INTRODUCTION
Hepatorenal syndrome (HRS), one of the most severe complications of liver failure (LF) and the leading cause of death in LF[1], is functional renal failure secondary to LF[2-10]. At present, the exact pathogenesis of HRS is still unclear, and the reduction in renal blood flow induced by renal vasoconstriction is considered to play a central role in the development of HRS[11,12]. Renal blood flow is regulated by the contraction and relaxation of vascular smooth muscle cells (VSMCs) of glomerular afferent arteries and glomerular mesangial cells (GMCs), while the contraction and relaxation of VSMCs and GMCs are regulated by intracellular Ca2+ concentrations[13,14]. GMCs are in direct contact with glomerular endothelial cells. When GMCs contract, glomerular mesangial volume decreases by 20%-25%, glomerular capillary plexuses are reduced, and the area for glomerular filtration is reduced. Inositol 1,4,5-trisphosphate (IP3) receptor (IP3RS) is the main Ca2+ release channel in cells. IP3 is an intercellular second messenger mediating transmembrane signal transmission. When binding to IP3RS, IP3 mediates intracellular calcium release and extracellular calcium influx[15,16]. VSMCs and GMCs transmit extracellular signals into the cell via the IP3-IP3R pathway, increasing intracellular Ca2+ concentrations[17,18]. Is high expression of renal IP3RS associated with HRS? To answer this question, in the present study we detected the expression of IP3RI protein and mRNA in the kidney of HRS rats to determine the relationship between IP3RI expression and HRS.
MATERIALS AND METHODS
Materials
Specific pathogen-free (SPF) Sprague-Dawley (SD) rats, weighing 220 g ± 20 g, were purchased from the Laboratory Animal Center of the Academy of Military Medical Sciences (Animal Certificate No. SCXK-2017-004; Beijing, China). Prior to experimentation, the rats were reared in separate cages at 23 °C ± 3 °C under a 12 h/12 h light/dark cycle, with free access to ordinary chow (purchased from the Laboratory Animal Center of China Medical University, Shenyang, China) and water. After one week of adaptation, the rats were used in the experiments.
D-galactosamine (D-GalN) and lipopolysaccharide (LPS) were purchased from Sigma (St. Louis, MO, United States). Anti-IP3RI antibody was obtained from US Biological (St. Salem, OR, United States). An enhanced chemiluminescence (ECL) kit was purchased from Pierce, Dallas, TX, United States. RNAisoTM plus, Prime Script TM RT Reagent Kit, and SYBR® Premix EX TagTM were purchased from TakaRa (Shiga, Japan).
Rat model of HRS
One hundred and twenty-five SD rats of SPF grade, weighing 220 ± 20 g, were randomly divided into four groups to receive an intravenous injection of D-GalN plus LPS (group G/L), D-GalN alone (group G), LPS alone (group L), and normal saline (group NS), respectively. Each group was further divided into five subgroups for testing at different time points (3, 6, 9, 12, and 24 h). Group G/L contained ten rats at each time point, and the other groups contained five rats at each time point. The rats were weighed and then injected with D-GalN (400 mg/kg body weight) and/or LPS (32 μg/kg) or NS (2 mL/kg) via the tail vein. Rats that died during the modeling process were excluded from the study. At 3, 6, 9, 12, and 24 h after modeling, the rats in groups G/L, G, and L were anesthetized with 0.8% pentobarbital sodium at 40 mg/kg via intraperitoneal injection and sacrificed to obtain liver and kidney tissues. A section of each tissue was fixed in formalin, and the remainder was preserved at -80 °C for Western blot and real-time PCR analysis of IP3RI protein and mRNA expression, respectively.
Western blot analysis
For total protein preparation, renal tissue was lysed for 15 min in a lysis solution containing 50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mmol/L EDTA, 5 mg/mL leupeptin, sodium orthovanadate, sodium fluoride, and 1 mmol/L PMSF, and then centrifuged at 12000 rpm for 12 min. The supernatant was collected and preserved at -80 °C.
After total protein concentration was determined using the bicinchoninic acid (BCA) method, the protein samples were mixed with 5 × loading buffer at a ratio of 4:1 (v/v), boiled for 5 min, resolved by 8% SDS-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membranes. The membranes were then blocked with 5% skimmed milk, Tris-buffered saline and Tween-20, and incubated with primary antibody against IP3RI (dilution, 1:1000) at 4 °C overnight. This was followed by incubation with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (dilution, 1:3000) for 2 h at room temperature. The immunoblots were visualized using an enhanced chemiluminescence system. The molecular weight of the target band was 230 kDa. β-actin (45 kDa) was used as an internal control. Digital imaging software was used for densitometry analyses, and the relative IP3RI level was calculated as IP3RI grey value divided by β-actin grey value.
Real-time PCR
Total RNA was prepared from renal tissue using Trizol according to the manufacturer’s instructions. After reverse transcription to cDNA in a 10-μL system containing 2.0 μL of 5 × Prime ScriptTM Buffer (for real time), 0.5 μL of Prime ScriptTM RT Enzyme Mi, 0.5 μL of Oligo dT Primer (50 μmol/L), 0.5 μL of Random 6-mers (100 μmol/L), 5.5 μL of RNase Free dH2O, and 1.0 μL of RNA (500 ng/μL), real-time PCR was performed in a 25-μL system containing 12.5 μL of 2 × SYBR Premix Ex TagTM, 0.5 μL of PCR Forward Primer (10 μmol/L), 0.5 μL of PCR Reverse Primer (10 μmol/L), 9.5 μL of RNase Free dH2O, and 2.0 μL of cDNA. Cycling parameters were 95 °C for 30 s and 45 cycles of 95 °C for 5 s, 57 °C for 20 s, and 72 °C for 30 s. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous reference. The primers used were as follows: forward, 5´-TCTGGCCAGCTGTCAGAACTAAAG-3´ and reverse, 5´-GTGGGTTGACATTCATGTGAGGA-3´ for IP3RI, and forward, 5´-GACAACTTTGGCATCGTGGA-3´ and reverse, 5´-GACAACTTTGGCATCGTGGA-3´ for GAPDH. The double-standard curve method was used to determine the relative IP3RI mRNA expression.
Statistical analysis
All statistical analyses were performed using SPSS 13.0 software. Numerical data, expressed as mean ± standard error of the mean, were compared using analysis of variance. P values < 0.05 were considered statistically significant.
RESULTS
Successful induction of HRS in rats with D-GalN/LPS
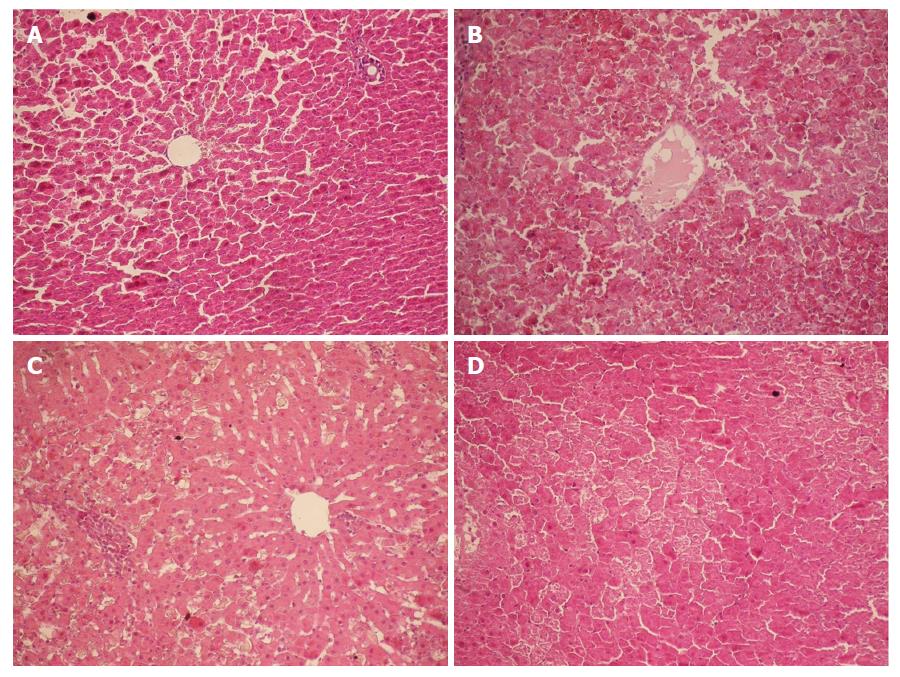
Following intravenous injection of D-GalN at 400 mg/kg body weight combined with LPS at 32 μg/kg in male SD rats, HRS was successfully induced. Twelve hours after injection, glomerular filtration rate (GFR) significantly decreased, liver and kidney function were severely impaired, and serum biochemical indices, such as alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine (Cr), exhibited significant changes. Hematoxylin-eosin staining showed massive hepatocyte necrosis with severe hemorrhage (Figure 1), while renal tissue had a normal morphology at the various time points (Figure 2). These changes were consistent with the clinical features of HRS.
Figure 1 Histopathology of the liver (HE staining, × 200).
A: Group Normal Saline (NS). Normal hepatocytes were arranged in cords; B: Group D-galactosamine (D-GalN) plus lipopolysaccharide (LPS) (G/L). At 12 h, massive hepatocyte necrosis with severe hemorrhage developed; C: Group D-GaIN (G). At 12 h, spotty hepatocyte necrosis was observed; C: Group LPS (L). At 12 h, hepatocytes began to develop necrosis, with incomplete necrosis visible.
Figure 2 Histopathology of the kidney.
A: The glomerular basement membrane of the kidney was intact, and the foot processes of podocytes and fenestra of endothelial cells were clearly visible; B: The basal part of proximal tubule cubical epithelial cells had abundant plasma membrane infolding, which was rich in longitudinally arranged mitochondria with intact cristae. On the free surface of proximal tubule cubical epithelial cells, microvilli were long and dense; C: The basal part of distal tubule cubical epithelial cells also had abundant plasma membrane infolding, which was rich in mitochondria. On the free surface of distal tubule cubical epithelial cells, microvilli were short and sparse.
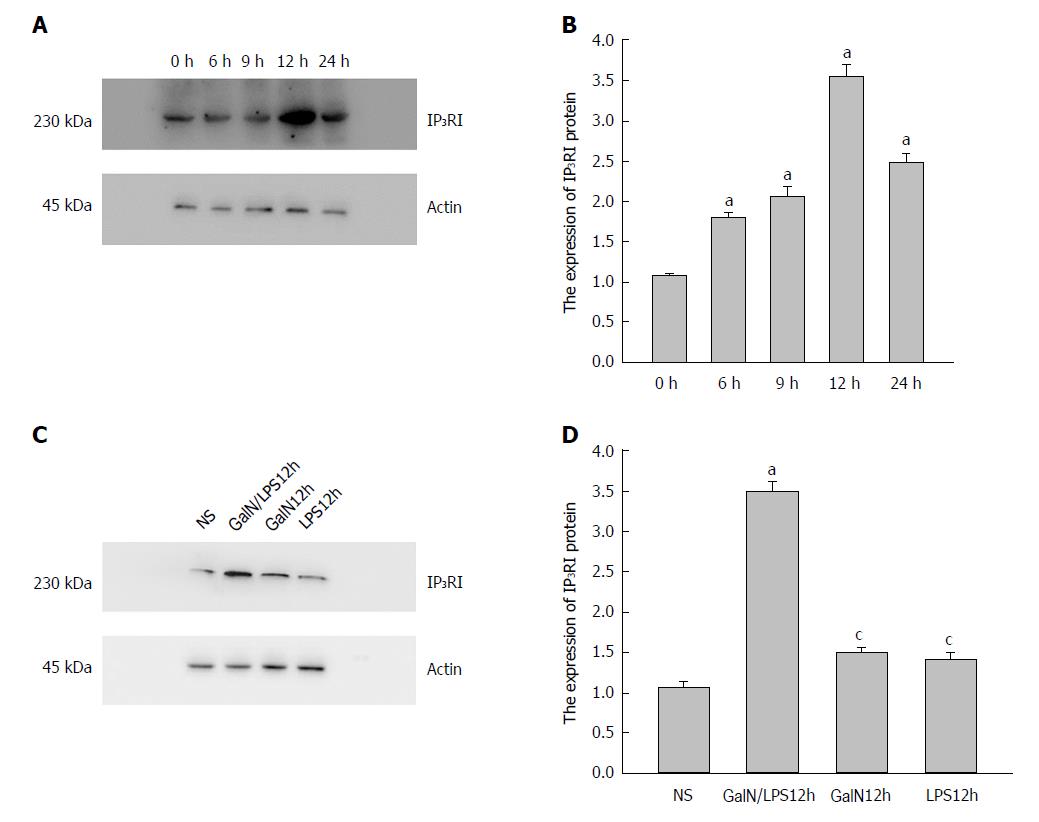
Western blot analysis of IP3RI protein expression
IP3RI (230 kDa) and β-actin (45 kDa) were detected in all groups. Densitometry analyses showed that IP3RI protein expression was significantly elevated in group G/L compared with group NS. This elevation began at 3 h (1.46 ± 0.07 vs 1.00 ± 0.05, P = 0.011), became obvious at 9 h, and reached a peak at 12 h (2.89 ± 0.14 vs 1.00 ± 0.05, P = 0.000) (Figure 3A).
Figure 3 Expression of type I inositol 1,4,5-trisphosphate receptor (IP3RI) protein in the kidney of rats in each group.
A: The expression of IP3RI protein in the kidney significantly increased in group D-galactosamine (D-GalN) plus lipopolysaccharide (LPS) (G/L), and was especially prominent at 12 h [aP < 0.05 vs group Normal Saline (NS)]. B: The expression of IP3RI protein in the kidney significantly increased in group G/L compared with the other groups (aP < 0.05 vs group NS, cP < 0.05 vs group G/L).
At 12 h, the expression of IP3RI protein in the kidney was significantly higher in group G/L than in groups G (1.17 ± 0.08) and L (1.02 ± 0.09) (P = 0.000 for both), thus excluding the impact of D-GalN or LPS on the expression of IP3RI protein. There was no significant difference in IP3RI protein expression between groups G and L (P = 0.245) or between group G or L and group NS (P > 0.05 for both) (Figure 3B).
RT-PCR analysis of IP3RI mRNA expression
IP3RI mRNA expression was significantly elevated in group G/L compared with group NS. This elevation began at 3 h (2.89 ± 0.51 vs 1.00 ± 0.00, P = 0.05), became obvious at 6 h (5.01 ± 0.38, P = 0.000), and reached a peak at 9 h (9.96 ± 0.63, P = 0.000). IP3RI mRNA expression began to decline at 12 h, and at 24 h, it returned to the level observed at 6 h. IP3RI mRNA expression at 9 h was significantly higher in group G/L than in groups G (1.43 ± 0.18) and L (1.29 ± 0.17) (P = 0.000 for both; Figure 4). IP3RI mRNA expression did not differ significantly between group G or L and group NS (P > 0.05 for both).
Figure 4 Expression of type I inositol 1,4,5-trisphosphate receptor (IP3RI) mRNA in the kidney of rats in group D-galactosamine (D-GalN) plus lipopolysaccharide (LPS) (G/L).
The expression of IP3RI mRNA in the kidney significantly increased in group G/L, and was especially prominent at 12 h (aP < 0.05 vs group Normal Saline (NS), cP < 0.05 vs group G/L).
DISCUSSION
HRS is one of the most common and severe complications of fulminant liver failure (FHF) and an advanced liver disease, with approximately 55% of FHF patients developing HRS[19,20]. The pathogenesis of HRS is still not completely clear, although it is believed to be associated with excessive renal vascular contraction, insufficient renal blood perfusion, sympathetic nervous system activation, and increased synthesis of vasoactive substances, all of which make the kidneys more sensitive to low perfusion[21,22]. Renal blood flow and GFR decrease significantly in HRS due to renal vasoconstriction, and many factors are involved in this process. A significant increase in vasocontracting factors [e.g., endothelin (ET) and angiotensin II] in the blood not only leads to renal vascular contraction, but also decreases the glomerular filtration coefficient (Kf) and GFR[23,24]. The contraction of VSMCs results in reduced renal blood flow, while GMC contraction reduces the glomerular filtration fraction and coefficient. As both VSMCs and GMCs are extremely sensitive to vasoactive substances, GFR is significantly decreased in HRS. ET and angiotensin II are important renal vasocontracting factors, and they activate Ca2+ channels via the IP3-IP3Rs pathway. IP3Rs is the intracellular calcium reservoir, which is present mainly in the endoplasmic reticulum and on the membrane, directly or indirectly mediating the calcium influx[25-27]. In addition, IP3Rs is also present in the nucleus, participating in nuclear calcium release and regulating gene expression[28,29]. When IP3 binds to IP3Rs, a conformational change in IP3Rs occurs, the calcium channel is open, and the calcium reserve in the endoplasmic reticulum is released into the cytoplasm. As a result, cytoplasmic free Ca2+ concentration ([Ca2+]i) increases, thus causing cell contraction[30-32]. Therefore, IP3Rs mediates an important Ca2+ signaling pathway in the cell, and the expression of IP3Rs is closely related to the sensitivity of the kidney to vasoconstrictors[33-35]. IP3Rs has four types of ligand binding sites associated with calcium channels[36-39], and renal IP3RI is mainly found in GMCs and VSMCs, and there is almost no IP3RI on the surface of other renal cells[40,41]. Therefore, the expression levels of IP3RI in renal GMCs and VSMCs may be related to renal vasoconstriction. As the opening of IP3-IP3Rs channels can increase intracellular [Ca2+]i, theoretically the expression level of IP3RI is closely related to the intracellular [Ca2+]i level. Wang et al observed increased expression of IP3RI in the glomerular capillary loops and anterior artery of rats with liver cirrhosis by immunohistochemistry. However, it is unknown whether the expression of IP3RI increases in FHF. To answer this question, we detected IP3RI expression in the renal tissue of a rat model of FHF at different time points at both the protein and mRNA levels using Western blot and real-time quantitative PCR, respectively.
Semi-quantitative Western blot analysis demonstrated that IP3RI protein expression was low in normal kidney tissue. Following treatment with D-GalN plus LPS, IP3RI protein expression began to rise at 3 h and reached a peak at 12 h. Interestingly, liver and kidney dysfunction and hepatocyte necrosis were most severe and blood TNF-α and ET-1 levels were highest at 12 h, which were concomitant with the elevation of IP3RI protein expression in the kidney[42,43]. By treating the animals with D-GalN or LPS alone, we excluded the effect of these drugs on IP3RI protein expression. As HRS developed 12 h after D-GalN/LPS administration, we compared the IP3RI protein expression at this time point among the groups. The results showed that IP3RI protein expression was high in group G/L and low in groups G, L, and NS.
In order to understand whether IP3RI protein expression in the kidney is regulated at the transcriptional level, real-time quantitative PCR was performed. The fluorescent dye SYBR Green I[44,45] added to the PCR reaction system can be incorporated into double-stranded DNA with PCR amplification and markedly enhance fluorescence[46-48]. The relative expression levels of these two parameters were calculated by the housekeeping gene GAPDH. It was found that the relative expression of IP3RI mRNA to GAPDH mRNA began to rise 3 h after D-GalN and LPS administration, but the protein level did not rise at this time point. At 9 h, the expression level of IP3RI mRNA reached the highest level. Although the protein level was also high at this time point, it was lower than that at 12 h. The expression of IP3RI mRNA began to decrease, but it was still significantly higher than that in the control group. These changes can be explained from two aspects. On the one hand, IP3RI mRNA expression may be prior to protein expression, which is associated not only with the translation efficiency and the speed of mRNA degradation, but also with the rate of protein degradation. On the other hand, the protein synthesis process also includes the assembly and translocation of proteins in ribosomes, which may affect the final expression of IP3RI protein.
In conclusion, joint D-GalN/LPS administration can induce HRS in SD rats at 12 h, which is concomitant with peak IP3RI protein expression in the kidney. Increased IP3RI protein expression may be regulated at the transcriptional level. Thus, increased expression of IP3RI may be closely associated with HRS development and progression.
ARTICLE HIGHLIGHTS
Research background
Hepatorenal syndrome (HRS) is one of the common and severe complications of liver failure and advanced liver disease, with approximately 55% of these patients developing this severe complication. At present, HRS has unclear pathogenesis, limited treatment options, and poor therapeutic efficacy. Once renal dysfunction aggravates rapidly, 60%-80% of patients with HRS will die. Therefore, elucidating the mechanism underlying the development and progression of HRS and taking effective preventive and therapeutic measures may improve the success rate of rescue, the incidence rate, and the mortality rate of HRS.
Research motivation
To detect the protein and mRNA expression of type I inositol 1,4,5-trisphosphate receptor (IP3RI) in the kidney of rats with HRS by Western blot and real-time PCR.
Research objectives
To explore whether high expression of renal IP3RI is associated with Ca2+ influx in vascular smooth muscle cells of glomerular afferent arteries and glomerular mesangial cells in rats with HRS.
Research methods
D-galactosamine (D-GalN) and/or lipopolysaccharide (LPS) were used to treat male Sprague-Dawley (SD) rats via the tail vein. Twelve hours after injection, massive hepatocyte necrosis with severe hemorrhage occurred in the liver, while renal tissue had a normal morphology. In addition, liver and kidney function was impaired severely, and serum biochemical indexes exhibited significant changes. These changes were consistent with the clinical features of HRS. Western blot and real-time PCR were then used to detect the protein and mRNA expression of renal IP3RI, respectively.
Research results
IP3RI protein expression was significantly elevated in rats with HRS. The elevation began at 3 h and reached the peak at 12 h. IP3RI mRNA expression was also significantly elevated in rats with HRS. The elevation began at 3 h and peaked at 9 h.
Research conclusions
Joint D-GalN/LPS administration can induce HRS in SD rats at 12 h, which is concomitant with peaked IP3RI protein and mRNA expression in the kidney. Increased expression of IP3RI may be closely associated with HRS development and progression.
Research perspectives
Our results suggest that IP3RI may be a signal molecule involved in the reduction of renal blood flow induced by renal vasoconstriction in HRS, thus providing a theoretical basis for further research of the pathogenesis of HRS. Gene silencing technology may be adopted to further elucidate the role of IP3RI in the pathogenesis of HRS.