Published online Jul 21, 2018. doi: 10.3748/wjg.v24.i27.3006
Peer-review started: May 4, 2018
First decision: May 17, 2018
Revised: June 5, 2018
Accepted: June 25, 2018
Article in press: June 25, 2018
Published online: July 21, 2018
Processing time: 77 Days and 17.1 Hours
To evaluate the efficacy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of pancreatic head cancer when pushing (push method) or pulling the echoendoscope (pull method).
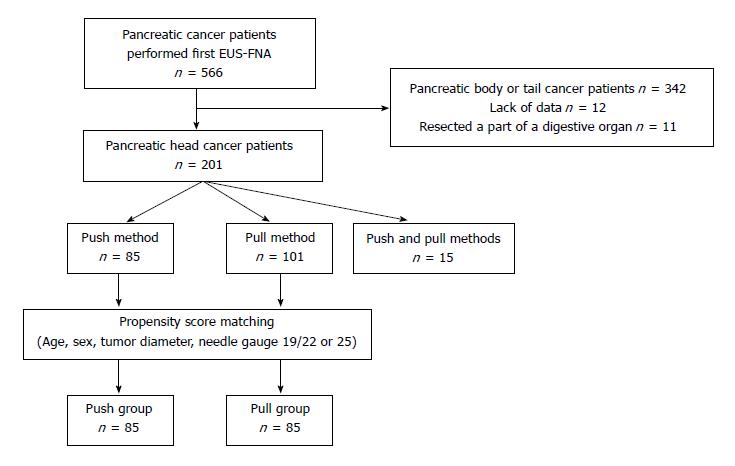
Overall, 566 pancreatic cancer patients had their first EUS-FNA between February 2001 and December 2017. Among them, 201 who underwent EUS-FNA for pancreatic head lesions were included in this study. EUS-FNA was performed by the push method in 85 patients, the pull method in 101 patients and both the push and pull methods in 15 patients. After propensity score matching (age, sex, tumor diameter, and FNA needle), 85 patients each were stratified into the push and pull groups. Patient characteristics and EUS-FNA-related factors were compared between the two groups.
Patient characteristics were not significantly different between the two groups. The distance to lesion was significantly longer in the push group than in the pull group (13.9 ± 4.9 mm vs 7.0 ± 4.9 mm, P < 0.01). The push method was a significant factor influencing the distance to lesion (≥ median 10 mm) (P < 0.01). Additionally, tumor diameter ≥ 25 mm (OR = 1.91, 95%CI: 1.02-3.58, P = 0.043) and the push method (OR = 1.91, 95%CI: 1.03-3.55, P = 0.04) were significant factors contributing to the histological diagnosis of malignancy.
The pull method shortened the distance between the endoscope and the lesion and facilitated EUS-FNA of pancreatic head cancer. The push method contributed to the histological diagnosis of pancreatic head cancer using EUS-FNA specimens.
Core tip: Solid pancreatic head lesions are punctured by the push or pull method. We evaluated the efficacy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) using the push and pull methods for pancreatic head cancer. After propensity score matching (age, sex, tumor diameter, and FNA needle), 85 patients each were stratified into the push and pull groups. Patient characteristics and EUS-FNA-related factors were compared between the two groups. The pull method shortened the distance between the endoscope and the lesion and facilitated EUS-FNA of pancreatic head cancer. The push method contributed to the histological diagnosis of pancreatic head cancer using EUS-FNA specimens.
- Citation: Sugimoto M, Takagi T, Suzuki R, Konno N, Asama H, Sato Y, Irie H, Watanabe K, Nakamura J, Kikuchi H, Waragai Y, Takasumi M, Hashimoto M, Hashimoto Y, Hikichi T, Ohira H. Push vs pull method for endoscopic ultrasound-guided fine needle aspiration of pancreatic head lesions: Propensity score matching analysis. World J Gastroenterol 2018; 24(27): 3006-3012
- URL: https://www.wjgnet.com/1007-9327/full/v24/i27/3006.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i27.3006
Endoscopic ultrasonography is superior to other imaging modalities for visualizing solid pancreatic lesions[1,2]. Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) plays an important role in the diagnosis of pancreatic cancer. The reported diagnostic sensitivity of EUS-FNA for solid pancreatic lesions is 79%-95.0%, the specificity is 75.0%-100%, and the accuracy is 78.0%-96.0%[3-8]. The use of EUS-FNA is widespread irrespective of the type of hospital[9].
However, EUS-FNA is difficult in some situations. Regarding the location of pancreatic lesions, some reports have described the difficulty of EUS-FNA for lesions in the pancreatic head. Haba et al[10] reported that the pancreatic head was an independent factor affecting the accuracy of EUS-FNA for solid pancreatic lesions. Tadic et al[11] reported that 69% of solid pancreatic lesions with intermediate or negative cytological diagnosis by initial EUS-FNA were localized in the pancreatic head. Varadarajulu et al[12] reported that six pancreatic cancers with false-negative results by EUS-FNA were localized in the pancreatic head. A lesion in the pancreatic head was reported to be an independent factor for the requirement for multiple needle passes[13].
There are two methods of puncturing the pancreatic head. Solid pancreatic head lesions are punctured in the duodenal bulbus by pushing (push method) or pulling (pull method) the echoendoscope after it is inserted into the second portion of the duodenum. It is not apparent which of the two methods is more effective. Therefore, we evaluated the efficacy of EUS-FNA of pancreatic head lesions in patients who underwent the push and/or pull method.
In this retrospective study, we compared the efficacy of EUS-FNA using the push and pull methods for pancreatic head lesions. This study was approved by the Institutional Review Board of Fukushima Medical University.
In all, 566 pancreatic cancer patients underwent their first EUS-FNA between February 2001 and December 2017 at Fukushima Medical University (Figure 1). Among these patients, 201 who underwent EUS-FNA of the pancreatic head were included in this study. EUS-FNA was performed using the push method in 85 patients, the pull method in 101 patients, both the push and pull methods in 15 patients.
Because of the retrospective design of the study, the push or pull method was not randomly assigned. Therefore, propensity score matching was used to reduce selection bias derived from patient background (age, sex, tumor diameter, and FNA needle type—19/22 or 25 G). Regarding the FNA needle, a 19-G needle was used in only 3 patients. Therefore, propensity score matching was performed based on whether the needle was 25 G. The propensity score was calculated by using logistic regression analysis. After propensity score matching, 85 patients each were stratified into the push and pull groups.
An echoendoscope was inserted into the patients after they were sufficiently sedated with midazolam. After the echoendoscope reached the antrum of the stomach or the duodenal bulbus, pancreatic head cancer was visualized by pushing the echoendoscope (push method). In contrast, when the echoendoscope reached the descending part of the duodenum, pancreatic head cancer was visualized by pulling the echoendoscope (pull method). After the lack of blood flow was confirmed on the puncture line by Doppler ultrasound, needle passes were started. Rapid onsite cytology (ROSE) of the EUS-FNA specimen was performed by the cytoscreener[14-19]. If the amount of the specimen was sufficient, the procedure was completed. However, if the amount of specimen was insufficient, more punctures were performed until sufficient specimen was collected, as determined by the pathologist. Class IV and V disease was characterized as a malignancy by cytology. Histology specimens were fixed overnight in 10% formalin solution. Specimens were sectioned onto slides and stained with hematoxylin and eosin or prepared for immunostaining (p53 or Ki-67) as necessary. Patients not diagnosed with pancreatic cancer by EUS-FNA were eventually diagnosed by EUS-FNA of lymph node metastases, second EUS-FNA, surgery, pathological autopsy, or biliary juice cytology.
The echoendoscope used in this study was GF-Y0005-UCT, GF-UC240AL-5, GF-UCT240AL-5, or GF-UCT260 (Olympus Medical Systems, Tokyo, Japan). The ultrasonography equipment used in this study was EU-ME1 or EU-ME2 (Olympus Medical Systems) or SSD5000 or ALOKA ProSound α-10 (ALOKA, Tokyo, Japan). The biopsy needles were Expect 22 or 25 G or Acquire 22 G (Boston Scientific, MA, USA); EZ Shot 22 G, EZ Shot 2 22 G, EZ Shot 3 plus 22 G, or NA11J-KB (Olympus Medical System); EchoTip 19, 22, or 25 G or Quick-Core 19 G (Cook Medical Inc., NC, United States); or Sonotip 22 or 25 G (Medi-Globe GmbH, Achenmühle, Germany).
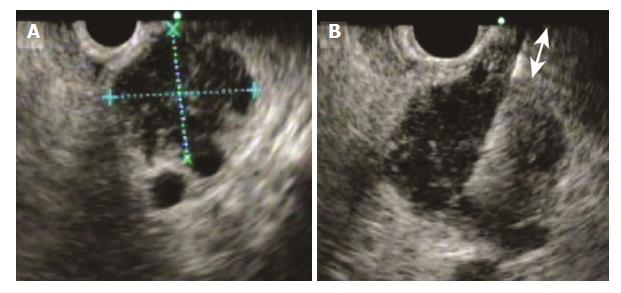
Patient characteristics (age, sex, and tumor diameter) and factors related to EUS-FNA (needle gauge, number of needle passes, distance to lesion, diagnosis of malignancy by cytology, diagnosis of malignancy by histology, overall diagnosis of malignancy, and adverse events) were compared between the push and pull groups. Tumor diameter and the distance to lesion were measured by EUS (Figure 2).
Student’s t test was used to compare continuous variables. Fisher’s exact test was used to compare nominal variables. Logistic regression was used to investigate factors that influenced the histological diagnosis of malignancy using EUS-FNA specimens. A P value < 0.05 indicated a significant difference. All statistical analyses were performed using the EZR platform (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, EZR is a modified version of the R commander that was designed to perform functions frequently used in biostatistics[20].
Patient characteristics were not significantly different between the two groups (Table 1). Regarding the factors related to EUS-FNA, the distance to lesion was significantly longer in the push group than in the pull group (13.9 ± 4.9 mm vs 7.0 ± 4.9 mm, P < 0.01). Other factors were not different between the two groups.
| Push group (n = 85) | Pull group (n = 85) | P value | |
| Patient characteristics | |||
| Age (yr), mean ± SD | 67.1 ± 10.2 | 67.6 ± 9.7 | 0.77 |
| Sex (male/female) | 48/37 | 46/39 | 0.88 |
| Tumor diameter (mm), mean ± SD | 25.9 ± 10.3 | 26.6 ± 10.1 | 0.67 |
| EUS-FNA results | |||
| Distance to lesion | 13.9 ± 4.9 | 7.0 ± 4.9 | < 0.01 |
| No. of needle passes of pancreas, mean ± SD | 2.4 ± 1.3 | 2.4 ± 1.3 | 0.86 |
| Needle gauge | 0.57 | ||
| 19 | 2 | 1 | |
| 22 | 32 | 38 | |
| 25 | 51 | 46 | |
| Diagnosis of malignancy by cytology, n (%) | 78 (91.8) | 78 (91.8) | 1 |
| Diagnosis of malignancy by histology, n (%) | 47 (55.3) | 34 (40.0) | 0.065 |
| Overall diagnosis of malignancy, n (%) | 80 (94.1) | 80 (94.1) | 1 |
| Adverse events | |||
| Abscess around pancreas, n (%) | 0 (0) | 1 (1.2) | 1 |
We investigated factors influencing the distance to lesion [≥ 10 mm (median)]. The push method was identified as significant in the univariate analysis (Table 2). Because no factors with P < 0.15 were identified, a multivariate analysis was not performed.
| Distance to lesion < 10 mm (n = 70) | Distance to lesion ≥ 10 mm (n = 100) | P value | |
| Age ≥ 68 yr | 32 (45.7) | 56 (56.0) | 0.21 |
| Sex, male | 35 (50.0) | 59 (59.0) | 0.28 |
| Tumor diameter ≥ 25 mm | 27 (38.6) | 45 (45.0) | 0.43 |
| Push method | 12 (17.1) | 73 (73.0) | < 0.01 |
| Needle gauge, 19 or 22 G | 32 (45.7) | 41 (41.0) | 0.64 |
We investigated factors influencing the histological diagnosis of malignancy. Among factors potentially influencing the histological diagnosis of malignancy, tumor diameter ≥ 25 mm and the push method were identified as significant in the univariate analysis (factors with P < 0.15 were selected for the multivariate analysis) (Table 3). Logistic regression was performed using these two items, and independent factors included tumor diameter ≥ 25 mm [odds ratio (OR) = 1.91, 95% confidence interval (CI): 1.02-3.58, P = 0.043] and the push method (OR = 1.91, 95%CI: 1.03-3.55, P = 0.04) (Table 4).
| Malignancy by histology(-) (n = 89) | Malignancy by histology(+) (n = 81) | P value | |
| Age ≥ 68 yr | 50 (56.2) | 38 (46.9) | 0.28 |
| Sex, male | 50 (56.2) | 44 (54.3) | 0.88 |
| Tumor diameter ≥ 25 mm | 45 (50.6) | 53 (65.4) | 0.062 |
| Push method | 38 (42.7) | 47 (58.0) | 0.065 |
| Needle gauge, 19 or 22 G | 37 (41.6) | 36 (44.4) | 0.76 |
| Needle passes ≥ 2 | 71 (80.0) | 59 (72.8) | 0.37 |
| OR | 95%CI | P value | |
| Tumor diameter ≥ 25 mm | 1.91 | 1.02-3.58 | 0.043 |
| Push method | 1.91 | 1.03-3.55 | 0.040 |
In this study, we investigated which method (push or pull method) of EUS-FNA was more effective in diagnosing pancreatic head cancer. The distance to lesion was significantly longer in the push group than in the pull group, and the push method was an independent factor contributing to the histological diagnosis of malignancy using EUS-FNA specimens.
As mentioned above, the ability to diagnose pancreatic masses by EUS-FNA has been reported to be excellent[3-8]. However, pancreatic head lesions were reported to be more difficult than lesions in other pancreatic locations to diagnose by EUS-FNA. Some reports have attempted to explain why EUS-FNA is difficult for pancreatic head lesions. One report indicated that pancreatic head lesions are visualized on the middle right of the EUS image, and the lesion is easily moved[21]. Another report suggested that because the tip of the echoendoscope is flexed, the passage of the needle is more difficult[22]. In this report, pancreatic head lesions were closer to the starting point when the needle was inserted by the pull method; however, the overall diagnosis of malignancy was not different between the push and pull groups. Thus, if a diagnosis of malignancy is needed or if the endoscopist is inexperienced, EUS-FNA of pancreatic head lesions should be performed by using the pull method.
The pull method better facilitates EUS-FNA for pancreatic head lesions than the push method; however, it has been reported that the echoendoscope position can become unstable[22]. In this report, the push method was an independent factor contributing to the histological diagnosis of pancreatic cancer. The direction in which the echoendoscope is pulled is opposite that of the lesion puncture, whereas the direction in which the echoendoscope is pushed is the same as that of lesion puncture. In addition, the echoendoscope is stable in the push method. These factors might explain why the push method contributed to the diagnosis of malignancy by histology of the EUS-FNA specimen. Therefore, the push method is recommended for patients requiring histology of EUS-FNA specimens, for example, patients who have a medical history of other cancers.
This study has some limitations. First, this study was retrospective and performed in a single center. We used propensity scoring to overcome this limitation; however, prospective and large-scale studies are warranted. Second, due to the retrospective nature of this study, EUS-FNA procedures were not performed by specific endoscopists. In this study, EUS-FNA was performed by specialists who had performed > 3000 pancreaticobiliary EUS procedures or by trainees under the guidance of specialists. Therefore, the quality of the EUS procedure was considered constant. Third, the difficulty of each method was not evaluated by Doppler ultrasound of blood flow on the puncture line. Because of the retrospective nature of this study, we were unable to identify the potential difficulty of either method by Doppler ultrasound of blood flow. Fourth, we used an EUS-guided trucut biopsy (EUS-TCB) needle or an EUS-guided fine needle biopsy (FNB) needle, such as the Quick-Core 19 G (Cook Medical Inc.) or Acquire 22 G needle (Boston Scientific). Although these needles have been reported to increase the yield of samples[23,24], EUS-TCB and EUS-FNB needles were used in only two patients. A Quick-Core needle (Cook Medical Inc.) was used in a patient in the push group, and an Acquire needle (Boston Scientific) was used in a patient in the pull group. Therefore, we do not believe that the use of EUS-TCB and EUS-FNB needles significantly influenced the results.
In conclusion, the pull method shortened the distance between the endoscope and the lesion and facilitated EUS-FNA of pancreatic head cancer. The push method contributed to the histological diagnosis of pancreatic head cancer using EUS-FNA specimens.
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) plays an important role in the diagnosis of pancreatic cancer. However, EUS-FNA of pancreatic head lesions is difficult. The pull and push methods are both used to diagnose pancreatic head lesions. It is unknown which method is more efficient.
We wanted to determine the appropriate puncture method for EUS-FNA of pancreatic head lesions.
The primary objective of this study was to reveal which method (push method or pull method) is more efficient for diagnosing pancreatic head cancer.
We placed 85 patients in each group (push group and pull group) using propensity score matching. Patient characteristics and some EUS-FNA-related factors were compared between the push and pull groups.
The distance to the pancreatic cancer was significantly longer in the push group than in the pull group. The push method was identified as a significant factor contributing to the histological diagnosis of malignancy.
The pull method shortened the distance between the echoendoscope and the lesion and facilitated EUS-FNA of pancreatic head cancer. The push method contributed to the histological diagnosis of pancreatic head cancer using EUS-FNA specimens.
If only a diagnosis of malignancy is needed or if the endoscopist is inexperienced, the pull method is recommended. However, the push method is recommended for patients requiring histological analysis of EUS-FNA specimens. Further prospective and large-scale studies on these methods are warranted.
We are grateful for the staff at the Department of Gastroenterology, Fukushima Medical University, School of Medicine; the medical staff at the Department of Endoscopy, Fukushima Medical University Hospital; and the medical staff at the Gastroenterology Ward at Fukushima Medical University Hospital.
| 1. | DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D, Kopecky K. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 335] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Tamm EP, Loyer EM, Faria SC, Evans DB, Wolff RA, Charnsangavej C. Retrospective analysis of dual-phase MDCT and follow-up EUS/EUS-FNA in the diagnosis of pancreatic cancer. Abdom Imaging. 2007;32:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Williams DB, Sahai AV, Aabakken L, Penman ID, van Velse A, Webb J, Wilson M, Hoffman BJ, Hawes RH. Endoscopic ultrasound guided fine needle aspiration biopsy: a large single centre experience. Gut. 1999;44:720-726. [PubMed] |
| 4. | Eloubeidi MA, Jhala D, Chhieng DC, Chen VK, Eltoum I, Vickers S, Mel Wilcox C, Jhala N. Yield of endoscopic ultrasound-guided fine-needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer. 2003;99:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 5. | Ryozawa S, Kitoh H, Gondo T, Urayama N, Yamashita H, Ozawa H, Yanai H, Okita K. Usefulness of endoscopic ultrasound-guided fine-needle aspiration biopsy for the diagnosis of pancreatic cancer. J Gastroenterol. 2005;40:907-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Yoshinaga S, Suzuki H, Oda I, Saito Y. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc. 2011;23 Suppl 1:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Uehara H, Ikezawa K, Kawada N, Fukutake N, Katayama K, Takakura R, Takano Y, Ishikawa O, Takenaka A. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J Gastroenterol Hepatol. 2011;26:1256-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Mohammad Alizadeh AH, Shahrokh S, Hadizadeh M, Padashi M, Zali MR. Diagnostic potency of EUS-guided FNA for the evaluation of pancreatic mass lesions. Endosc Ultrasound. 2016;5:30-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Singh S, Purohit T, Aoun E, Patel Y, Carleton N, Mitre M, Morrissey S, Dhawan M, Thakkar S. Comparison of the outcomes of endoscopic ultrasound based on community hospital versus tertiary academic center settings. Dig Dis Sci. 2014;59:1925-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Haba S, Yamao K, Bhatia V, Mizuno N, Hara K, Hijioka S, Imaoka H, Niwa Y, Tajika M, Kondo S. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Tadic M, Kujundzic M, Stoos-Veic T, Kaic G, Vukelic-Markovic M. Role of repeated endoscopic ultrasound-guided fine needle aspiration in small solid pancreatic masses with previous indeterminate and negative cytological findings. Dig Dis. 2008;26:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728-736; quiz 751, 753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 262] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Uehara H, Sueyoshi H, Takada R, Fukutake N, Katayama K, Ashida R, Ioka T, Takenaka A, Nagata S, Tomita Y. Optimal number of needle passes in endoscopic ultrasound-guided fine needle aspiration for pancreatic lesions. Pancreatology. 2015;15:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Logroño R, Waxman I. Interactive role of the cytopathologist in EUS-guided fine needle aspiration: an efficient approach. Gastrointest Endosc. 2001;54:485-490. [PubMed] |
| 15. | Klapman JB, Logrono R, Dye CE, Waxman I. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 361] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 16. | Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Yamao K, Sawaki A, Mizuno N, Shimizu Y, Yatabe Y, Koshikawa T. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol. 2005;40:1013-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Hikichi T, Irisawa A, Bhutani MS, Takagi T, Shibukawa G, Yamamoto G, Wakatsuki T, Imamura H, Takahashi Y, Sato A. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol. 2009;44:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Dumonceau JM, Koessler T, van Hooft JE, Fockens P. Endoscopic ultrasonography-guided fine needle aspiration: Relatively low sensitivity in the endosonographer population. World J Gastroenterol. 2012;18:2357-2363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 14540] [Article Influence: 1118.5] [Reference Citation Analysis (0)] |
| 21. | Yasuda I, Iwashita T, Doi S. Tips for endoscopic ultrasound-guided fine needle aspiration of various pancreatic lesions. J Hepatobiliary Pancreat Sci. 2014;21:E29-E33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kedia P, Gaidhane M, Kahaleh M. Technical Advances in Endoscopic Ultrasound (EUS)-Guided Tissue Acquisition for Pancreatic Cancers: How Can We Get the Best Results with EUS-Guided Fine Needle Aspiration? Clin Endosc. 2013;46:552-562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Thomas T, Kaye PV, Ragunath K, Aithal G. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: a large tertiary referral center experience. Am J Gastroenterol. 2009;104:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Mukai S, Itoi T, Yamaguchi H, Sofuni A, Tsuchiya T, Tanaka R, Tonozuka R, Honjo M, Fujita M, Yamamoto K. A retrospective histological comparison of EUS-guided fine-needle biopsy using a novel franseen needle and a conventional end-cut type needle. Endosc Ultrasound. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kir G, Singh S, Smith RC S- Editor: Gong ZM L- Editor: Logan S E- Editor: Huang Y