Published online Jul 21, 2018. doi: 10.3748/wjg.v24.i27.3030
Peer-review started: April 14, 2018
First decision: May 21, 2018
Revised: June 15, 2018
Accepted: June 25, 2018
Article in press: June 25, 2018
Published online: July 21, 2018
Processing time: 96 Days and 15.7 Hours
To evaluate the long-term efficacy of endoscopic resection (ER) for small (≤ 4.0 cm) gastric gastrointestinal stromal tumors (GISTs) originating from the muscularis propria layer.
Between June 2005 and February 2015, we retrospectively analyzed 229 consecutive patients with gastric MP-GISTs who underwent ER with a follow-up at least 36 mo. The main outcome measurements included complete resection rate, complications, and long-term follow-up outcomes.
ER included endoscopic muscularis excavation in 179 cases, endoscopic full-thickness resection in 32 cases, and submucosal tunneling endoscopic resection in 18 cases. The median size of GISTs was 1.90 cm. Of the 229 GISTs, 147 were very low risk, 72 were low risk, 8 were intermediate risk, and 2 were high risk. Short-term outcomes showed the complete resection rate was 96.5%, and 8 patients (3.5%) had complications. Of the 8 patients with complications, only one patient required surgical intervention. Long-term outcomes showed 225 patients were actively followed-up until composition of this manuscript. The remaining 4 patients were lost because of unrelated death. During the follow-up period (median, 57 mo), no residual, recurrent lesions, or distant metastasis were detected in any patients. Binary logistic regression analysis showed tumor size was a risk factor associated with a high mitotic index (≥ 5/50 HPF) of GISTs (P = 0.002).
ER seems to be an effective and safe method for gastric MP-GISTs ≤ 4.0 cm, and, for some intermediate or high risk GISTs, adjuvant therapy and/or additional surgery might be required to reduce the risk of recurrence or metastasis.
Core tip: The long-term effectiveness of endoscopic resection for small (≤ 4.0 cm) gastric gastrointestinal stromal tumors originating from the muscularis propria layer (MP-GISTs) still remains debatable. In our study, we included a larger sample size with a longer follow-up period to assess the long-term safety and efficacy of ER for gastric MP-GIST. Long-term outcomes showed 225 patients were actively followed-up until composition of this manuscript. The remaining 4 patients were lost because of unrelated death. During the follow-up period (median, 57 mo), no residual, recurrent lesions, or distant metastasis were detected in any patients. Endoscopic resection seems to be an effective and safe method for gastric MP-GISTs ≤ 4.0 cm, and, for some intermediate or high risk GISTs, adjuvant therapy and/or additional surgery might be required to reduce the risk of recurrence or metastasis.
- Citation: Zhang Y, Mao XL, Zhou XB, Yang H, Zhu LH, Chen G, Ye LP. Long-term outcomes of endoscopic resection for small (≤ 4.0 cm) gastric gastrointestinal stromal tumors originating from the muscularis propria layer. World J Gastroenterol 2018; 24(27): 3030-3037
- URL: https://www.wjgnet.com/1007-9327/full/v24/i27/3030.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i27.3030
Recently, endoscopic resection (ER) has become one of the prevailing treatments for small (≤ 4.0 cm) gastric gastrointestinal stromal tumors originating from the muscularis propria layer (MP-GISTs), in many countries[1-6]. However, its long-term effectiveness still remains debatable. Some surgeons have posited that ER can result in insufficient margins that would likely increase the risk of tumor recurrence or implantation metastasis. Thus, laparoscopic wedge resection (LWR) is still recommended for treating small gastric MP- GISTs[7-9]. Nevertheless, LWR is associated with a number of serious long-term complications. Especially when the tumor is located near or in the gastric cardia or pylorus, resection of the gastric cardia or pylorus might lead to irreparable damage to the cardioesophageal sphincter or pylori sphincter, leaving patients prone to certain diseases associated with digestive fluid reflux[7,9,10].
Although recurrent tumors or implantation metastasis events were extremely rare according to the available literature where ER has been used for small gastric MP-GISTs, such study series were relatively small or only had a short follow-up period of less than 36 mo[2,11-14]. Since June 2005, our endoscopy center has been using ER for small (≤ 4.0 cm) upper gastrointestinal subepithelial tumors originating from the muscularis propria layer (MP-SETs). Up to December 2017, we had completed 1021 cases of ER for small (≤ 4.0 cm) upper gastrointestinal MP-SETs. Within those 1021 cases, we selected 229 consecutive patients who had gastric MP-GISTs less than 4.0 cm with at least 36 mo of follow-up after ER, and demonstrated the long-term safety and efficacy of ER for this type of tumor.
This retrospective cohort study was approved by the Ethics Committee of Taizhou Hospital, Wenzhou Medical College. Data from patients with gastric MP-GISTs who underwent ER were reviewed between June 2005 and February 2015 at the Information Systems Department of Taizhou Hospital of Zhejiang Province. Patients were included if they met all of the following criteria: (1) The patient had a gastric MP-GIST that was diagnosed histopathologically as a GIST after ER; (2) the tumors were 1.0-4.0 cm in size and did not exhibit bleeding, ulceration, or scarring; and (3) the patient had no evidence of lymph node metastasis assessed by endoscopic ultrasonography (EUS) or computed tomography (CT) examination preoperatively and also did not have other malignant tumors. According to the inclusion criteria, 229 consecutive patients with gastric MP-GISTs were included. Before the endoscopic procedure, informed consent was obtained from all patients in accordance with the institutional protocol.
ER included endoscopic muscularis excavation (EME), endoscopic full-thickness resection (EFTR), and submucosal tunneling endoscopic resection (STER). Between June 2005 and July 2011, patients with gastric MP-GISTs usually were treated by EME. If the tumor had the characteristic of extensive connection to the underlying MP or serosal layer, EFTR was performed to resect it completely. Since August 2011, if the tumor was located in the cardia, the proximal body, or fundus of the stomach near the cardia where a submucosal tunnel can be established, we applied the STER technique to resect it[1].
All ER procedures were performed under general anesthesia by a highly skilled endoscopist (LPY) in the operating room. The basic equipment and accessories included a single-channel endoscope (Q-260 J, Olympus) and/or a dual-channel endoscope (GIF-2T240, Olympus), an electrosurgical generator (ICC 200; ERBE, Tübingen, Germany), argon plasma coagulation (APC 300, ERBE), and a carbon dioxide insufflator (Olympus Optical).
When performing EME, injection solution (100 mL saline plus 2 mL indigo carmine and 1 mL epinephrine) was used to make a submucosal elevation after placing marking several dots around the tumor. A cross or circumferential mucosal incision was made to reveal the lesion with a hook knife (KD-620LR; Olympus), and then a circumferential resection was performed along the edge of the lesion until the lesion was completely excavated from the muscularis propria (MP) layer with an insulated-tip knife (KD-611L, IT2; Olympus) or a hybrid knife (ERBE)[15].
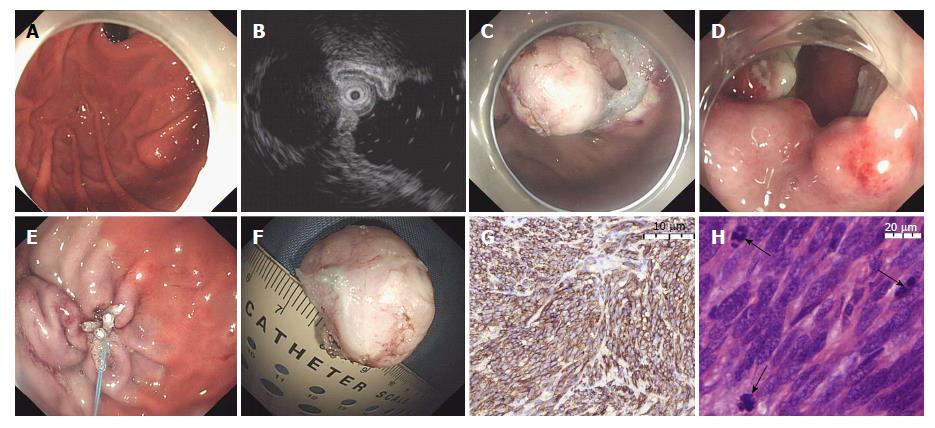
When performing EFTR (Figure 1), several dots and a submucosal elevation were made similar to the EME procedure, and then a circumferential incision was made along the marking dots until the intraluminal side of the lesion was fully revealed. Subsequently, a small puncture was first made in the seromuscular layer of the lesion with an insulated-tip knife (KD-611L, IT2; Olympus), and then the resection was continuously performed along the puncture. A snare resection was made to completely remove the lesion after three-quarters of the circumference of the tumor was resected. Finally, the gastric wall defect was closed with several clips and an endoloop or an over-the-scope clip (OTSC) after tumor removal[15].
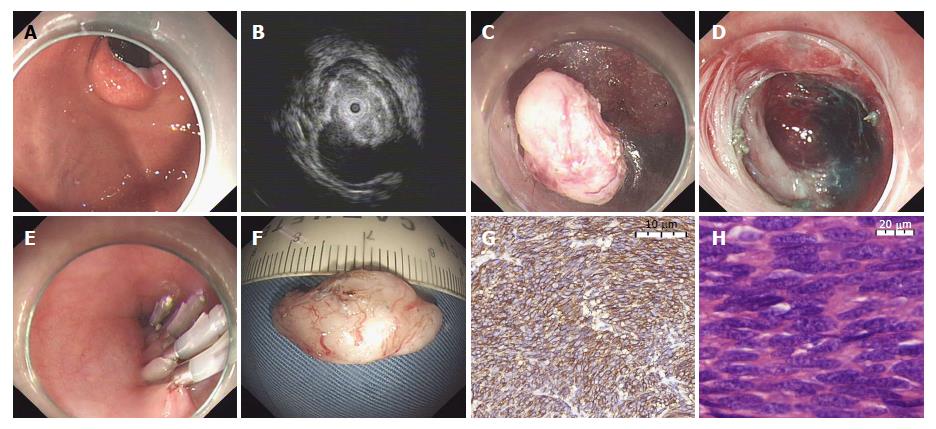
When performing STER (Figure 2), the lesion was first marked by submucosal injection of methylene blue, and then a 2 cm longitudinal mucosal incision was made 5 cm above the lesion with a hook knife (KD-620LR, Olympus), which was used as the entry point. Subsequently, a submucosal tunnel was created between the submucosal and muscular layers to the lesion using a hybrid knife, and the lesion was excavated from the MP layer through the submucosal tunnel until complete lesion separation. Lastly, the mucosal incision site was closed with clips after adequate hemostasis and tumor removal[15].
The main measurements of this study were divided into two parts: short-term outcomes and long-term outcomes. Short-term outcomes included complete resection and complications. Complete resection refers to tumors removed en bloc with no apparent residual tumor at the resection site and with negative margins upon pathologic examination[1]. In this study, complications were identified as perioperative bleeding, delayed bleeding, localized peritonitis, or perforation requiring surgical intervention. Perforation that did not require surgical intervention and could be closed by endoscopic methods was not considered a complication[2]. Long-term outcomes included rates of local recurrence and distant metastasis in patients who were followed up for more than 3 years. Local recurrence was defined as the identification of a lesion located on or adjacent to the scar of the previous ER, which was subsequently confirmed via biospy[1].
All 229 patients were included in a prospectively maintained database at the Medical Information Centre of Taizhou Hospital of Zhejiang Province. Every patient with a gastric MP-GIST was examined with gastroscopy to check for wound healing at 3 mo and 6 mo after ER, and every patient also underwent EUS to check for residual lesions at 3 mo. Subsequently, gastroscopy and/or EUS was performed to look for local recurrent lesions, and abdominal ultrascan and/or CT were used to check distant metastasis every year, indefinitely.
Data were analyzed using SPSS 20.0 software (SPSS Inc., Chicago, IL, United States). For descriptive statistics, the mean was used in the case of a normal distribution of variables, whereas the median (interquartile range) was used for variables with a skewed distribution. Binary logistic regression analysis was used to test for effect associations among independent variables and a high mitotic index (5/50 HPF) of GISTs. A P value < 0.05 was considered statistically significant.
A total of 229 consecutive patients with gastric MP-GISTs were included in this study (143 females, 86 males; mean age 54.9 ± 10.8 years old). As displayed in Table 1, 29 GISTs were located in the gastric cardia, 118 in the gastric fundus, 72 in the gastric body, and 10 in the antrum. The median size of GIST specimens was 1.90 cm (interquartile range 1.55-2.40 cm). All 229 GISTs had a low-level echo, including a homogeneous echo in 197 cases and an inhomogeneous echo in 32 cases.
| Parameters | Endoscopic resection (n = 229) |
| Age (yr), mean ± SD | 54.9 ± 10.8 |
| Gender | |
| Male | 86 |
| Female | 143 |
| Tumor size (cm), median (interquartile range) | 1.90 (1.55-2.40) |
| Tumor location | |
| Cardia | 29 (12.7) |
| Fundus | 118 (51.5) |
| Body | 72 (32.1) |
| Antrum | 10 (3.3) |
| Tumor growth pattern | |
| Intraluminal growth | 178 (77.7) |
| Extraluminal growth | 51 (22.3) |
| EUS characteristics | |
| Homogeneous echo | 197 (86.0) |
| Inhomogeneous echo | 32 (14.0) |
| Mitotic index | |
| < 5/50 HPF | 219 (95.6) |
| 5-10/50 HPF | 8 ( 3.5) |
| ≥ 10/50 HPF | 2 ( 0.9) |
| NIH risk classification | |
| Very low | 147 (64.2) |
| Low risk | 72 (31.4) |
| Intermediate risk | 8 ( 3.5) |
| High risk | 2 ( 0.9) |
219 out of 229 GISTs had a mitotic count of < 5 per 50 high-power fields, 8 out of 229 GISTs had a count of 5-10 per 50 high-power fields, and 2 out of 229 GISTs had a count of ≥ 10 per 50 high-power fields. According to the NCCN guidelines[16], 147 GISTs were very low risk, 72 were low risk, 8 were intermediate risk, and 2 were high risk.
In this study, 179 GISTs were treated with EME, 32 GISTs were treated with EFTR, and 18 GISTs were treated with STER. The mean time of ER procedure was 52.8 ± 16.1 min. Complete resection by ER was achieved in 221 lesions (96.5%). Among the other 8 GISTs without complete resection, 3 GISTs were resected in one piece during EME technique, but the tumor margin could not be evaluated definitively because of electrocautery, and the other 5 GISTs were resected piecemeal during the EME procedure. Of the 8 GISTs, 5 were located in the gastric fundus and 3 in the gastric body. The size of these GISTs in diameter was ranged 2.7 cm to 3.6 cm. According to the NCCN guidelines, these 8 GISTs were all low risk. Because those patients were unwilling to accept the potential risk of the surgery, further surgical resection was not performed after pathological examination. The median time from endoscopic treatment to a no-residue diet was 4 d (range 1-10 d, interquartile range 3-6 d). The median length of hospital stay after ER was 5 d (range 1-14 d, interquartile range 3-7 d).
In this study, 8 patients had complications (3.5%, 8/229), including 5 patients with perioperative bleeding (2.2%, 5/229), 2 patients with localized peritonitis (0.9%, 2/229), and one patient with delayed bleeding (0.4%, 1/229). Of the 8 patients with complications, only one patient with perioperative bleeding was converted to laparoscopic surgery (0.4%), and the other 7 patients were managed successfully by endoscopic methods and conservative treatment (gastrointestinal decompression, the intravenous infusion of antibiotics and esomeprazole). No patients had other serious complications.
In this study, 225 out of 229 patients with gastric MP-GISTs were actively followed-up until composition of this manuscript. The remaining 4 patients were lost to follow-up because of unrelated death. Two patients with very low risk GISTs died of cordis and cerebral accident at 53 and 86 mo post-endoscopic procedure, respectively. Another patient with a low risk GIST died of pneumonia at 47 mo, and the last patient with a very low risk GIST died from traffic-accident at 59 mo.
The median follow-up period after ER was 57 mo (range 36-132 mo; interquartile range 46-71 mo. In this study, 2 patients with high-risk GISTs took imatinib mesylate to prevent recurrence or metastasis, whereas the other 8 patients with intermediate-risk GISTs were unable to take imatinib mesylate because they were unable afford the medication. During the follow-up period, no residual, recurrent lesions, or distant metastasis were detected in any patients, including 8 patients without complete resection. Furthermore, all 229 patients who underwent ER maintained their previous quality of life.
Risk factors analyzed included: Age (≤ 40 years old, 41-60 years old, 60 years old), gender (male, female), tumor size (< 2.0, 2.0-3.0, and ≥ 3.0 cm), tumor location (antrum with an OR value defined as 1, compared with body, fundus, and cardia), and tumor growth pattern (intraluminal, extraluminal). Among the analyzed factors, tumor size was the only risk factor associated with a high mitotic index (≥ 5/50 HPF) of GISTs (OR = 6.675; 95%CI: 2.047-21.771; P = 0.002; Table 2).
| High mitotic index (> 5/50 HPF) | |||
| Odds ratio | 95%CI | P value | |
| Age, yr (≤ 40, 40 to ≤ 60, and > 60) | - | - | 0.756 |
| Gender (male, female) | - | - | 0.982 |
| Tumor size, cm (< 2.0, 2.0-3.0, and ≥ 3.0) | 6.675 | 2.047-21.771 | 0.002 |
| Tumor location (cardia, fundus, body, and antrum) | - | - | 0.505 |
| Tumor growth pattern (intraluminal, extraluminal) | - | - | 0.069 |
Gastric MP-GISTs are among the most common gastric MP-SETs, which account for about 13.0%-71.4% of all gastric SETs[1,5,10,17,18]. A proportion of gastric MP-GISTs are known to possess some degree of malignant potential, which usually cannot be diagnosed accurately before surgical or endoscopic removal. Resection is often recommended because it not only provides an accurate diagnosis, but also may be curative if the lesion is removed completely[10,15]. Currently, ER is being increasingly used for gastric MP-GIST removal. However, additional evidence is required to support the long-term effectiveness of ER for the treatment of gastric GISTs. Compared with the published studies, our study included a larger sample size with a longer follow-up period to assess the long-term safety and efficacy of ER for gastric MP-GIST, which would increase the evidence and support for the use of ER to treat/remove gastric MP-GISTs.
Short-term outcomes of this study showed complete resection by ER was achieved in 221 lesions (96.5%), and complications occurred in 8 patients (3.5%), including 5 patients with perioperative bleeding (2.2%), 2 patients with localized peritonitis (0.9%), and one patient with delayed bleeding (0.4%, 1/229). Among the 8 patients with complications, only one patient with perioperative bleeding required a conversion to laparoscopic surgery. No patients had other serious complications. These short-term outcomes were consistent with previously published findings from a number studies evaluating the use of ER for the treatment of gastric GISTs[2,4,11,12,14]. According to these short-term outcomes, ER is a feasible treatment for gastric MP-GISTs.
The risk of local recurrence associated with ER for gastric GISTs was a major concern of some surgeons. Recently, a number of studies using ER to treat gastric GISTs have shown that the recurrence rate ranged from 0 to 6.7% (Table 3)[2-4,11-14,19]. In 3 of these studies with a mean/median follow-up period of ≥ 36 mo, the recurrence rate ranged between 2.2% and 6.7%[3,4,19]. In our study, no patient experienced a local recurrence or distant metastasis during a median follow-up period of 57 mo. Some differences in the rate of recurrence might be explained by the single-center retrospective design, different inclusion criteria applied in different studies, different pathologic risk grades of gastric GISTs, and different ER methods performed in different endoscopic centers.
| Author | Publication year | Study design | Case n | Endoscopic procedure | Tumor size, mean ± SD (cm) | Pathologic risk grade, n | Complete resection n (%) | surgical intervention n (%) | Recurrence n (%) | Follow-up (mo) |
| Feng et al[14] | 2015 | RS | 50 | Endoscopic resection | < 2 | MI ≤ 5, 41 MI > 5, 9 | NA | 1 (2.0) | 0 | 321 (12-65) |
| Shen et al[13] | 2015 | RS | 32 | ER | 1.70 ± 0.36 | Very low, 9 low, 18 intermediate, 3 high, 2 | 32 (100) | 1 (3.1) | 1 (3.1) | 31.51 (2-53) |
| Joo et al[4] | 2016 | RS | 90 | ESD, 72 STER, 8 EMR, 7 EFTR, 2 Polypectomy, 1 | 2.3 ± 1.2 | Very low, 45 Low, 28 Intermediate, 1 High, 6 | 88 (97.8) | 5 (5.6) | 2 (2.2) | 46.0 ± 28.5 |
| Tan et al[21] | 2017 | RS | 52 | STER, 20 EFTR, 32 | STER, 17.8 ± 7.2; EFTR, 15.4 ± 6.6 | Low, 26 Intermediate, 26 | En bloc 50 (96.2) | 1 (1.9) | 1 (1.9) | 10.9 ± 7.8 23.8 ± 18.6 |
| An et al[11] | 2017 | RS | 168 | ESD | 1.51 (0.5-6) | Very low, 117 Low, 37 Intermediate, 14 | En bloc 168 (100) | 0 | 0 | 251 (6-67) |
| Balde et al[18] | 2017 | RS | 30 | ESD | 1.51 (1.0-1.8) | Very low, 22 Low, 4 Intermediate, 4 | 27 (90.0) | NA | 2 (6.7) | 57.9 (± 28.9) |
| Meng et al[19] | 2017 | RS | 75 | ESD | 1.44 ± 0.67 | NA | NA | NA | 2 (2.7) | 3.3 yr1 1-7 yr |
| Andalib et al[2] | 2018 | RS | 12 | EN, 5 EFTR, 7 | 2.41 (1.0 - 5.0) | Low, 11 Intermediate, 1 | 11 (91.7) | 0 | 0 | 121 (6.5-24) |
| This study | - | RS | 229 | EME, 179 EFTR, 32 STER, 18 | 1.901 (1.0-4.0) | Very low, 147 Low, 72 Intermediate, 8 High, 2 | 221 (96.5) | 1 | 0 | 571 (46-71) |
Complete resection might be a key factor associated with a low recurrence rate in patients with gastric GISTs. Our experience of complete resection for gastric MP-GISTs can be concluded as follows. First, the tumor size of gastric MP-GISTs might be no more than 4.0 cm in diameter. When the tumor size is > 4 cm in diameter, it is very difficult to remove the tumor en bloc with an endoscopic approach, because of the limitations of the cardia and esophagus space[1,2]. Meanwhile, larger tumor size is associated with certain disadvantages, such as a narrower endoscopic view, higher complication rate, and longer endoscopic resection time. Therefore, in our endoscopy center, surgical resection still is the first choice for patients with gastric GISTs > 4 cm. Second, the tumor capsule must be kept intact during ER. Submucosal injection solution was repeatedly injected into the surrounding tissue, which allowed for the differentiation of the MP layer from the tumor mass, which avoided tumor capsule rupture when making a circular resection around the lesion. Third, the tumor should be assessed by EUS and CT before ER. For some lesions with high risk features (irregular border, cystic spaces, echogenic foci, and internal heterogeneity) identified on EUS or metastasis confirmed by CT, ER is absolutely contraindicated. Finally, EFTR is recommended when the gastric MP-GIST has extraluminal growth or is tightly connected to the underlying MP or serosal layer.
According to several previous studies, the mitotic index is an important prognostic factor for GISTs[4,13,20]. Several previous studies reported that even small GISTs (< 2.0 cm) have malignant potential with a high mitotic index[4,13]. In this study, we found 8 out of 229 GISTs had an index of 5-10 per 50 high-power fields, and 2 out of 229 GISTs had an index of ≥ 10 per 50 high-power fields. Subsequently, we analyzed the risk factors associated with the high mitotic index (≥ 5/50 HPF) and found that tumor size was significantly and independently related to a high mitotic index (OR = 6.675, P = 0.002). Thus, tumor size is still another important factor associated with the malignant potential of small gastric GISTs, which might affect the long term prognosis of patients with gastric GISTs. For some GISTS, classified as intermediate or high risk, adjuvant therapy (imatinib mesylate, etc.) and/or additional surgery are recommended to reduce the risk of recurrence after ER[21,22].
However, this study had a few limitations. First, this is a single-center, retrospective cohort study, and a selection bias may be present, even though the data were collected from a prospectively maintained database. Second, the lack of randomization might be another factor contributing to selection bias. Third, 4 out of 229 patients discontinued follow-up because of unrelated death. Finally, our institution is a tertiary endoscopic center in Zhejiang Province, and all endoscopic operations were performed by an experienced endoscopist. Thus, the results in this study might not apply to all centers. Therefore, a randomized, controlled, multicenter study is needed to evaluate the safety of ER for small (≤ 4.0 cm) gastric GISTs.
In conclusion, ER might be an effective and safe therapeutic method for patients with gastric MP-GISTs ≤ 4.0 cm, and for some patients with intermediate or high risk GISTs, adjuvant therapy (imatinib mesylate, etc.) and/or additional surgery might be required to reduce the risk of recurrence.
The long-term effectiveness of endoscopic resection for small gastric gastrointestinal stromal tumors originating from the muscularis propria layer (MP-GISTs) still remains debatable.
A larger sample size with a longer follow-up period were included to assess the long-term safety and efficacy of ER for gastric MP-GIST
Evaluate the long-term efficacy of ER for small gastric gastrointestinal stromal tumors originating from the muscularis propria layer.
We retrospectively analyzed 229 consecutive patients with gastric MP-GISTs who underwent ER with a follow-up at least 36 mo, between June 2005 and February 2015. The main outcome measurements included complete resection rate, complications, and long-term follow-up outcomes.
The outcomes showed that 225 patients were actively followed-up. The remaining 4 patients were lost because of unrelated death. During the follow-up period, no residual, recurrent lesions, or distant metastasis were detected in any patients.
Endoscopic resection seems to be an effective and safe method for gastric MP-GISTs ≤ 4.0 cm, and for some intermediate or high risk GISTs, adjuvant therapy might be required to reduce the risk of recurrence or metastasis.
Endoscopic resection might be an effective and safe therapeutic method for patients with gastric MP-GISTs ≤ 4.0 cm, and for some patients with intermediate or high risk GISTs, adjuvant therapy (imatinib mesylate, etc.) and/or additional surgery might be required to reduce the risk of recurrence.
| 1. | Ye LP, Zhang Y, Luo DH, Mao XL, Zheng HH, Zhou XB, Zhu LH. Safety of Endoscopic Resection for Upper Gastrointestinal Subepithelial Tumors Originating from the Muscularis Propria Layer: An Analysis of 733 Tumors. Am J Gastroenterol. 2016;111:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Andalib I, Yeoun D, Reddy R, Xie S, Iqbal S. Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: methods and feasibility data. Surg Endosc. 2018;32:1787-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Balde AI, Chen T, Hu Y, Redondo N JD, Liu H, Gong W, Yu J, Zhen L, Li G. Safety analysis of laparoscopic endoscopic cooperative surgery versus endoscopic submucosal dissection for selected gastric gastrointestinal stromal tumors: a propensity score-matched study. Surg Endosc. 2017;31:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 4. | Joo MK, Park JJ, Kim H, Koh JS, Lee BJ, Chun HJ, Lee SW, Jang YJ, Mok YJ, Bak YT. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc. 2016;83:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 5. | Huang LY, Cui J, Lin SJ, Zhang B, Wu CR. Endoscopic full-thickness resection for gastric submucosal tumors arising from the muscularis propria layer. World J Gastroenterol. 2014;20:13981-13986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Schmidt A, Bauder M, Riecken B, von Renteln D, Muehleisen H, Caca K. Endoscopic full-thickness resection of gastric subepithelial tumors: a single-center series. Endoscopy. 2015;47:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Kim DJ, Lee JH, Kim W. Laparoscopic resection for 125 gastroduodenal submucosal tumors. Ann Surg Treat Res. 2014;86:199-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Dressler JA, Palazzo F, Berger AC, Stake S, Chaudhary A, Chojnacki KA, Rosato EL, Pucci MJ. Long-term functional outcomes of laparoscopic resection for gastric gastrointestinal stromal tumors. Surg Endosc. 2016;30:1592-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Cui JX, Gao YH, Xi HQ, Cai AZ, Zhang KC, Li JY, Wei B, Chen L. Comparison between laparoscopic and open surgery for large gastrointestinal stromal tumors: A meta-analysis. World J Gastrointest Oncol. 2018;10:48-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Li QL, Yao LQ, Zhou PH, Xu MD, Chen SY, Zhong YS, Zhang YQ, Chen WF, Ma LL, Qin WZ. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc. 2012;75:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | An W, Sun PB, Gao J, Jiang F, Liu F, Chen J, Wang D, Li ZS, Shi XG. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc. 2017;31:4522-4531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Tan Y, Tang X, Guo T, Peng D, Tang Y, Duan T, Wang X, Lv L, Huo J, Liu D. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc. 2017;31:3376-3382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Shen C, Chen H, Yin Y, Chen J, Han L, Zhang B, Chen Z, Chen J. Endoscopic versus open resection for small gastric gastrointestinal stromal tumors: safety and outcomes. Medicine (Baltimore). 2015;94:e376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Feng F, Liu Z, Zhang X, Guo M, Xu G, Ren G, Hong L, Sun L, Yang J, Zhang H. Comparison of Endoscopic and Open Resection for Small Gastric Gastrointestinal Stromal Tumor. Transl Oncol. 2015;8:504-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Ye LP, Mao XL. Endoscopic treatments for small gastric subepithelial tumors originating from muscularis propria layer. World J Gastroenterol. 2015;21:9503-9511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1-41; quiz S42-4. [PubMed] |
| 17. | He Z, Sun C, Wang J, Zheng Z, Yu Q, Wang T, Chen X, Liu W, Wang B. Efficacy and safety of endoscopic submucosal dissection in treating gastric subepithelial tumors originating in the muscularis propria layer: a single-center study of 144 cases. Scand J Gastroenterol. 2013;48:1466-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, Ławniczak M, Starzyńska T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc. 2012;75:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 19. | Meng Y, Li W, Han L, Zhang Q, Gong W, Cai J, Li A, Yan Q, Lai Q, Yu J. Long-term outcomes of endoscopic submucosal dissection versus laparoscopic resection for gastric stromal tumors less than 2 cm. J Gastroenterol Hepatol. 2017;32:1693-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Jeong IH, Kim JH, Lee SR, Kim JH, Hwang JC, Shin SJ, Lee KM, Hur H, Han SU. Minimally invasive treatment of gastric gastrointestinal stromal tumors: laparoscopic and endoscopic approach. Surg Laparosc Endosc Percutan Tech. 2012;22:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Tan Y, Tan L, Lu J, Huo J, Liu D. Endoscopic resection of gastric gastrointestinal stromal tumors. Transl Gastroenterol Hepatol. 2017;2:115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Zhang Q, Gao LQ, Han ZL, Li XF, Wang LH, Liu SD. Effectiveness and safety of endoscopic resection for gastric GISTs: a systematic review. Minim Invasive Ther Allied Technol. 2018;27:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Arigami T, Chiu CC, Piccinni G, Rodrigo L, Shida A S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y