-flap group between operators of different experience. Importantly, although operators scored superiority of the clip-flap method in 30% of ESD procedures in the visual analogue scale, in 25% of cases the operators evaluated the clip-flap method as inferior, usually because the head of the endoclip interfered with the cutting edge, making ESD more difficult. In our experience, the best way to do this method is to property place the clip on the lesion. Yamamoto et al[20-22] recommended the use of one endoclip for each tumor, adding additional endoclips (i.e., a single endoclip at a different point or a cross pattern of endoclips at same point) as needed. The endoclip cross pattern further stabilized the visual field by providing good countertraction when the attachment was slipped under the endoclip. In this study, however, we used one endoclip according to first protocol. The characteristics of tumors and required techniques of ESD differ between colorectal and gastric tumors. Although fine visualization is required to perform ESD, because ESD for patients with gastric tumors is easy compared with that for colorectal tumors, any merit of the clip-flap method for gastric tumors may be minor. When considering clip-flap-assisted ESD, endoscopists should select patients, especially cases in which it is difficult to ensure a fine visualization, and should apply the endoclip carefully so that the head of the endoclip pushes downward.
There were any limitations in this study, as below. First, this study is single-center study and non double-blinded study. Second, sample power is insufficient. Although 200 patients with gastric tumors were required for the desired power of 80% with a significance level of 0.05 in a two-sided test, because there was no significant difference of efficacy between both regimens at an intermediate analysis, we decided to stop the examination according to initial protocol. Because the power of conclusions are insufficient, we think that it will be required to plan further study that investigate efficacy of the clip-flap assisted ESD by multi-center study enrolled many patients.
We conducted a prospective randomized study to investigate the efficacy and safety of the clip-flap method-assisted ESD for patients with early-stage gastric tumors. We demonstrated similar resection times between the conventional group and the clip-flap group and failed to show any benefits of the clip-flap method for patients with early-stage gastric tumors. However, the clip-flap method prevented poor visualization in the cutting area and also saved procedure time. Although, compared with the conventional method without traction, the clip-flap method has been proven to be advantageous for patients with early-stage colorectal tumors, the superiority of clip-flap method-assisted ESD in the stomach is unproven.
The endoscopic submucosal dissection (ESD) for early-stage gastric cancer is first-line endoscopic therapy in Japan, because of en bloc resection and a lower local recurrence rate of gastric cancer. However, ESD often causes development of adverse events, such as gastric bleeding and perforation. When ESD is performed for gastric cancer, poor visualization in the resection area during ESD procedure results in longer procedure times and their associated development of above adverse events. Now, the gold standardized method for resection by ESD for all of patients with early-stage gastric cancer and adenoma has not been established in point of continuous clear visualization in the resection area.
To keep clear visualization at early-phase after starting ESD procedure, endoscopists are required to create a mucosal flap. Of several traction systems to create mucosal flap, recently, the clip-flap method is focused, because of safety and correctly compared with other methods. However, it is unknown whether the clip-flap method is appropriate for patients with early-stage gastric tumors.
The main objective was to investigate prospectively the efficacy (the rate of en bloc resection and procedure time of ESD) and safety (gastric bleeding and perforation) of clip-flap assisted ESD for gastric cancer and adenoma.
We enrolled 104 patients with gastric cancer or adenoma scheduled for ESD. Inclusion criteria were age > 20 years and the diagnosis of gastric adenoma or clinical early-stage gastric cancer. Early-stage gastric cancers were clinically diagnosed using endoscopy, endoscopic ultrasonography, histopathology, and computed tomography. We randomized patients into two subgroups using the minimization method based on location of the tumor, tumor size and ulcer status: ESD using an endoclip (the clip-flap group) and ESD without an endoclip (the conventional group). Therapeutic efficacy and safety were assessed.
No significant differences in en bloc resection rate (P = 1.00), curative endoscopic resection rate (P = 0.85), procedure time (P = 0.69), area of resected specimen (P = 0.49), delayed bleeding rate (P = 0.49), or perforation rate (P = 0.93) were found between the clip-flap group and the conventional group.
For patients with early-stage gastric cancer and adenoma, the clip-flap method has no advantage in efficacy or safety compared with the conventional method. Although operators scored superiority of the clip-flap method in 30% of ESD procedures, in 25% of cases the operators evaluated the clip-flap method as inferior, usually because the head of the endoclip interfered with the cutting edge, making ESD more difficult. Therefore, the best way to do this method is to property place the clip on the lesion.
Although the clip-flap method has been proven to be advantageous for patients with early-stage colorectal tumors compared with the conventional method, the superiority of clip-flap method-assisted ESD in the stomach is unproven. When considering clip-flap-assisted ESD, endoscopists should select patients, especially cases in which it is difficult to ensure a fine visualization, and should apply the endoclip carefully so that the head of the endoclip pushes downward.
| 1. | Fujishiro M. Endoscopic submucosal dissection for stomach neoplasms. World J Gastroenterol. 2006;12:5108-5112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Sugimoto M, Jang JS, Yoshizawa Y, Osawa S, Sugimoto K, Sato Y, Furuta T. Proton Pump Inhibitor Therapy before and after Endoscopic Submucosal Dissection: A Review. Diagn Ther Endosc. 2012;2012:791873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 515] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 4. | Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 415] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 5. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakamura K, Hirano M, Esaki M. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? A multi-center retrospective study in Japan. J Gastroenterol. 2017;52:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 6. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A. Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy. 2006;38:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77 Suppl 1:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (2)] |
| 9. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 484] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 10. | Ban H, Sugimoto M, Otsuka T, Murata M, Nakata T, Hasegawa H, Fukuda M, Inatomi O, Bamba S, Kushima R. Letter: a potassium-competitive acid blocker vs a proton pump inhibitor for healing endoscopic submucosal dissection-induced artificial ulcers after treatment of gastric neoplasms. Aliment Pharmacol Ther. 2017;46:564-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Lim JH, Kim SG, Choi J, Im JP, Kim JS, Jung HC. Risk factors of delayed ulcer healing after gastric endoscopic submucosal dissection. Surg Endosc. 2015;29:3666-3673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Yoshizawa Y, Sugimoto M, Sato Y, Sahara S, Ichikawa H, Kagami T, Hosoda Y, Kimata M, Tamura S, Kobayashi Y. Factors associated with healing of artificial ulcer after endoscopic submucosal dissection with reference to Helicobacter pylori infection, CYP2C19 genotype, and tumor location: Multicenter randomized trial. Dig Endosc. 2016;28:162-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Toyonaga T, Nishino E, Man-I M, East JE, Azuma T. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc. 2012;45:362-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Saito Y, Emura F, Matsuda T, Uraoka T, Nakajima T, Ikematsu H, Gotoda T, Saito D, Fujii T. A new sinker-assisted endoscopic submucosal dissection for colorectal cancer. Gastrointest Endosc. 2005;62:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Gotoda T, Oda I, Tamakawa K, Ueda H, Kobayashi T, Kakizoe T. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos). Gastrointest Endosc. 2009;69:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 16. | Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc. 2012;45:375-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 17. | Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Mori H, Kawabe M, Nagahara A, Otaka M, Ogihara T. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc. 2009;69:1370-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 132] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Parra-Blanco A, Nicolas D, Arnau MR, Gimeno-Garcia AZ, Rodrigo L, Quintero E. Gastric endoscopic submucosal dissection assisted by a new traction method: the clip-band technique. A feasibility study in a porcine model (with video). Gastrointest Endosc. 2011;74:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 19. | Neuhaus H, Costamagna G, Devière J, Fockens P, Ponchon T, Rösch T; ARCADE Group. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the “R-scope”). Endoscopy. 2006;38:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Yamamoto K, Hayashi S, Nakabori T, Shibuya M, Ichiba M, Inada M. Endoscopic submucosal dissection using endoclips to assist in mucosal flap formation (novel technique: “clip flap method”). Endoscopy. 2012;44 Suppl 2 UCTN:E334-E335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Yamamoto K, Hayashi S, Nishida T, Saiki H, Naito M, Michida T, Ito T. Effective use of the “clip-flap” method for the endoscopic submucosal dissection of a difficult-to-approach superficial gastric tumor. Endoscopy. 2015;47 Suppl 1 UCTN:E318-E319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Yamamoto K, Hayashi S, Saiki H, Indo N, Nakabori T, Yamamoto M, Shibuya M, Nishida T, Ichiba M, Inada M. Endoscopic submucosal dissection for large superficial colorectal tumors using the “clip-flap method”. Endoscopy. 2015;47:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Kamada T, Haruma K, Inoue K, Shiotani A. [Helicobacter pylori infection and endoscopic gastritis -Kyoto classification of gastritis]. Nihon Shokakibyo Gakkai Zasshi. 2015;112:982-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 24. | Sugimoto M, Ban H, Ichikawa H, Sahara S, Otsuka T, Inatomi O, Bamba S, Furuta T, Andoh A. Efficacy of the Kyoto Classification of Gastritis in Identifying Patients at High Risk for Gastric Cancer. Intern Med. 2017;56:579-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Matowicka-Karna J, Sitarz R, Zhang CW S- Editor: Wang XJ L- Editor: A E- Editor: Yin SY