Published online Aug 7, 2018. doi: 10.3748/wjg.v24.i29.3302
Peer-review started: April 4, 2018
First decision: April 19, 2018
Revised: May 12, 2018
Accepted: June 16, 2018
Article in press: June 16, 2018
Published online: August 7, 2018
Processing time: 121 Days and 5.6 Hours
To assess the efficacy and safety of fourth-generation quinolones for Helicobacter pylori (H. pylori) eradication, we conducted this systematic review and meta-analysis of randomized clinical trials.
Major literature databases (PubMed, EMBASE and the Cochrane Central Register of Controlled Trials) were searched for relevant articles published prior to February 2018. We performed a meta-analysis of all randomized clinical trials that examined the efficacy of H. pylori eradication therapies and included fourth-generation quinolones in the experimental arm. Subgroup analyses by regions and different types of fourth-generation quinolones were also performed.
Ten studies including a total of 2198 patients were assessed. A meta-analysis of randomized controlled trials showed that the eradication rate of therapies containing non-fourth-generation quinolones was significantly lower than that of therapies containing fourth-generation quinolones by intention-to-treat (ITT) analysis [75.4% vs 81.8%; odds ratio (OR) = 0.661; 95% confidence interval (CI): 0.447-0.977; P = 0.038]. This analysis also showed that the eradication rate of the therapies containing non-fourth-generation quinolones was inferior to that of therapies containing fourth-generation quinolones by per-protocol analysis (79.1% vs 84.7%; OR = 0.663; 95%CI: 0.433-1.016; P = 0.059). Moreover, the occurrence of side effects was significantly different between the control and experimental groups by ITT analysis (30.6% vs 19.5%; OR = 1.874; 95%CI: 1.120-3.137; P = 0.017). The sub-analyses also showed significant differences in moxifloxacin therapies vs other fourth-generation quinolone therapies (84.3% vs 71.9%) and in Asian vs European groups (76.7% vs 89.1%).
Therapies containing fourth-generation quinolones achieved a poor eradication rate in the treatment of H. pylori infection. Such regimens might be useful as a rescue treatment based on antimicrobial susceptibility testing. Different antibiotics should be chosen in different regions.
Core tip: With the increase in the Helicobacter pylori (H. pylori) resistance rate, eradication is becoming increasingly challenging. This is the first meta-analysis comprehensively focused on fourth-generation quinolones for the treatment of H. pylori infection. Additionally, we found that fourth-generation quinolones had a higher eradication rate (81.8%) and a lower rate of incidence of side effects (19.5%). These findings will provide a specific basis for the clinical use of fourth-generation quinolones for H. pylori eradication.
- Citation: An Y, Wang Y, Wu S, Wang YH, Qian X, Li Z, Fu YJ, Xie Y. Fourth-generation quinolones in the treatment of Helicobacter pylori infection: A meta-analysis. World J Gastroenterol 2018; 24(29): 3302-3312
- URL: https://www.wjgnet.com/1007-9327/full/v24/i29/3302.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i29.3302
Helicobacter pylori (H. pylori) infection plays a crucial role in the pathogenesis of gastrointestinal diseases, such as gastritis, non-ulcer dyspepsia, peptic ulcer diseases, and gastric cancer[1]. H. pylori infection affects approximately 50% of the population worldwide[2]. Its prevalence is approximately 70% in developing nations and approximately 20%-30% in developed nations[3]. Eradication of H. pylori facilitates peptic ulcer healing, reduces ulcer relapse rates, and prevents gastric cancer[4]. In the past, the recommended treatment for eradicating H. pylori was 7 d of standard triple therapy (STT) consisting of a proton pump inhibitor (PPI) with clarithromycin (CAM) and amoxicillin (AMPC)[5]. However, with the wide use of the STT regimen, the eradication rate of H. pylori has declined to unacceptable levels over the last decade (< 80%) due to high resistance to metronidazole and clarithromycin[6]. A recent study on H. pylori resistance to antimicrobial agents reported that clarithromycin resistance has rapidly increased in many countries over the past decade, with resistance rates of approximately 18% in Europe, 30% in Japan, 40% in Turkey, and 50% in China; limited data are available for the United States[7-10]. The prevalence of H. pylori resistance to metronidazole is 33% in Europe and 40% in the United States, with a high resistance rate (50%-80%) in developing countries[10]. To overcome these difficulties, there is a need to evaluate novel regimens and antibiotics to identify effective alternative treatment strategies. Levofloxacin-based therapy is recommended by the Maastricht IV[11] and Maastricht V Consensus Reports[12]. Nonetheless, according to studies, the resistance rate to levofloxacin is approximately 22.1% in Italy and 36.9% in China; a recent study surprisingly reported a resistance rate of 31.9% in the United States[13-15]. Jeong et al[16] reported that the eradication rate was 57.1% when levofloxacin was used. Fourth-generation quinolones, including moxifloxacin, sitafloxacin, gemifloxacin, and gatifloxacin, which have broad-spectrum antibacterial activity, are active against a variety of gram-negative and gram-positive bacteria[17]. Recent studies have shown that fourth-generation quinolones can increase drug penetration into bacterial cells, improve the strength of activity and have better bioavailability. This group of drugs inhibits the metabolism of bacterial cells by inhibiting DNA replication and therefore enhances antibacterial activity[18]. Furthermore, treatment with fourth-generation quinolones has achieved a high H. pylori eradication rate and has been recommended in some studies[19-22]. Nevertheless, Chung et al[23] reported that the eradication rate was not satisfactory when using fourth-generation quinolones.
To evaluate the efficacy and safety of therapies containing fourth-generation quinolones, we conducted a systematic review and meta-analysis of the available data. The primary outcome measures we assessed were eradication rates, side effects, and compliance of the therapies containing fourth-generation quinolones compared with those of therapies containing non-fourth-generation quinolones. Our outcomes will provide useful evidence for clinical practice[24].
We searched the PubMed (to February 2018), EMBASE (to February 2018), and Cochrane Central Register of Controlled Trials (Issue 2, 2018) databases. The following search terms were used for all databases: (“Helicobacter pylori” OR H. pylori) AND (Moxifloxacin OR Sitafloxacin OR Gemifloxacin OR Gatifloxacin); the search terms varied slightly among these databases. This meta-analysis was conducted according to the Preferred Reporting Item for Systematic Reviews and Meta-Analyses (PRISMA)[25].
Articles eligible for inclusion in the meta-analysis met the following criteria: (1) randomized controlled trial (RCT) conducted; (2) methods used for the diagnosis of H. pylori, including the urea breath test (UBT), the rapid urease test (RUT), bacterial culture, histology, and/or fecal antigen test; (3) eradication rate made available; (4) eradication testing with UBT and/or histology performed at least 4 wk after the completion of therapy; and (5) eradication regimens in the experimental arm included fourth-generation quinolones.
Studies were excluded under the following circumstances: (1) eradication data could not be confirmed; (2) articles and abstracts were written in a language other than English; (3) fourth-generation quinolones were included in two treatment arms; and (4) the experimental group and the control group included more than one variable (for example, the comparison of triple therapy and quadruple therapy; antibiotics and duration were different in both groups).
Three authors (An Y, Wang Y, and Wu S) independently extracted data from the selected studies. Any disagreements were resolved by consensus.
The extracted data included the following: the study design; number of enrolled patients in each treatment arm; diagnostic methods for confirming H. pylori infection before enrolling and re-checking strategies after completing the eradication study; publication time; name of the authors; location of the trial; drug regimens; duration of treatment; eradication rates by intention-to-treat (ITT) analysis and per-protocol (PP) analysis; number of successful and failed eradications; and percentage of adverse effects.
To avoid duplication of data, if a trial was repeatedly published by the same authors or institutions, only the most recently published or most informative study was included.
The quality of RCTs with available full text was assessed using the risk of bias assessment tool developed by the Cochrane Handbook for Systematic Reviews of Interventions[26]. Two independent reviewers assessed the risk of bias through six domain-based evaluations, including selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other bias. Each indicator was scored by low risk of bias, unclear risk of bias, and high risk of bias. Any disagreement was discussed and decided by a third reviewer. We also employed a funnel plot and Egger’s test to assess the presence of publication bias.
The statistical analysis was performed using the meta-analysis software STATA12.0 (StataCorp LP, College Station, TX, United States). The primary outcomes of the meta-analysis were the H. pylori eradication rate and therapy-related side effects among the trials comparing the control and experimental groups based on ITT analysis. For each trial, we calculated the odds ratio (OR) for the primary measure. The ORs were presented with 95% confidence intervals (CIs); in addition, a P-value < 0.05 was considered significant. The degree of heterogeneity among the trial results was estimated using the χ2 statistic (P-value < 0.10 considered significant) and the I2 test (0%-25%, 25%-50%, 50%-75%, and > 75% represented insignificant, low, moderate, and high heterogeneity, respectively). If significant heterogeneity (P < 0.10 or I2 > 50%) was achieved, we employed the random effects model to combine the effect sizes of the included studies. When no significant heterogeneity was found, we used fixed effects to pool the data. Additionally, subgroup analyses were performed based on the location and different types of fourth-generation quinolones.
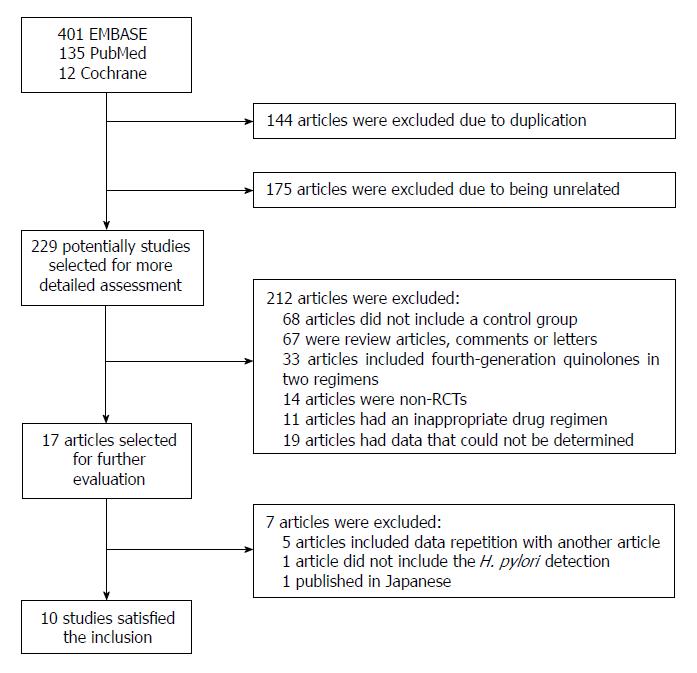
The bibliographical search yielded a total of 548 studies from PubMed, Embase, and the Cochrane Central Register of Controlled Trials. Among these articles, we excluded 144 due to duplication and 175 that were unrelated. We selected 229 potential studies for detailed assessment, among which 68 were excluded because there was no control group. We also excluded 67 review articles, comments, or letters. Thirty-three articles were excluded because of the inclusion of fourth-generation quinolones in two regimens, 14 articles were non-RCTs, 11 articles were excluded due to an inappropriate drug regimen, and 19 articles had data that could not be determined. Then, 17 articles were selected for further evaluation. Five articles were excluded because the data repeated those in other studies, one study did not state the methods for diagnosis of H. pylori, and one study was published in Japanese. Ultimately, 10 studies (two abstracts and eight full-text articles) met the inclusion criteria and were included in the systematic review and meta-analysis (Figure 1). These 10 studies[27-36] are summarized in Table 1 based on our meta-analysis. The quality assessment is reported in Table 2.
| Year-Author | Location | H. pylori infection initial diagnosis/re-checking | Control group-Day | Fourth-generation quinolone group-Day | Eradication rate (ITT)(control group/fourth- generation quinolone group) | Eradication rate (PP)(control group/fourth- generation quinolone group) | Compliance | Side effects |
| 2017-Mansour Ghanaei, F | Iran | 14C-UBT or histology | BPAC-10 | BPAG-10 | 89% (81/91)/77% (70/91) | 91% (81/90)/77.8% (70/90) | ||
| 2015-Masoodi, M | Iran | 13C-UBT, RUT pathology test | OBAC-10 | OBAG-10 | 61.6% (37/60)/66.6% (40/60) | 67.2% (37/55)/72.7% (40/55) | 97.1%/98.3% | 37/19 |
| 2014-Rakici, H | Turkey | pistology, stool antigen test | LanAL-10 | LanAM-10 | 89.4% (92/103)/87.8% (93/106) | 92% (92/100)/91.8% (93/102) | 96%/95.1% | - |
| 2013-Murakami, K | Japan | culture method, RUT, UBT | LanAL-7 | LanAS-7 | 43.1% (28/65)/70% (49/70) | 43.7% (28/84)/72.1% (49/68) | 98.4%/94.1% | 11/11 |
| 2012-Zeng, Z | China | 14C-UBT | EAC-7 | EAM-7 | 78.9% (180/228)/79.4% (181/228) | 82.9% (180/217)/84.2% (181/215) | - | - |
| 2009-Lu, NH | China | 14C-UBT | EAC-7 | EAM-7 | 90.3% (28/31)/85.7% (24/28) | - | - | - |
| 2008-Kilic, ZM | Turkey | gastroscopy, histology, RUT, 13C-UBT | RBCAC-14 EAC-14 | RBCAM-14 | 76.7% (23/30)/66.7% (20/30) | 76.7% (23/30)/66.7% (20/30) | 100%/100% | 11/13 |
| EAM-14 | 63.3% (19/30)/53.3% (16/30) | 63.3% (19/30)/53.3% (16/30) | 100%/100% | 17/21 | ||||
| 2007-Bago, P | Croatia | RUT, histology, culture test, 13C-UBT | LanMetC-7 LanAC-7 | LanMetM-7 | 70.4% (50/71)/93.5% (58/62) | 75.8% (50/66)/96.7% (58/60) | - | |
| LanAM-7 | 78.2% (61/78)/86.4% (57/66) | 80.2% (61/76)/90.5% 57/63) | ||||||
| 2005-Kist, M | Germany | 13C-UBT | EAC-7 ETC-7 | EAM-7 | 72.5% (58/80)/87.5% (70/80) | 78% (58/74)/89% (70/79) | - | - |
| ETM-7 | 75% (60/80)/90% (72/80) | 79% (60/76)/92% (72/78) | ||||||
| 2005-Nista, EC | Italy | Histological examination, 13C-UBT | ETC-7 EAC-7 | ETM-7 | 75% (60/80)/90% (72/80) | 78.9% (60/76)/92.3% (72/78) | - | 29/11 |
| EAM-7 | 72.5% (58/80)/87.5% (70/80) | 78.4% (58/74)/88.6% (70/79) | 26/10 |
| Year-Author | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data (attrition bias) | Selective reporting (reporting bias) | Other bias |
| 2017-Mansour Ghanaei, F | L | H | H | L | L | L | L |
| 2015-Masoodi, M | L | L | L | H | L | L | L |
| 2014-Rakici, H | L | H | H | H | L | U | L |
| 2013-Murakami, K | L | H | L | H | L | L | L |
| 2012-Zeng, Z | U | U | U | U | L | U | U |
| 2009-Lu, NH | U | U | U | U | L | U | U |
| 2008-Kilic, ZM | L | H | H | H | L | L | U |
| 2007-Bago, P | L | L | L | H | L | L | L |
| 2005-Kist, M | H | H | H | H | L | H | L |
| 2005- Nista, EC | L | H | H | H | L | L | L |
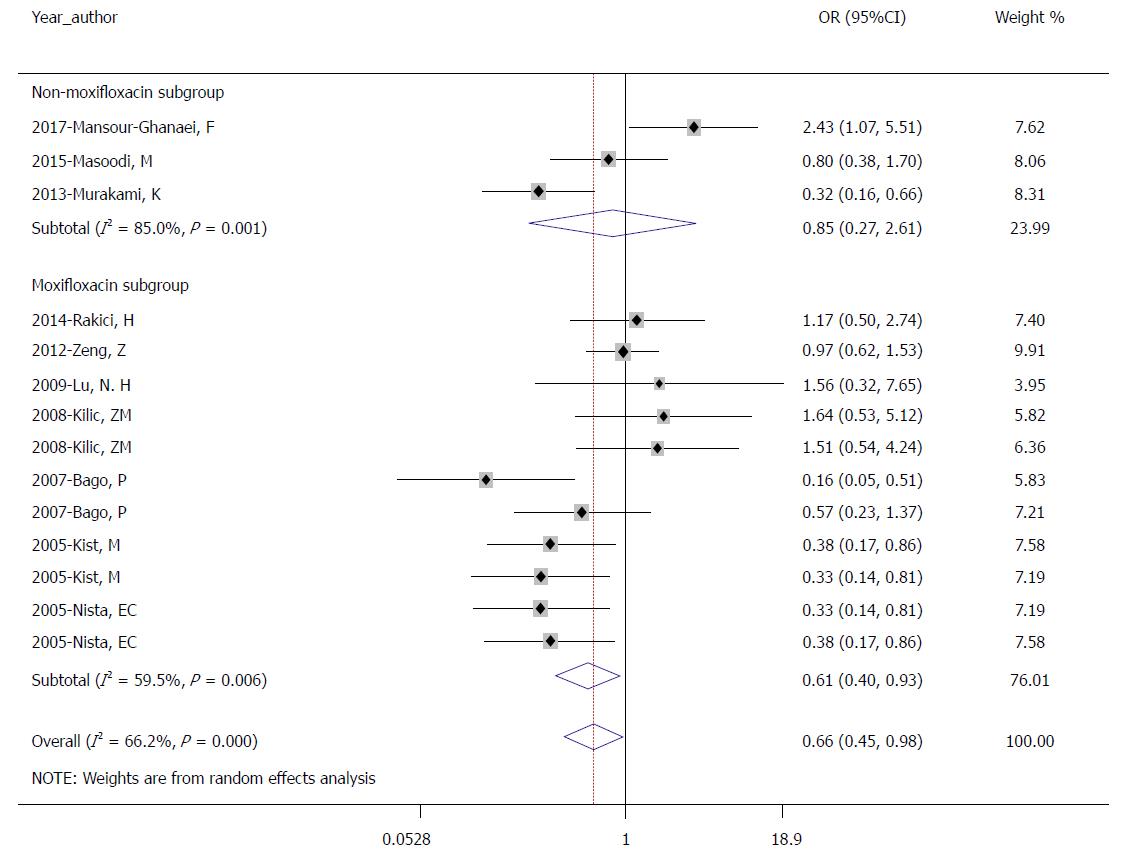
There were 10 studies with a total of 2198 patients in our meta-analysis; of these patients, 1107 received therapy without fourth-generation quinolone and 1091 received therapy with fourth-generation quinolone. The pooled eradication rates were 75.4% (835/1107) in the control group and 81.8% (892/1091) in the experimental group by ITT analysis. The pooled OR was 0.661 (95%CI: 0.447-0.977; P = 0.038) using the random effects model (I2 = 66.2%, P = 0.000; Figure 2).
Moreover, the pooled eradication rates were 79.1% (835/1055) in the control group and 84.7% (892/1053) in the experimental group by PP analysis. The pooled OR was 0.663 (95%CI: 0.433-1.016; P = 0.059) using the random effects model (I2 = 64.4%, P = 0.000).
The results of ITT showed that the eradication rates of therapies containing non-fourth-generation quinolones was significantly lower than those of therapies containing fourth-generation quinolones.
Additional subgroup analyses for the meta-analysis were performed due to heterogeneity. We analyzed different types of fourth-generation quinolones, covering seven moxifloxacin trials and three other trials (including 1 sitafloxacin and 2 gemifloxacin). In the moxifloxacin subgroup, the pooled eradication rates were 77.3% (689/891) in the control group and 84.3% (733/870) in the experimental group (OR = 0.614, 95%CI: 0.403-0.935; P = 0.023; Figure 3) by ITT analysis, and the rates were 81.3% (689/847) in the control group and 87.3% (689/891) in the experimental group by PP analysis (OR = 0.614, 95%CI: 0.395-0.956; P = 0.031). In the other subgroup, the pooled eradication rates were 67.6% (146/216) in the control group and 71.9% (159/221) in the experimental group (OR = 0.846, 95%CI: 0.274-2.614; P = 0.772; Figure 3) by ITT analysis, and the rates were 70.2% (146/208) in the control group and 74.6% (159/213) in the experimental group by PP analysis (OR = 0.860, 95%CI: 0.239-3.091; P = 0.817). This subgroup analysis showed that the regimen with moxifloxacin achieved a higher eradication rate than the regimen without moxifloxacin. However, there was no significant difference in the eradication rate in the other subgroup.
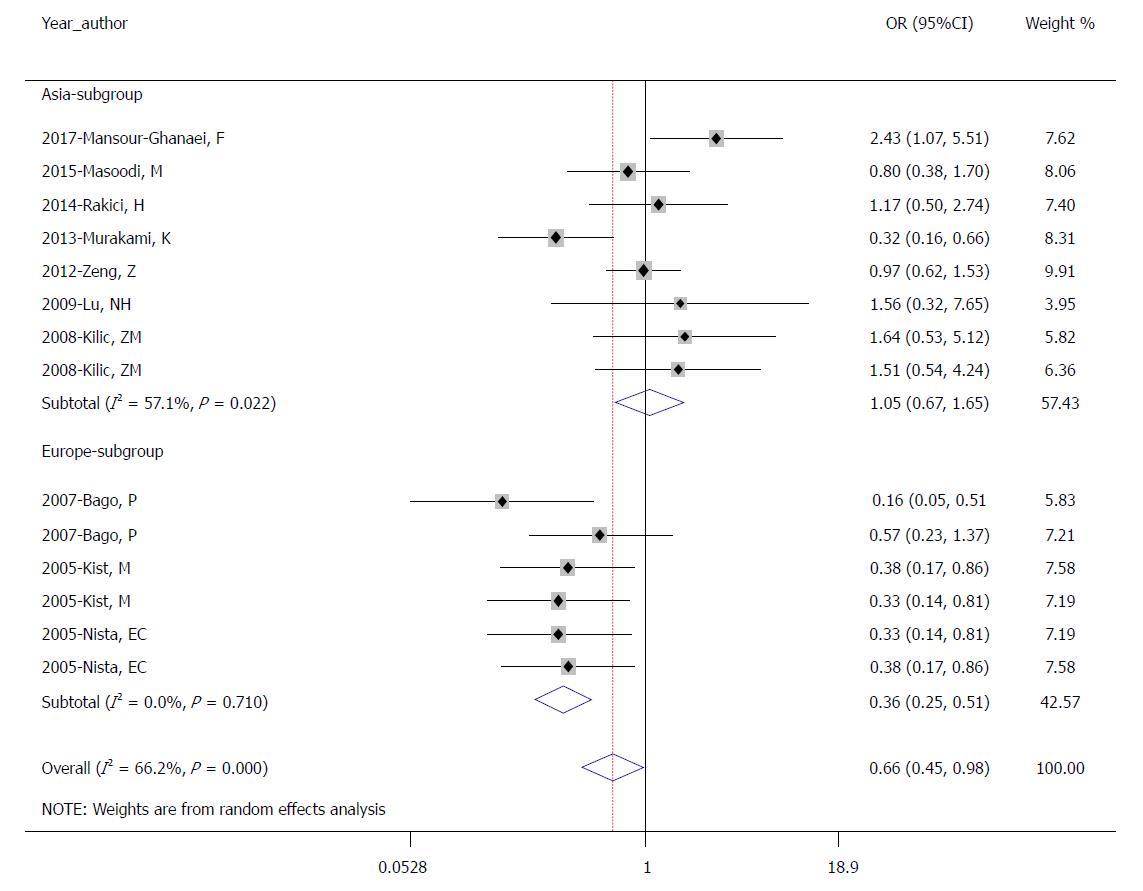
We also conducted subgroup analysis by region (seven trials in Asia and three trials in Europe). In the Asian subgroup, the pooled eradication rates of the control group and the experimental group were 76.5% (488/638) and 76.7% (493/643), respectively, by ITT analysis (OR = 1.051; 95%CI: 0.671-1.646; P = 0.827; Figure 4) and 79.6% (488/613) and 80.0% (493/616), respectively, by PP analysis (OR = 1.072; 95%CI: 0.627-1.833; P = 0.800). In the European subgroup, the pooled eradication rates of the control group and the experimental group were 74.0% (347/469) and 89.1% (399/448), respectively, by ITT analysis (OR = 0.661; 95%CI: 0.447-0.977; P = 0.000; Figure 4) and 78.5% (347/442) vs 91.3% (399/437), respectively, by PP analysis (OR = 0.361; 95% CI: 0.240-0.544; P = 0.000). The results showed that therapies containing fourth-generation quinolones may not be advisable treatments for H. pylori infection in Asia. However, the use of fourth-generation quinolones in Europe can significantly improve the eradication rate.
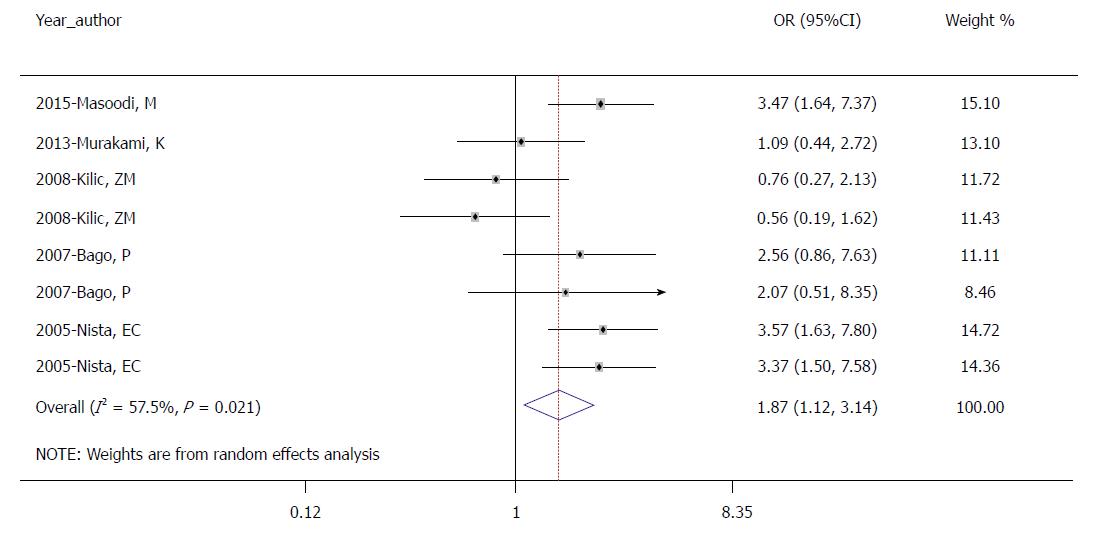
Of the 10 studies, four studies provided data regarding side effects. The results showed that common symptoms included nausea, diarrhea, black stool, and taste disturbance. The occurrence of total side effects in the control group was significantly higher than that in the experimental group by ITT analysis (30.6% vs 19.5%, OR = 1.874; 95%CI: 1.120-3.137; P = 0.017; Figure 5).
Four studies included in the meta-analysis provided information about compliance. The results showed high compliance (> 95%), and there were no significant differences between the study groups.
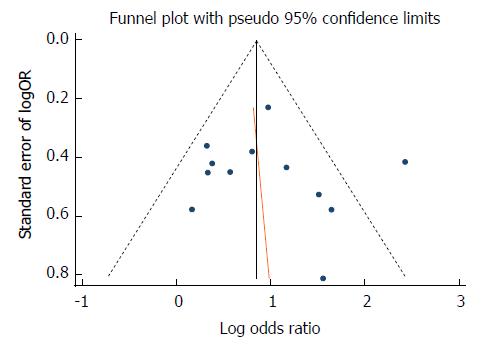
Egger’s regression test suggested that there was no significant bias (P = 0.725) in the ITT analysis, while the funnel plot showed a slightly asymmetrical distribution (Figure 6).
H. pylori infection is marked by a vast prevalence and strong association with various gastric diseases[37]. Therapeutic regimens range from STT to the present novel regimens, such as quadruple therapy with bismuth, sequential treatment, concomitant therapy, and hybrid therapy[38,39]. However, the treatment effects are still not ideal due to bacterial antibiotic resistance[40]. Thus, it is necessary to evaluate novel regimens or antibiotics[41]. With the resistance rate to the third-generation quinolone levofloxacin continuing to increase, resulting in a low eradication rate[16], therapies containing fourth-generation quinolones might be suitable for the treatment of H. pylori infection.
This meta-analysis indicated that therapies containing fourth-generation quinolones had a higher clearance rate than other therapies by ITT and PP analyses. The mechanism of action of fourth-generation quinolones against H. pylori is to inhibit bacterial DNA gyrase, thus interfering with bacterial DNA replication[42]. These fourth-generation quinolones embed in the broken DNA chain and form complexes to inhibit nicking and closing activity, achieving a bactericidal effect[43]. However, according to Graham, who had given a report card to grade H. pylori therapy by ITT, the eradication rate is still poor (grade D, 81%-84%)[44]. This may be related to the low compliance of patients[27,33]. The choice of fourth-generation quinolones, the duration of treatment, and the difference in PPI also influenced the pooled eradication rates of therapies containing fourth-generation quinolones.
The subgroup analyses of antibiotic species conducted in this study demonstrated that regimens containing moxifloxacin were superior to those not containing moxifloxacin (84.3% vs 71.9%). This finding might be consistent with a previous systematic review[45], but the eradication rate was still less than 85% by ITT analysis. The main reason was that the resistance rate of H. pylori to moxifloxacin was higher, even reaching up to 27.0% when analyzed by the E-test[31]. This phenomenon reminds us that it is best to conduct a susceptibility test to choose antibiotics reasonably.
We also conducted subgroup analysis by region. The eradication rate of fourth-generation quinolone treatments in Europe was much higher than that in Asia (89.1% vs 76.7%). This difference may be due to the low utilization rate of antibiotics in Europe[8]. In Asia, the abuse of antibiotics is very common, which leads to a high drug resistance rate of H. pylori. According to a multiregion prospective 7-year study by Liu et al[46], the prevalence of H. pylori after moxifloxacin treatment was 17.2%. Resistance to moxifloxacin was reported to be similar to that of levofloxacin, ranging from 14.9% to 20.0% in Turkey[29]. The increasing antibiotic resistance rate makes the eradication of H. pylori more difficult.
The rate of incidence of adverse events in the control groups was higher than that in the experimental groups. The pooled OR (1.874) indicated that the use of fourth-generation quinolones in the treatment of H. pylori infection can reduce the incidence of adverse reactions. This result indicates that therapies containing fourth-generation quinolones are safer.
The main limitation of this meta-analysis is potential biases. On the one hand, the largest number of studies was conducted using moxifloxacin; only one study used sitafloxacin, and two used gemifloxacin. This selection had a certain effect on the pooled eradication rate and may also be a particularly important issue in the use of a single antibiotic to eradicate H. pylori for clinical treatment. On the other hand, all included studies were performed in Europe and Asia, with no studies conducted in Africa or America. Because H. pylori infection occurs worldwide, our results may not be appropriate for global generalization. These two factors lead to the bias of conclusion. In addition, most of the studies in our meta-analysis had problems with concealment of allocation and blinding, which caused the selection bias. The restrictions on the language of publication also imply other bias, and thus our meta-analysis may not reflect all the outcomes.
Our analysis also implied other limitations. Most articles reporting a control arm were conducted using clarithromycin; our analysis is therefore especially lacking detailed data on levofloxacin. Two of the 10 included studies were abstracts, generating concerns regarding the data extraction and quality assessment of these studies and affecting the reliability of our results.
In conclusion, this meta-analysis indicates that therapies containing fourth-generation quinolones can achieve a higher eradication rate of H. pylori infection, but the eradication rate remains poor. In the absence of other drug options or in cases of patient allergy to penicillin, such regimens might be considered as a rescue treatment based on antimicrobial susceptibility testing. Further investigation is necessary to draw more solid conclusions about the use of fourth-generation quinolones in the treatment of H. pylori infection. In addition, we will study more effective therapies for H. pylori infection if necessary.
The resistance of Helicobacter pylori (H. pylori) to antibiotics is increasing and often leads to the failure of eradication treatment. Recent studies have reported that therapies containing fourth-generation quinolones remain effective against antibiotic-resistant H. pylori. However, the efficacy and safety of these therapies require further study. This is the first meta-analysis comparing the curative effect of fourth-generation quinolones with that of other therapies in regard to eradicating H. pylori.
In the Maastricht IV and Maastricht V Consensus Reports, levofloxacin-based therapy is recommended when the first treatment fails. Therapies containing fourth-generation quinolones are not mentioned. Our meta-analysis focused on eradication rates, side effects and compliance of therapies containing fourth-generation quinolones when compared with therapies using non-fourth-generation quinolones.
This meta-analysis aimed to clarify the effect of fourth-generation quinolones on the eradication of H. pylori infection and provide some evidence for clinical practice.
The meta-analysis was conducted according to the PRISMA criteria. We searched the PubMed, EMBASE, and Cochrane Library databases. The outcome was to calculate the pooled eradication rate and therapy-related side effects among the trials, comparing the control and experimental groups. We calculated the odds ratio of each trial for the primary measure. The odds ratios were presented with 95% confidence intervals, and a P-value < 0.05 was considered significant. This methodology was also performed for subgroup analysis.
Available data from 10 studies showed that treatment with a fourth-generation quinolone could achieve a higher H. pylori eradication rate and decrease the side effects, but the eradication rate is less than acceptable. Fourth-generation quinolones can significantly improve the eradication rate in Europe but not in Asia.
Quinolone resistance increases with age and duration of use. It is essential for practitioners to use quinolone antibiotics in the clinic reasonably. This study comprehensively analyzed the role of fourth-generation quinolone in the treatment of H. pylori infection. Our results suggested that fourth-generation quinolones are not ideal for eradication of H. pylori. Treatment based on antibiotic susceptibility testing might be more valid and obtain a higher rate of eradication of H. pylori infection, particularly in areas where resistance to antibiotics develops rapidly.
According to reports that mutations at positions 87 and 91 of gyrA are the main cause of H. pylori resistance to fourth-generation quinolones, we will continue to pay attention to the resistance rate to fourth-generation quinolones globally. We will also focus on rapid genotyping methods, such as detecting gyrA mutations in H. pylori. Further studies of sitafloxacin, gemifloxacin, and gatifloxacin are imperative to draw more solid conclusions about the use of fourth-generation quinolones for the eradication of H. pylori infection.
| 1. | Liou JM, Lin JT, Chang CY, Chen MJ, Cheng TY, Lee YC, Chen CC, Sheng WH, Wang HP, Wu MS. Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomised comparative trial with crossover design. Gut. 2010;59:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia-Abadia E, Vinagre-Rodriguez G, Martinez-Alcala C, Hernandez-Alonso M. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology. 2013;121-128.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 3. | Song M, Ang TL. Second and third line treatment options for Helicobacter pylori eradication. World J Gastroenterol. 2014;20:1517-1528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 4. | Wang B, Lv ZF, Wang YH, Wang H, Liu XQ, Xie Y, Zhou XJ. Standard triple therapy for Helicobacter pylori infection in China: a meta-analysis. World J Gastroenterol. 2014;20:14973-14985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 559] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 6. | Molina-Infante J, Pazos-Pacheco C, Vinagre-Rodriguez G, Perez-Gallardo B, Dueñas-Sadornil C, Hernandez-Alonso M, Gonzalez-Garcia G, Mateos-Rodriguez JM, Fernandez-Bermejo M, Gisbert JP. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter. 2012;17:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Mitui M, Patel A, Leos NK, Doern CD, Park JY. Novel Helicobacter pylori sequencing test identifies high rate of clarithromycin resistance. J Pediatr Gastroenterol Nutr. 2014;59:6-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y; Study Group participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 643] [Article Influence: 49.5] [Reference Citation Analysis (3)] |
| 9. | Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Iizuka Y, Fukuda K, Fujita Y, Katsurahara M, Ito T. Annual change of primary resistance to clarithromycin among Helicobacter pylori isolates from 1996 through 2008 in Japan. Helicobacter. 2009;14:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, Valasek MA. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43:514-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 584] [Article Influence: 58.4] [Reference Citation Analysis (2)] |
| 11. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1612] [Article Influence: 115.1] [Reference Citation Analysis (7)] |
| 12. | Malfertheiner P, Megraud F, O’Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 2079] [Article Influence: 231.0] [Reference Citation Analysis (1)] |
| 13. | Saracino IM, Zullo A, Holton J, Castelli V, Fiorini G, Zaccaro C, Ridola L, Ricci C, Gatta L, Vaira D. High prevalence of primary antibiotic resistance in Helicobacter pylori isolates in Italy. J Gastrointestin Liver Dis. 2012;21:363-365. [PubMed] |
| 14. | Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Shiota S, Reddy R, Alsarraj A, El-Serag HB, Graham DY. Antibiotic Resistance of Helicobacter pylori Among Male United States Veterans. Clin Gastroenterol Hepatol. 2015;13:1616-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Jeong MH, Chung JW, Lee SJ, Ha M, Jeong SH, Na S, Na BS, Park SK, Kim YJ, Kwon KA. [Comparison of rifabutin- and levofloxacin-based third-line rescue therapies for Helicobacter pylori]. Korean J Gastroenterol. 2012;59:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Mah FS. Fourth-generation fluoroquinolones: new topical agents in the war on ocular bacterial infections. Curr Opin Ophthalmol. 2004;15:316-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Kłosińska-Szmurło E, Grudzień M, Betlejewska-Kielak K, Pluciński F, Biernacka J, Mazurek AP. Physicochemical properties of lomefloxacin, levofloxacin, and moxifloxacin relevant to the biopharmaceutics classification system. Acta Chim Slov. 2014;61:827-834. [PubMed] |
| 19. | Hirata Y, Serizawa T, Shichijo S, Suzuki N, Sakitani K, Hayakawa Y, Yamada A, Koike K. Efficacy of triple therapy with esomeprazole, amoxicillin, and sitafloxacin as a third-line Helicobacter pylori eradication regimen. Int J Infect Dis. 2016;51:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Mahmoudi L, Farshad S, Seddigh M, Mahmoudi P, Ejtehadi F, Niknam R. High efficacy of gemifloxacin-containing therapy in Helicobacter Pylori eradication: A pilot empirical second-line rescue therapy. Medicine (Baltimore). 2016;95:e4410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Sugimoto M, Sahara S, Ichikawa H, Kagami T, Uotani T, Furuta T. High Helicobacter pylori cure rate with sitafloxacin-based triple therapy. Aliment Pharmacol Ther. 2015;42:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Gisbert JP, Romano M, Molina-Infante J, Lucendo AJ, Medina E, Modolell I, Rodríguez-Tellez M, Gomez B, Barrio J, Perona M. Two-week, high-dose proton pump inhibitor, moxifloxacin triple Helicobacter pylori therapy after failure of standard triple or non-bismuth quadruple treatments. Dig Liver Dis. 2015;47:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Chung KH, Dong HL, Kim N, Shin CM, Jin HH, Sang HL, Lee D, Hong SO, Jin EH. Su1696 Efficacy of Second-Line Treatment for Helicobacter pylori Infection: Moxifloxacin-Containing Triple Therapy vs. Bismuth-Containing Quadruple Therapy. Gastroenterology. 2012;142:S-483-S-484. [DOI] [Full Text] |
| 24. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48537] [Article Influence: 2855.1] [Reference Citation Analysis (3)] |
| 25. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8333] [Article Influence: 520.8] [Reference Citation Analysis (2)] |
| 26. | Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011;. |
| 27. | Mansour-Ghanaei F, Pedarpour Z, Shafaghi A, Joukar F. Clarithromycin versus Gemifloxacin in Quadruple Therapeutic Regimens for Helicobacter Pylori Infection Eradication. Middle East J Dig Dis. 2017;9:100-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Masoodi M, Talebi-Taher M, Tabatabaie K, Khaleghi S, Faghihi AH, Agah S, Asadi R. Clarithromycin vs. Gemifloxacin in Quadruple Therapy Regimens for Empiric Primary Treatment of Helicobacter pylori Infection: A Randomized Clinical Trial. Middle East J Dig Dis. 2015;7:88-93. [PubMed] |
| 29. | Rakici H, Ayaz T, Akdogan RA, Bedir R. Comparison of levofloxacin- and moxifloxacin-based triple therapies with standard treatment in eradication of Helicobacter pylori as first-line therapy. Digestion. 2014;90:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 30. | Murakami K, Furuta T, Ando T, Nakajima T, Inui Y, Oshima T, Tomita T, Mabe K, Sasaki M, Suganuma T. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J Gastroenterol. 2013;48:1128-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Zeng Z, Lv N, Hu F, Si J, Wu K, Jiang B, Liu W, Zhang J, Chen M, Hu P. Moxifloxacin-based triple therapy for Helicobacter pylori eradication: A multicenter randomized parallel-controlled study. J Gastroenterol Hepatol. 2012;27:3. |
| 32. | Lu NH, Xie Y, Zhu X, Chen YX, Ma JH, He XX. Eradication therapy for Helicobacter pylori infection in patients with duodenal ulcers based on moxifloxacin triple therapy: a randomized controlled trial. J Gastroenterol Hepatol. 2009;24:A15-A15. |
| 33. | Kiliç ZM, Köksal AS, Cakal B, Nadir I, Ozin YO, Kuran S, Sahin B. Moxifloxacine plus amoxicillin and ranitidine bismuth citrate or esomeprazole triple therapies for Helicobacter pylori infection. Dig Dis Sci. 2008;53:3133-3137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Bago P, Vcev A, Tomic M, Rozankovic M, Marusić M, Bago J. High eradication rate of H. pylori with moxifloxacin-based treatment: a randomized controlled trial. Wien Klin Wochenschr. 2007;119:372-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Kist M. How effective is moxifloxacin for the first-line treatment of patients with Helicobacter pylori infection? Nat Clin Pract Gastroenterol Hepatol. 2005;2:450-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 36. | Nista EC, Candelli M, Zocco MA, Cazzato IA, Cremonini F, Ojetti V, Santoro M, Finizio R, Pignataro G, Cammarota G. Moxifloxacin-based strategies for first-line treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 2005;21:1241-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Thamphiwatana S, Gao W, Obonyo M, Zhang L. In vivo treatment of Helicobacter pylori infection with liposomal linolenic acid reduces colonization and ameliorates inflammation. Proc Natl Acad Sci U S A. 2014;111:17600-17605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 38. | Apostolopoulos P, Koumoutsos I, Ekmektzoglou K, Dogantzis P, Vlachou E, Kalantzis C, Tsibouris P, Alexandrakis G. Concomitant versus sequential therapy for the treatment of Helicobacter pylori infection: a Greek randomized prospective study. Scand J Gastroenterol. 2016;51:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Alhooei S, Tirgar Fakheri H, Hosseini V, Maleki I, Taghvaei T, Valizadeh SM, Bari Z. A Comparison between Hybrid and Concomitant Regimens for Helicobacter Pylori Eradication: A Randomized Clinical Trial. Middle East J Dig Dis. 2016;8:219-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Egan BJ, Marzio L, O’Connor H, O’Morain C. Treatment of Helicobacter pylori infection. Helicobacter. 2008;13 Suppl 1:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Kwon YH, Kim N, Lee JY, Choi YJ, Yoon K, Nam RH, Suh JH, Lee JW, Lee DH. Comparison of the efficacy of culture-based tailored therapy for Helicobacter pylori eradication with that of the traditional second-line rescue therapy in Korean patients: a prospective single tertiary center study. Scand J Gastroenterol. 2016;51:270-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Moore RA, Beckthold B, Wong S, Kureishi A, Bryan LE. Nucleotide sequence of the gyrA gene and characterization of ciprofloxacin-resistant mutants of Helicobacter pylori. Antimicrob Agents Chemother. 1995;39:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Lee JW, Kim N, Nam RH, Park JH, Kim JM, Jung HC, Song IS. Mutations of Helicobacter pylori associated with fluoroquinolone resistance in Korea. Helicobacter. 2011;16:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol. 2014;12:177-186.e3; Discussion e12-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 45. | Zhang G, Zou J, Liu F, Bao Z, Dong F, Huang Y, Yin S. The efficacy of moxifloxacin-based triple therapy in treatment of Helicobacter pylori infection: a systematic review and meta-analysis of randomized clinical trials. Braz J Med Biol Res. 2013;46:607-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Liu DS, Wang YH, Zeng ZR, Zhang ZY, Lu H, Xu JM, Du YQ, Li Y, Wang JB, Xu SP. Primary antibiotic resistance of Helicobacter pylori in Chinese patients: a multiregion prospective 7-year study. Clin Microbiol Infect. 2018;24:780.e5-780.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chmiela M, Slomiany BL, Tongtawee T S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y