INTRODUCTION
Hepatitis B virus/hepatitis C virus (HBV/HCV) dual infection is common in regions with a high HBV prevalence, as HBV and HCV share a similar mode of transmission[1]. The global prevalence of HBV/HCV dual infection is approximately 5%-10% in patients with chronic HCV infection, and the prevalence is reported to be 8.4% in China and 12%-14% in East Asia[2]. HBV/HCV-coinfected patients tend to have more severe liver fibrosis and a higher risk of hepatocellular carcinoma than those without coinfection[3].
Recently, interferon (IFN)-based treatment for HCV infection has been gradually replaced by direct-acting antiviral agent (DAA)-based therapy due to the higher HCV sustained virologic response (SVR) rate and greater tolerability of DAA-based therapy. However, concerns have been raised regarding HBV reactivation in patients receiving DAA therapy. HBV reactivation may occur after immunosuppression therapy and chemotherapy and may result in reactivation-related hepatitis, liver failure, need for liver transplantation, and even death[4]. Similarly, HBV reactivation can occur in HBV/HCV-coinfected patients undergoing IFN-based or DAA-based treatment[5]. The United States Food and Drug Administration (FDA) has reported 29 cases of HBV reactivation, and liver failure occurred in three of these cases after DAA-based treatment[6]. As a result, the FDA introduced a black box warning regarding HBV reactivation risk in HBV/HCV-coinfected patients receiving DAA therapy[7].
A previous meta-analysis reported the proportion of HBV reactivation in HBV/HCV-coinfected individuals, but only two of the included publications involved HBV reactivation with DAA-based therapy. Moreover, the need for preemptive anti-HBV therapy remains controversial[8,9]. Therefore, we conducted a comprehensive meta-analysis to assess the incidence of hepatitis B reactivation in patients receiving DAA-based therapy or IFN-based therapy and the effectiveness of preemptive anti-HBV therapy for preventing HBV reactivation.
MATERIALS AND METHODS
Data search strategy
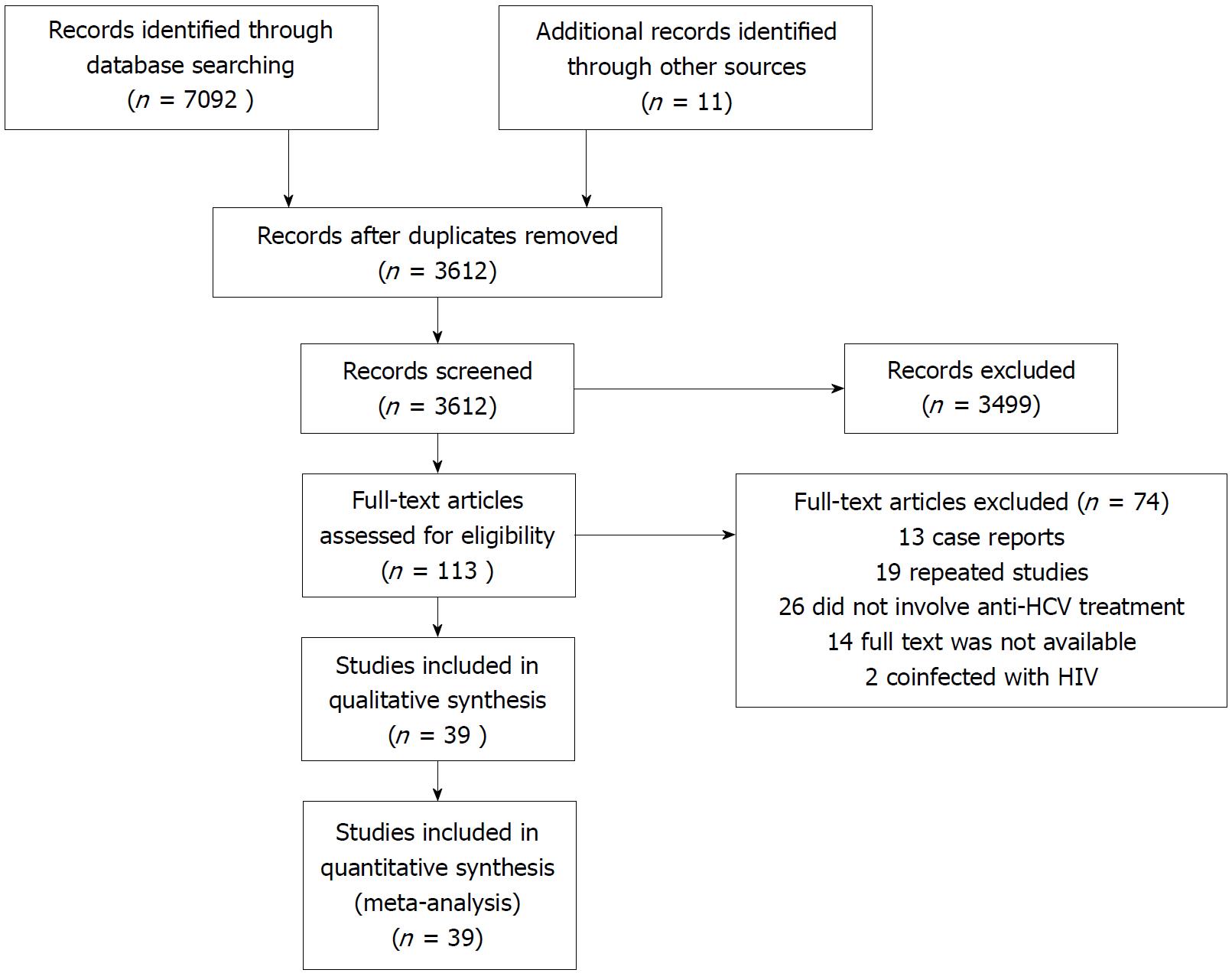
This systematic review was based on published literature and included 39 studies. Figure 1 shows the literature search process for the meta-analysis. Relevant publications were extracted from the PubMed, MEDLINE and EMBASE databases from inception to December 30, 2017 using the following key words and subject terms: hepatitis B virus, hepatitis C virus, HBV and HCV dual infection, coinfection and reactivation. The data search was not limited by language or study type. In addition, the reference lists of relevant reviews and online conference abstracts were also screened. When two publications investigated the same population, only the most recent study or the more detailed study was included. Our review adhered to the PRISMA guidelines[10], and the protocol was registered on PROSPERO (registration number: CRD42018085920).
Figure 1 Literature search process for the systematic review and meta-analysis.
HCV: Hepatitis C virus; HIV: Human immunodeficiency virus.
Study selection
Studies included in the meta-analysis were required to meet the following criteria: (1) Conducted in patients treated with DAA-based therapy or IFN-based therapy; and (2) involved with HBV reactivation or HCV SVR after anti-HCV treatment. The following studies were excluded: (1) Studies in patients with human immunodeficiency virus coinfection; and (2) case reports and studies with no extractable data.
The primary outcome was HBV reactivation in accordance with the American Association for the Study of Liver Diseases (AASLD) criteria[11]: (1) Chronic HBV infection (HBsAg-positive; increase in HBV DNA > 2 log IU/mL from baseline level or HBV DNA > 100 IU/mL in patients with previously undetectable HBV DNA); and (2) previous HBV infection (HBsAg-negative, HBcAb-positive; HBsAg changed from negative to positive or HBV DNA ≥ 20 IU/mL during treatment). The secondary outcomes included HBV reactivation-related hepatitis, the HCV SVR at the end of anti-HCV treatment and the efficacy of preemptive anti-HBV treatment with nucleos(t)ide analogues (NUCs). A hepatitis flare was defined as a concomitant increase in alanine aminotransferase of at least twice the upper limit of normal.
Data extraction and quality assessment
Qualitative studies were selected for the meta-analysis, and both reviews and case reports were excluded. All data were extracted by two independent investigators. The data included the following: Study design, study year, country, patient age and sex, total number of patients treated, number of patients with HBV reactivation and reactivation-related hepatitis, number of patients with SVR, HBV DNA level and HBV marker status at baseline, and treatment regimen and duration.
The quality assessment tools (Systematic Evidence Review from the Risk Assessment Work Group) from the National Institutes of Health were used to assess the methodological quality of the cohort studies and randomized controlled trials. Each study was assessed as “good”, “fair”, or “poor” quality using the items included in each tool. Studies of “poor” quality suggested a high risk of bias[12].
Statistical analysis
The meta-analysis was conducted in Stata version 14.0 (StataCorp, College Station, TX, United States) using the metan, metaprop, metareg and metabias commands. The incidence rates of HBV reactivation and hepatitis flare were calculated using the random effects model, and the Freeman-Tukey double arcsine transformation was used to stabilize the variance of the raw data[13]. The pooled relative risk (RR) with 95%CIs for HBV reactivation and hepatitis flare was calculated according to the HBV DNA level with an inverse variance approach. The heterogeneity in the pooled studies was estimated with the I2 statistic. Significant heterogeneity was investigated using meta-regression and subgroup analyses for treatment regimen (IFN-based therapy vs DAA-based therapy), HBV DNA level (detectable HBV DNA vs undetectable HBV DNA), study sample size (n < 30 vs n ≥ 30) and race (Asian vs non-Asian). A two-sided P value less than 0.05 was regarded as statistically significant. Publication bias was tested using Egger’s test and was assessed by funnel plots.
RESULTS
Study characteristics and quality assessment
The systematic review identified 7092 articles. After the elimination of duplicates, the titles and abstracts were screened, and 39 full articles met the inclusion criteria. These studies were conducted between 1997 and 2017, and included eight prospective cohort studies[14-21], twenty-seven retrospective cohort studies[22-48], three randomized controlled trials[49-51] and one case-control study[52]. A total of 3468 patients with a median age of 53 years, were enrolled, including 1060 HBsAg-positive patients and 2408 patients with previous HBV infection. Thirty-five of these studies reported the number of HBV reactivations, thirty-five studies reported the number of hepatitis flares, and thirty-six studies reported the HCV SVR rates. HCV SVR with DAA-based therapy was achieved in 202 of 203 HBsAg-positive patients and 486 of 507 HBsAg-negative but HBcAb-positive patients. The baseline characteristics are shown in supplementary Table 1.
All articles were assessed as being of fair to good quality. However, studies with a small number of chronic HBV infections may have a potential risk of bias. The small-number effect on the pooled effect size was tested by meta-regression. The quality assessment of the included studies is shown in supplementary Table 2.
HBV reactivation
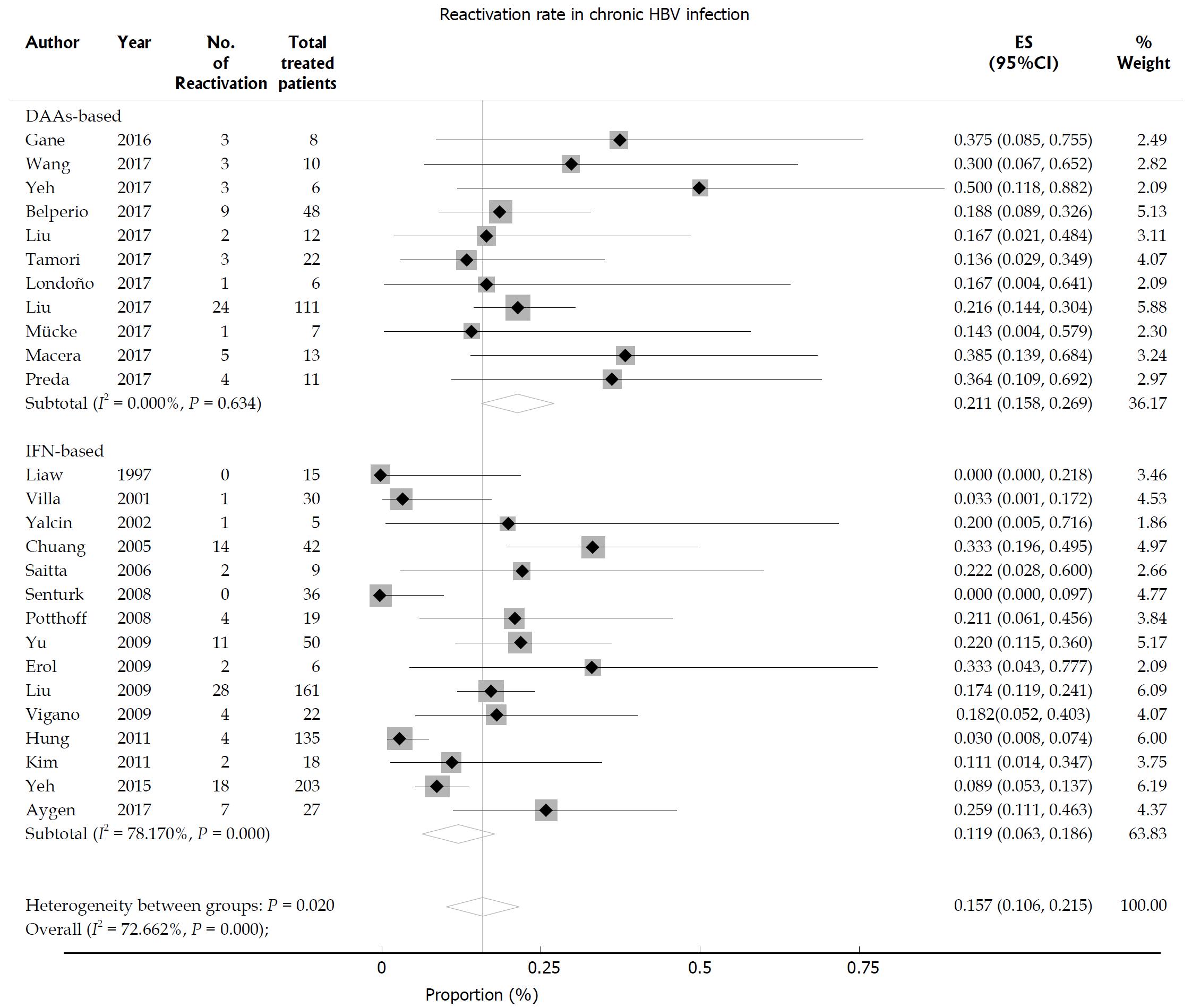
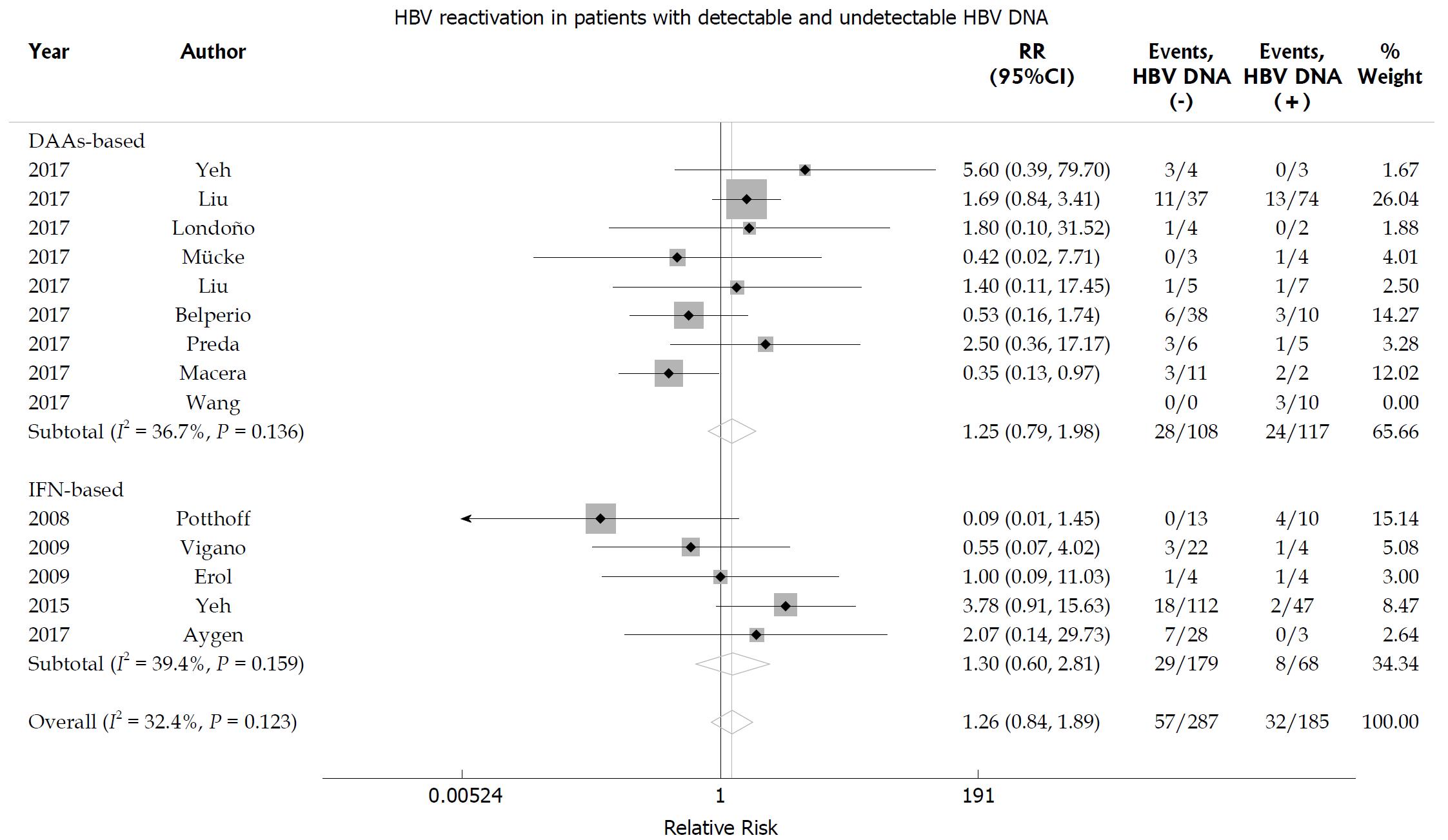
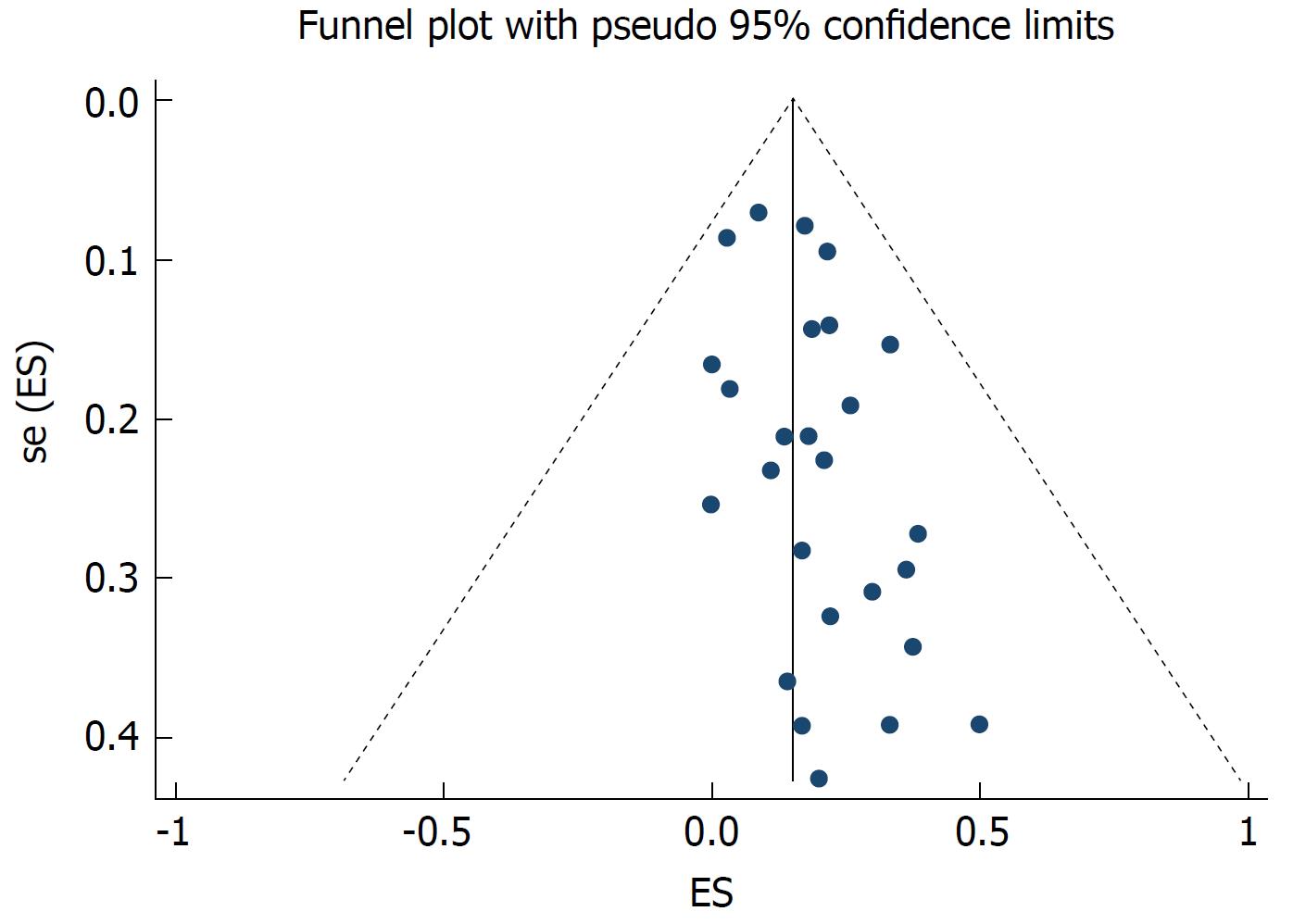
The random effects pooled overall HBV reactivation rate was 15.7% (95%CI: 10.6-21.5) in HBsAg-positive patients receiving anti-HCV treatment with statistical heterogeneity among the studies (I2 = 72.6%; P < 0.001; Figure 2). Subgroup analysis was performed according to the treatment regimen. The HBV reactivation rate was higher in the DAA-treated group (21.1%, 95%CI: 15.8-26.9) than in the IFN-treated group (11.9%, 95%CI: 6.3-18.6; P = 0.02 for subgroup differences). The HBV reactivation risk was further analyzed based on the HBV DNA level. We found that the HBV reactivation rate in patients with undetectable HBV DNA did not differ from that in those with detectable HBV DNA receiving either DAA-based or IFN-based therapy [relative risk (RR) 1.26, 95%CI: 0.84-1.89; P = 0.255]. No significant heterogeneity was found among the studies (I2 = 32.4%; P = 0.123; Figure 3). Egger’s test showed a certain publication bias (P = 0.03; Figure 4). Univariate meta-regression analysis of the HBV reactivation rate showed that no baseline characteristics influenced the between-subgroup variation (all P > 0.05)
Figure 2 Hepatitis B virus reactivation in hepatitis C virus patients with chronic hepatitis B virus infection.
Pooled estimates of hepatitis B virus reactivation in hepatitis C virus patients with chronic hepatitis B surface antigen-positive hepatitis B virus infection.
Figure 3 Hepatitis B virus reactivation according to baseline hepatitis B virus DNA level in hepatitis C virus patients with chronic hepatitis B virus infection.
Relative risk of hepatitis B virus (HBV) reactivation according to baseline HBV DNA level in hepatitis C virus patients with chronic hepatitis B surface antigen-positive HBV infection.
Figure 4 Funnel plot of 26 studies estimating the hepatitis B virus reactivation rates in hepatitis C virus patients with chronic hepatitis B virus infection.
The vertical line corresponds to the summary hepatitis B virus reactivation rates as estimated from the random effect model. The publication bias is presented; P = 0.03, as tested by Egger’s test.
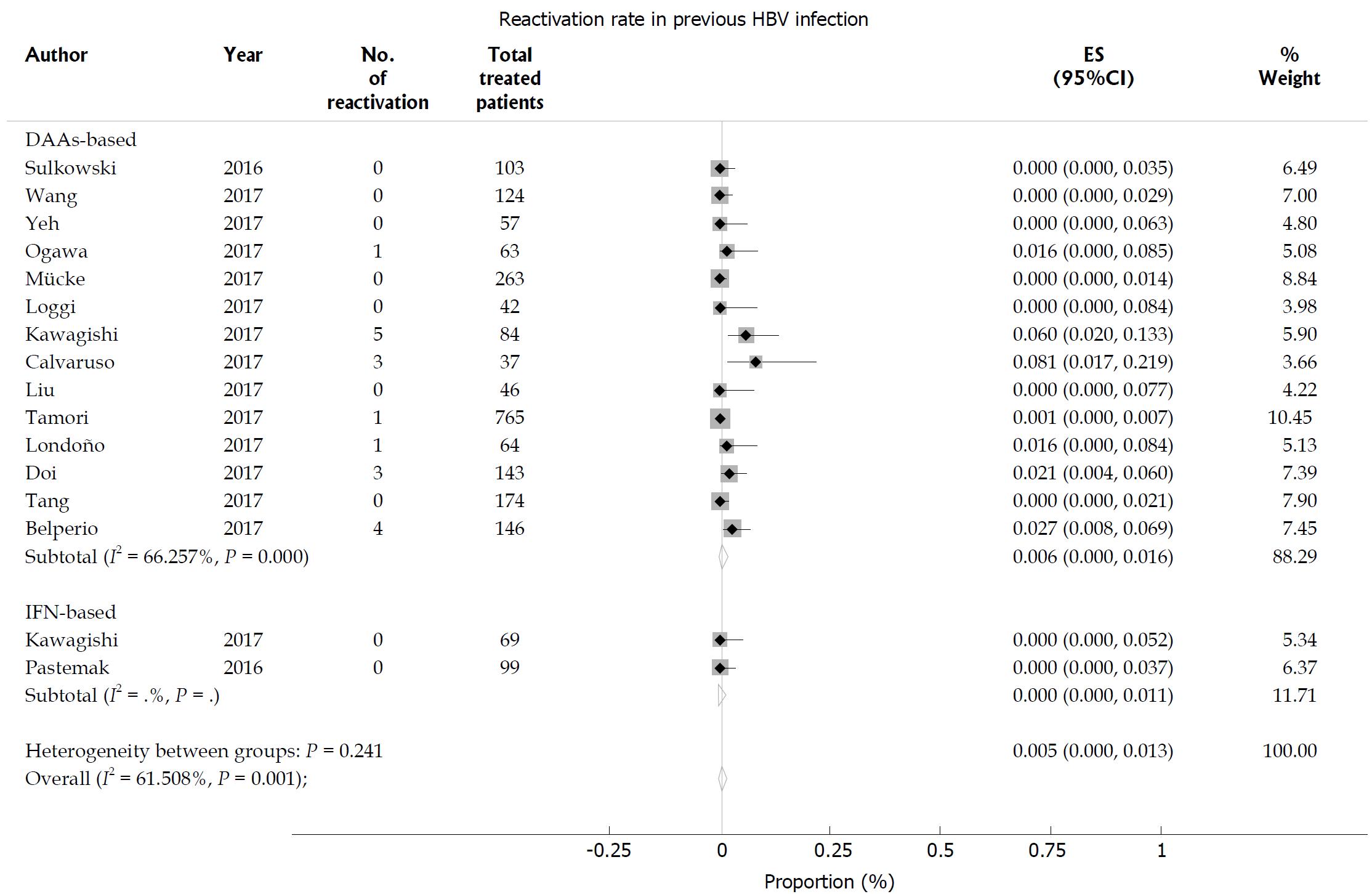
In patients with previous HBV infection, the HBV reactivation rate was 0.5% (95%CI: 0-1.3), with significant heterogeneity among the studies (I2 = 61.5%; P = 0.001; Figure 5). In the subgroup analysis, the incidence of HBV reactivation was not different between the DAA-treated group (0.6%, 95%CI: 0-1.6) and the IFN-treated group (0, 95%CI: 0-1.1; P = 0.241 for subgroup differences). No significant publication bias was found using Egger’s test (P = 0.115).
Figure 5 Hepatitis B virus reactivation in hepatitis C virus patients with previous hepatitis B virus infection.
Pooled estimates of hepatitis B virus (HBV) reactivation in hepatitis C virus patients with previous hepatitis B surface antigen-negative but HBcAb-positive HBV infection.
HBV reactivation-related hepatitis
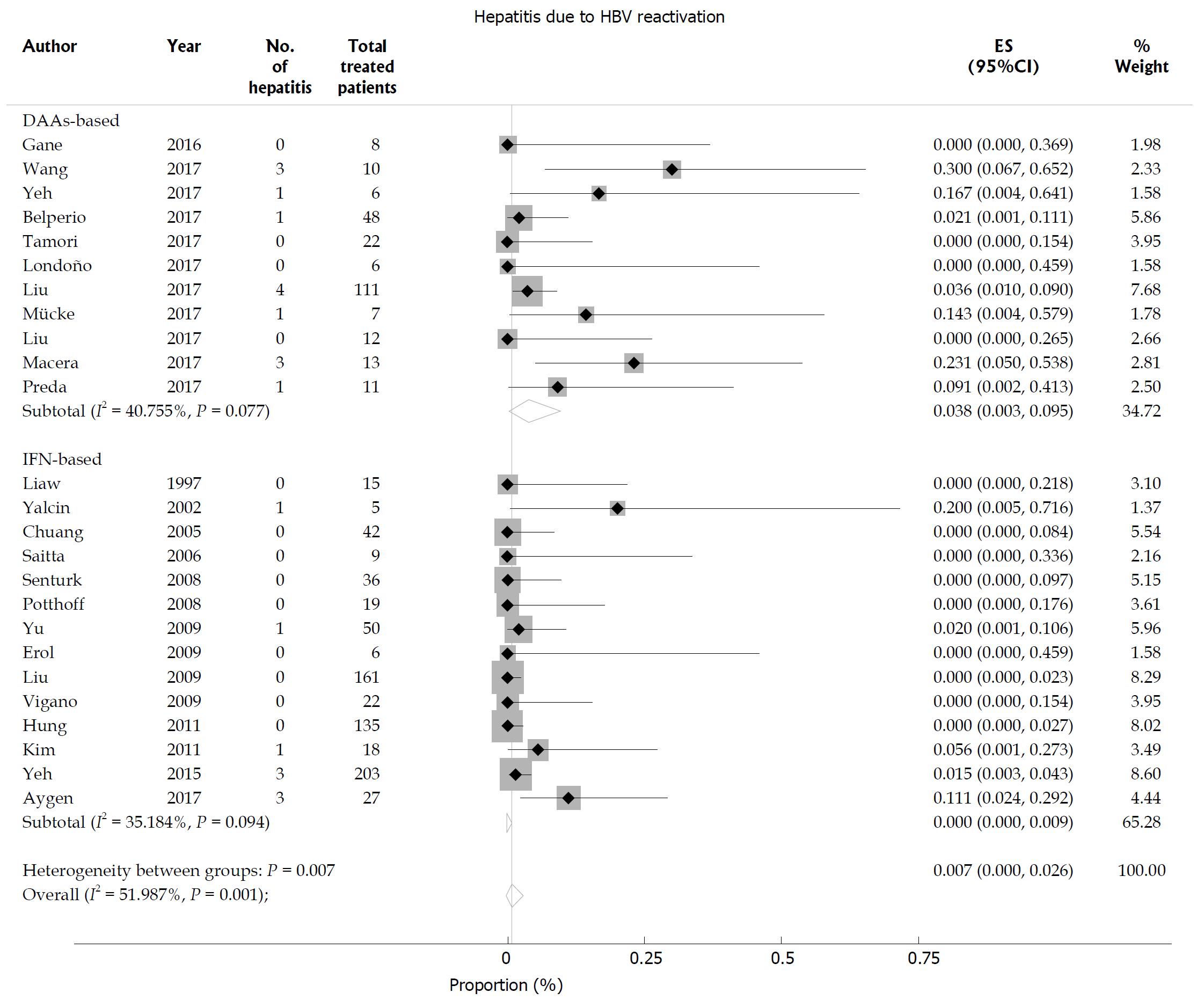
The incidence of hepatitis related to HBV reactivation was 0.7% (95%CI: 0-2.6) in HBsAg-positive patients with statistical heterogeneity among the studies (I2 = 51.9%; P = 0.001; Figure 6). Subgroup analysis revealed that HBsAg-positive patients receiving DAA therapy (3.8%, 95%CI: 0.3-9.5) had a higher rate of HBV reactivation-related hepatitis than those receiving IFN-based therapy (0, 95%CI: 0-0.9; P = 0.007 for subgroup differences). Egger’s test showed a significant publication bias (P = 0.015). None of the patients with previous HBV infection experienced hepatitis related to HBV reactivation.
Figure 6 Hepatitis B virus reactivation-related hepatitis in hepatitis C virus patients with chronic hepatitis B virus infection.
Overall risk of hepatitis B virus (HBV) reactivation-related hepatitis in hepatitis C virus patients with chronic hepatitis B surface antigen-positive HBV infection.
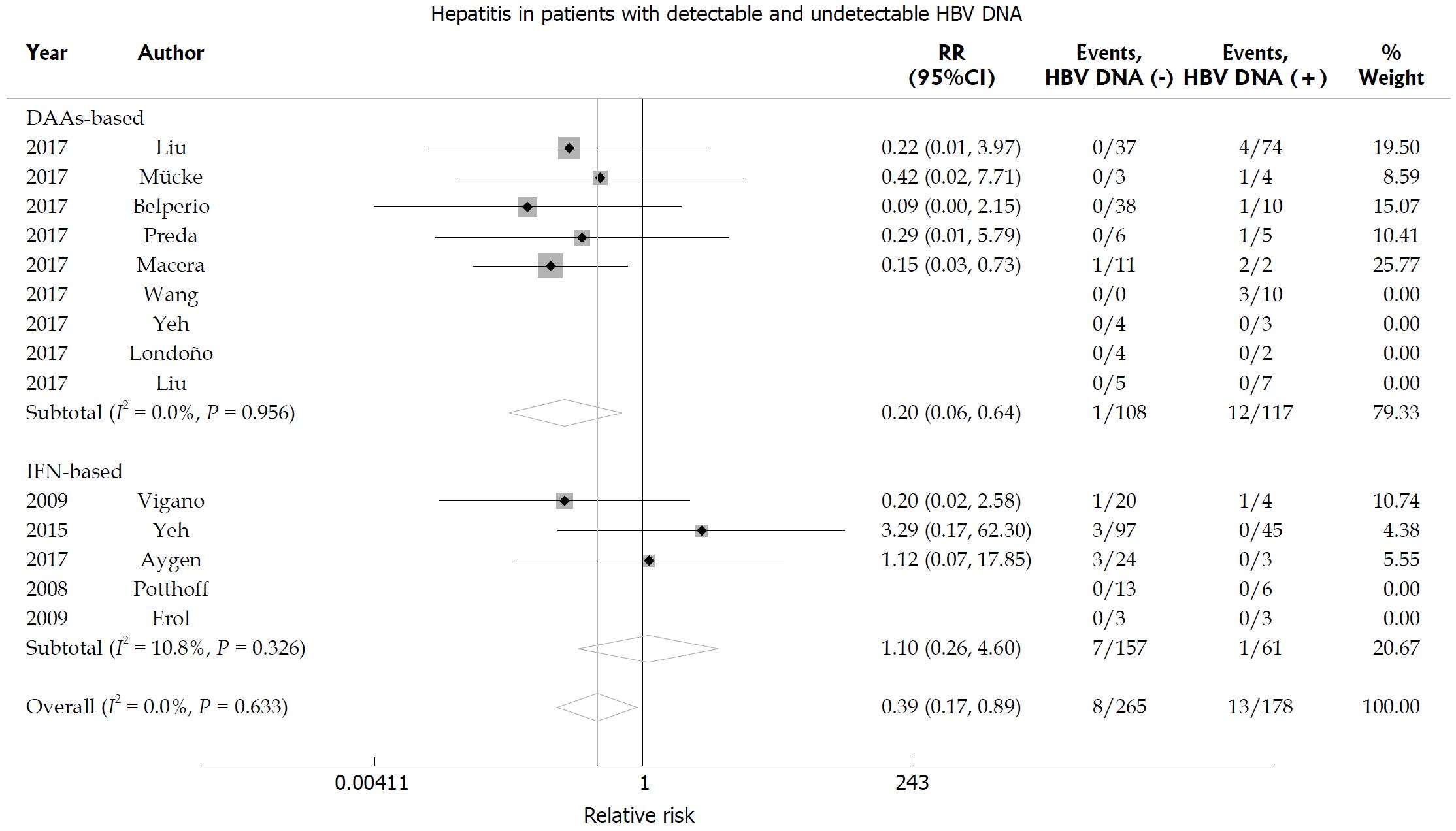
According to baseline HBV DNA level, the rate of hepatitis was lower in patients with undetectable HBV DNA than in patients with detectable HBV DNA (RR = 0.39, 95%CI: 0.17-0.89; P = 0.025; Figure 7). Subgroup analysis revealed that the rate of hepatitis was lower in patients with undetectable HBV DNA than in patients with detectable HBV DNA in the DAA-treated group (RR = 0.20, 95%CI: 0.06-0.64; P = 0.007). In addition, the incidence of hepatitis was similar in IFN-treated patients with undetectable or detectable HBV DNA (RR = 1.10, 95%CI: 0.26-4.60; P = 0.895). No significant heterogeneity was found among the studies (I2 = 0; P = 0.633).
Figure 7 Hepatitis B virus reactivation-related hepatitis according to baseline hepatitis B virus DNA level.
Relative risk of hepatitis B virus (HBV) reactivation-related hepatitis according to baseline HBV DNA level in hepatitis C virus patients with chronic hepatitis B surface antigen-positive HBV infection.
HBV reactivation with and without antiviral prophylaxis
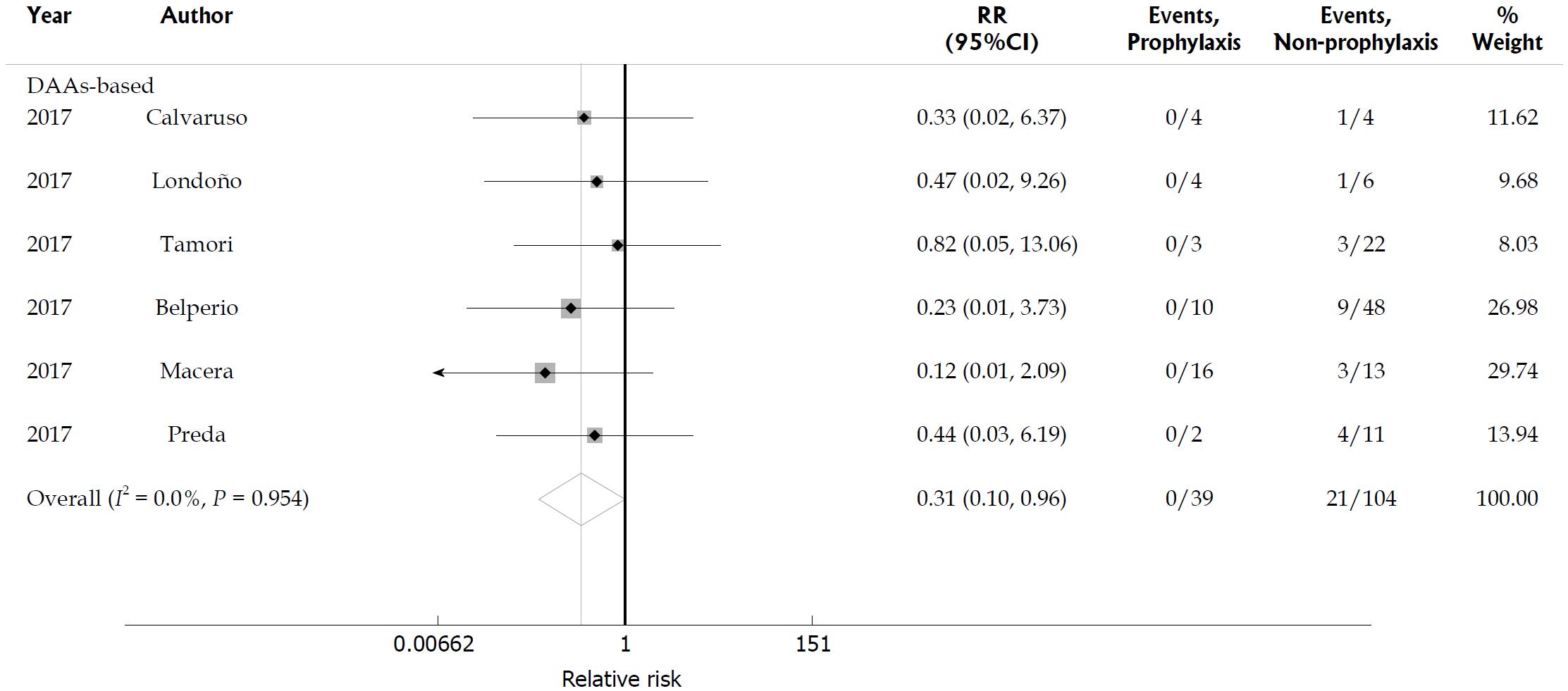
We identified six studies that compared the HBV reactivation rate in HBsAg-positive patients with and without antiviral prophylaxis during DAA-based therapy; these studies included 39 patients who received preemptive anti-HBV treatment at baseline and 104 patients who did not receive preemptive anti-HBV therapy[16,17,22,23,36,41]. None of the 39 patients receiving entecavir or tenofovir developed HBV reactivation, but 21 of the 104 HBsAg-positive patients who did not receive antiviral prophylaxis developed HBV reactivation and five patients developed HBV reactivation-related hepatitis while undergoing DAA therapy. This meta-analysis showed that preemptive anti-HBV therapy with entecavir or tenofovir significantly reduced the risk of HBV reactivation in patients receiving DAA-based treatment (RR = 0.31, 95%CI: 0.1-0.96; P = 0.042). No statistical heterogeneity was found among the included studies (I2 = 0; P = 0.954; Figure 8).
Figure 8 Hepatitis B virus reactivation in hepatitis B surface antigen-positive patients with and without antiviral prophylaxis.
Relative risk of hepatitis B virus reactivation in hepatitis B surface antigen-positive patients with antiviral prophylaxis and without prophylaxis. HBV: Hepatitis B virus.
DISCUSSION
Multiple studies have reported HBV reactivation in HCV/HBV coinfected patients treated with DAA, but the reactivation rate is unclear, and the clinical outcome can range from ALT flares to liver failure and even death[16]. We found that the HBV reactivation rate was higher in patients receiving DAA-based therapy than in those receiving IFN-based therapy. A previous study showed an HBV reactivation rate of 12.2% in DAA-treated patients, but only 18 patients treated with DAA were enrolled in the study, and the definition of HBV reactivation was vastly different[5]. The uniform definition of HBV reactivation according to the AASLD criteria was used in our study, and the HBV reactivation rate was higher than that reported by Chen et al[5]. Furthermore, our review showed that the rate of hepatitis was 3.8% in patients who received DAA-based therapy, whereas the incidence was 0 in patients who received IFN-based therapy. Although the HBV reactivation rate was not different between patients with undetectable or detectable serum HBV DNA at baseline, patients with detectable HBV DNA who received DAAs tended to have a higher proportion of HBV reactivation-related hepatitis. Wang et al[15] reported three patients who had clinically apparent HBV reactivation, and one with hepatic failure. Macera et al[16] reported five HBsAg-positive patients with hepatitis, two of whom developed hepatic failure despite rescue treatment with NUCs. In addition, the FDA reported three of twenty-nine patients with HBV reactivation who developed liver failure during treatment with DAAs[6]. HBV reactivation occurred more frequently and the clinical outcome was more severe in patients treated with DAAs. This effect may be explained as follows: HBV viral replication can be suppressed in patients with HCV infection and the rapid suppression of HCV viral load by DAA treatment may create a permissive environment for HBV replication, resulting in HBV reactivation[53]. In addition, IFN exerts an antiviral effect to suppress HBV replication and delay the time to HBV reactivation[54], but DAAs do not have any effect on the innate antiviral immune response.
Our meta-analysis revealed that preemptive anti-HBV therapy with NUCs significantly decreased the HBV reactivation rate. None of the patients receiving entecavir or tenofovir treatment experienced HBV reactivation while undergoing DAA therapy. The AASLD guideline recommends anti-HBV treatment for patients with active HBV infection[9]. However, the European Association for the Study of the Liver guideline recommends that HBsAg-positive patients treated with DAAs should receive concomitant antiviral prophylaxis at least 12 wk after DAA therapy[8]. Our study shows that preemptive anti-HBV treatment with NUCs is effective in preventing HBV reactivation in HBsAg-positive patients, especially in those with detectable HBV DNA who have a high risk of reactivation-related hepatitis.
We found that HBV reactivation was rare in patients with previous HBV infection, that the HBV reactivation rate was not different between patients receiving DAA-based treatment or IFN-based treatment, and that none of these patients experienced HBV reactivation-related hepatitis. Ogawa et al[34] reported that the anti-HBs titre may be associated with a risk of HBV reactivation, and that patients with previous HBV infection and negative anti-HBs or very low-titre anti-HBs are at risk of HBV reactivation. According to the results of our study, the HBV DNA level should be monitored during DAA therapy in patients with previous HBV infection, especially those with negative anti-HBs, even though the occurrence of HBV reactivation is rare in these patients.
There are several limitations in this meta-analysis. First, only four randomized controlled trials were included, and most of the studies were cohort studies with a lower data quality for analysis. Second, substantial heterogeneity was observed between the studies and may have caused overestimation or underestimation of the HBV reactivation risk. Third, there was an absence of unified assays for HBV DNA testing in several studies, especially in the IFN-treated group. In addition, we were unable to extract some detailed information that might have had an effect on HBV reactivation such as HBsAg level, anti-HBs titre, and HBV genotype. Further studies should be conducted to obtain relevant information about these parameters. Finally, the effect of the small number studies in our meta-analysis may have influenced the statistical accuracy of the study outcomes.
In conclusion, the present study found that HBV reactivation and hepatitis flare occurred more frequently in HBsAg-positive patients receiving DAA-based anti-HCV treatment than in patients receiving IFN-based therapy. The incidence of HBV reactivation was rare in patients with previous HBV infection. In accordance with the guideline recommendations, our study showed that preemptive anti-HBV therapy with NUCs was effective in preventing HBV reactivation in HBsAg-positive patients, especially those with detectable serum HBV DNA. In patients with previous HBV infection receiving DAA treatment for HCV, especially those with negative anti-HBs, the ALT level and HBV DNA level should be monitored. Further studies are required to assess the cost effectiveness of preemptive anti-HBV therapy in HBsAg-positive patients receiving DAA-based therapy.
ARTICLE HIGHLIGHTS
Research background
Hepatitis B virus/hepatitis C virus (HBV/HCV) dual infection is common in regions with high HBV prevalence, as HBV and HCV share a similar mode of transmission. HBV/HCV-coinfected patients tend to have more severe liver fibrosis and a higher risk of hepatocellular carcinoma than those without coinfection. HBV reactivation may occur after patients receive direct-acting antiviral agent (DAA)-based therapy or interferon (IFN)-based therapy for hepatitis C. Several studies have reported HBV reactivation but a meta-analysis to determine the proportion of HBV reactivation is still lacking.
Research motivation
Although previous studies reported HBV reactivation in patients undergoing DAA-based therapy or IFN-based therapy, the results indicate contradictory HBV reactivation rates. Moreover, the need for preemptive anti-HBV therapy remains controversial.
Research objectives
The main objectives of the systematic review and meta-analysis were to evaluate the incidence of HBV reactivation in patients receiving DAA-based therapy or IFN-based therapy for hepatitis C and the effectiveness of preemptive anti-HBV therapy for preventing HBV reactivation.
Research methods
Relevant publications were searched in the PubMed, MEDLINE and EMBASE databases with the indicated key words and subject terms. The data were extracted, and statistical analysis was conducted in Stata to assess the incidence of HBV reactivation and reactivation-related hepatitis. Significant heterogeneity was investigated using meta-regression and subgroup analyses for treatment regimen, HBV DNA level, study sample size, and race. Publication bias was tested using Egger’s test and was assessed by funnel plots.
Research results
The systematic review identified 7092 articles and enrolled 39 full articles that met the inclusion criteria. The pooled random effects overall HBV reactivation rate was 15.7% (95%CI: 10.6-21.5) in hepatitis B surface antigen (HBsAg)-positive patients. The HBV reactivation rate was higher in the DAA-treated group (21.1%, 95%CI: 15.8-26.9) than in the IFN-treated group (11.9%, 95%CI: 6.3-18.6). The incidence of hepatitis related to HBV reactivation was 0.7% (95%CI: 0-2.6) in HBsAg-positive patients and patients receiving DAA therapy (3.8%, 95%CI: 0.3-9.5) had a higher rate of HBV reactivation-related hepatitis than those receiving IFN-based therapy (0, 95%CI: 0-0.9). Preemptive anti-HBV therapy with entecavir or tenofovir significantly reduced the risk of HBV reactivation in patients receiving DAA-based treatment (RR = 0.31, 95%CI: 0.1-0.96).
Research conclusions
Our study found that HBV reactivation and hepatitis flare occurred more frequently in HBsAg-positive patients receiving DAA-based anti-HCV treatment than in patients receiving IFN-based therapy. HBV reactivation was rare in patients with previous HBV infection. Preemptive anti-HBV therapy with NUCs was effective in HBsAg-positive patients to prevent HBV reactivation, especially in those with detectable serum HBV DNA.
Research perspectives
Our study found the high risk of HBV reactivation in patients receiving DAA-based therapy for hepatitis C. Preemptive anti-HBV treatment proved to be effective in preventing HBV reactivation during DAA therapy. However, more high quality randomized controlled trials are needed to assess the risk of HBV reactivation in patients with HBV/HCV coinfection. Further investigation is required to assess the cost effectiveness of treating HBV/HCV-coinfected patients with NUCs prior to initiating DAA therapy.