Published online Jul 7, 2018. doi: 10.3748/wjg.v24.i25.2776
Peer-review started: April 28, 2018
First decision: May 24, 2018
Revised: May 26, 2018
Accepted: June 9, 2018
Article in press: June 9, 2018
Published online: July 7, 2018
Processing time: 68 Days and 7.9 Hours
Liposarcoma of the stomach is extremely rare, and only 37 cases have been reported worldwide. We herein report two cases of liposarcoma of the stomach. The first patient was referred to our hospital with upper abdominal discomfort. The endoscopic examination revealed a tumor mass about 3 cm in diameter. The patient underwent a partial gastrectomy and had an uneventful recovery. The histopathological examination revealed a well-differentiated liposarcoma. The second patient had symptoms of upper abdominal discomfort combined with nausea and anorexia. Several palpable masses were found with endoscopy. Endoscopic submucosal dissection was the treatment used, and the postoperative course was uneventful. The histopathological diagnosis was a well-differentiated liposarcoma. The two patients did not undergo any adjuvant therapy. They are both currently in good condition without recurrence. Therefore, we believe that the outcome of liposarcoma of the stomach is positive, and surgical resection may be the first choice for treatment at present.
Core tip: Liposarcoma of the stomach is extremely rare, and only 37 cases have been reported in the literature. We herein report two cases and review the literature. These cases might contribute to improving our understanding of the etiology, diagnosis, treatment strategies, and outcome of liposarcoma of the stomach. This report can also serve as a reminder to gastroenterologists, surgeons, and pathologists who encounter liposarcoma of the stomach in their clinical practice.
- Citation: Kang WZ, Xue LY, Wang GQ, Ma FH, Feng XL, Guo L, Li Y, Li WK, Tian YT. Liposarcoma of the stomach: Report of two cases and review of the literature. World J Gastroenterol 2018; 24(25): 2776-2784
- URL: https://www.wjgnet.com/1007-9327/full/v24/i25/2776.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i25.2776
Liposarcoma is one of the most common mesenchymal neoplasms[1], and liposarcomas are classified histologically into five subtypes[2]. However liposarcoma of the stomach is rare, and only 37 cases have been reported in the literature. Liposarcoma of the stomach is mainly located in the antrum, and it is usually of submucosal origin. Definitive diagnosis is reached only by histopathological examination. Because of the low incidence of this tumor, treatment of gastric liposarcoma is still not well-standardized. However, the prognosis may remain satisfactory if the condition is diagnosed early and treated appropriately. Herein, two cases of liposarcoma of the stomach are described, and we also discuss the histopathological types, etiology, diagnosis, and treatment strategies in this report.
In the Chinese Academy of Medical Sciences Cancer Hospital we encountered two cases, one in 2009 and one in 2016.
The first patient was a 45-year-old woman who presented with the symptom of upper abdominal discomfort, which she had experienced for 6 mo. She complained of abdominal pain without any fever or gastrointestinal bleeding. During the physical examination, no special physical signs were found.
The gastroscopy revealed a large tumor mass about 5 cm in diameter located in the junction of the body and fundus of the stomach; it had been considered a benign tumor. Computed tomography confirmed a spherical tumor in the stomach, which was approximately 5.6 cm × 4.2 cm × 3.5 cm in size. The border of the tumor was clear and presented a significantly strengthened edge, and the center of the tumor was inhomogeneous. There were no visible signs of metastatic disease. Upper gastrointestinal imaging also found a circular tumor with smooth edges. The patient had no distinctive past medical history and denied any relevant family history. On March 30, 2009, the patient underwent a resection of the stomach tumor, and surgeons resected part of the omentum. An intraoperative pathology freezing study revealed mesenchymal neoplasms.
She had an uneventful recovery and was discharged after 9 d. The patient did not undergo any adjuvant treatment. She has remained under close follow-up supervision and is currently disease free.
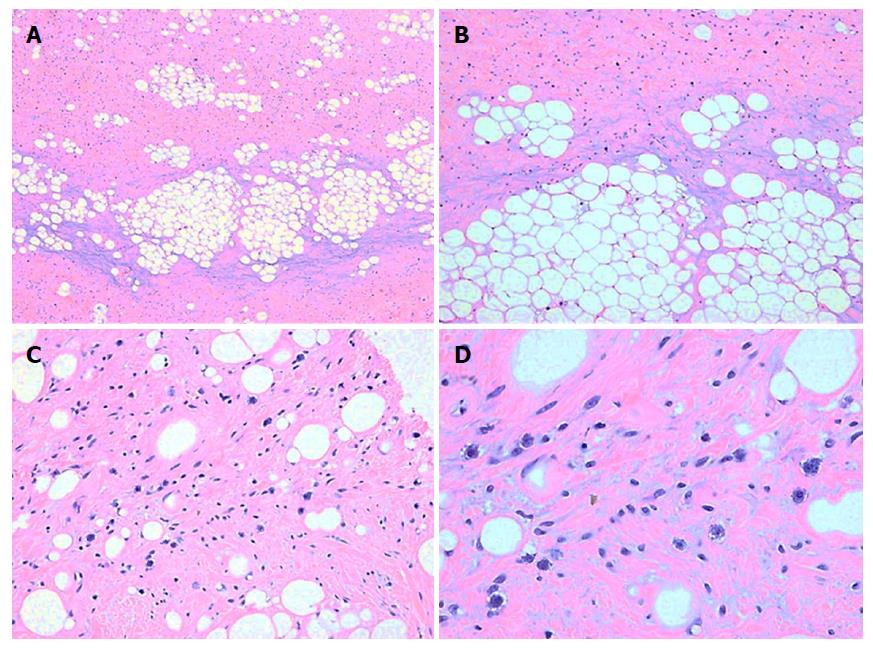
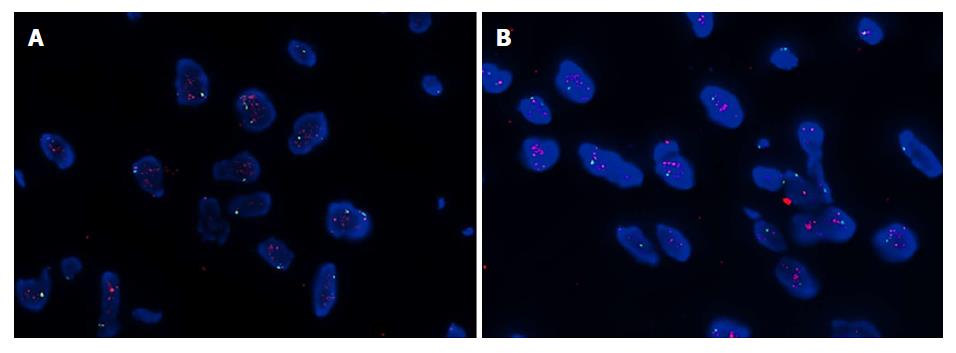
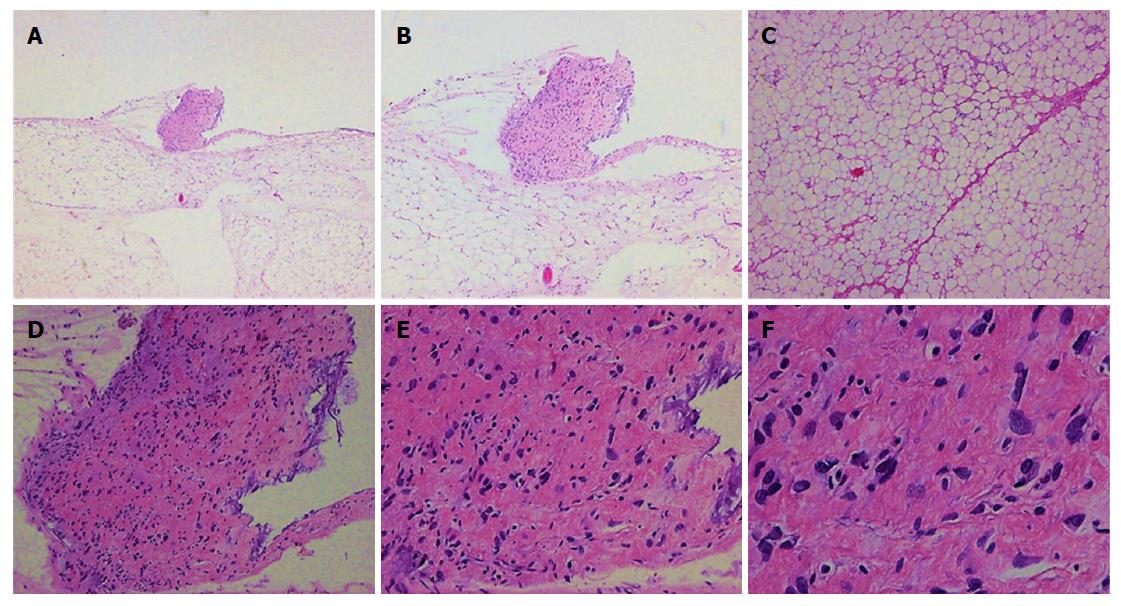
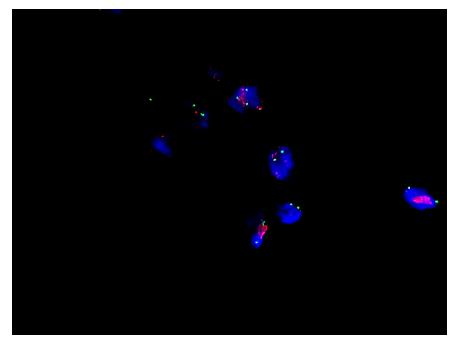
The histopathological examination revealed a well-differentiated liposarcoma measuring 6 cm × 5 cm × 4 cm, which had infiltrated the muscle and serosal layers of the gastric wall (Figure 1). The immunohistochemistry finds were S-100+, CD34++, SMA+, Desmin++, CD117-, HMB45-, and Ki-67 < 1%. Fluorescent in situ hybridization (FISH) detection showed amplification of the MDM2 gene (Figure 2).
A 69-year-old man was admitted to our department because of upper abdominal discomfort combined with nausea and anorexia that he had been experiencing for about 6 mo. During this period he lost 10 kg in weight. At first he pursued treatment with traditional Chinese medicine, and his symptoms were relieved. He underwent pituitary surgery in 2014 because of a pituitary tumor, and he had suffered from hypertension for 30 years. As a result of regular medication, his blood pressure was well controlled. His family history was unremarkable.
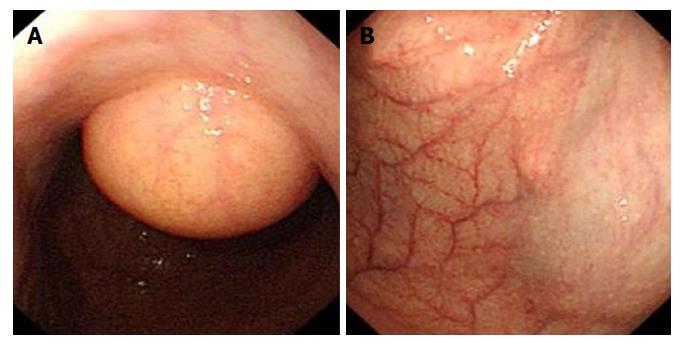
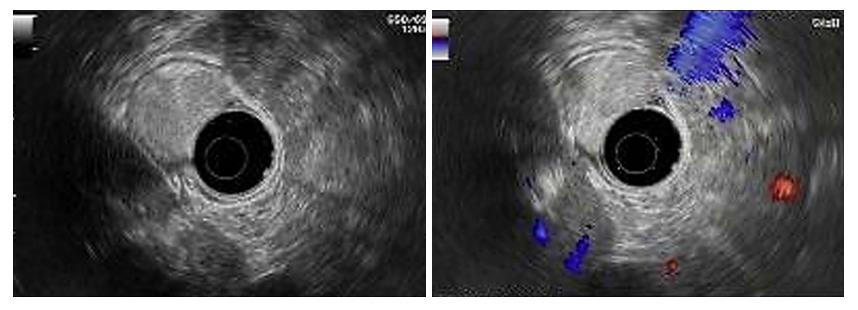
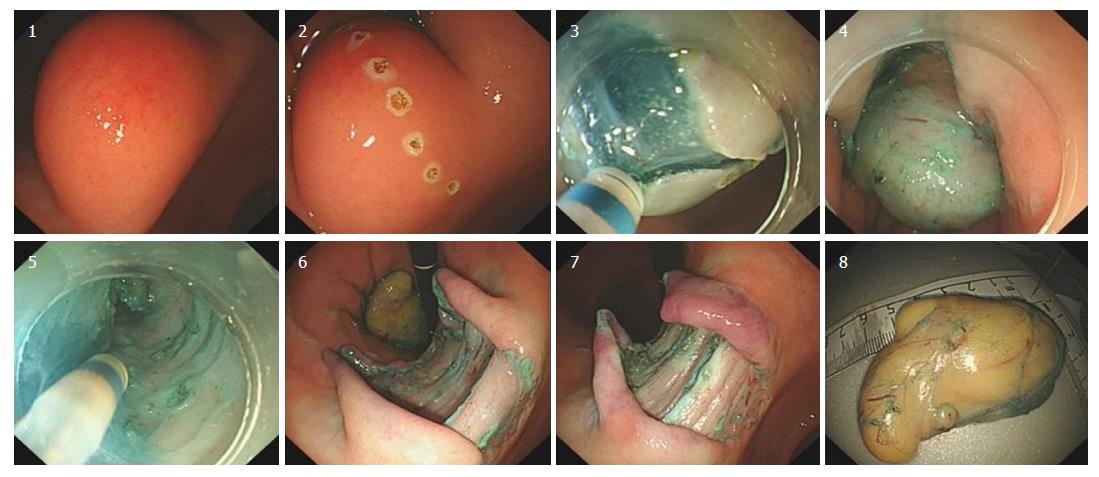
Our hospital’s endoscopic examination showed that a limited knurl was distributed from the lower part of the gastric body to the corner of the stomach (Figure 3A), and a knurl was also found in the gastric fundus (figure 3B). Multiple biopsies were obtained, but they were all superficial and showed only unspecific inflammation of the gastric mucosa. Gastric endoscopic ultrasound (EUS) examination revealed that the tumor was mainly located in the submucosa of the gastric wall and was potentially a liposarcoma (Figure 4). Computed tomography confirmed a fat density tumor about 5.1 cm × 2.8 cm in size. No hepatic metastasis or nodal involvements were detected.
On August 1, 2016, an endoscopic submucosal dissection (ESD) was performed (Figure 5). During the operation, we found that the surface of the tumor was complete and smooth, and the substrate was sturdy. The operation was successful without any complications. The postoperative course was uneventful, and the patient was discharged on postoperative day 7. He did not undergo any adjuvant treatment and remained free of metastasis 20 mo after surgery.
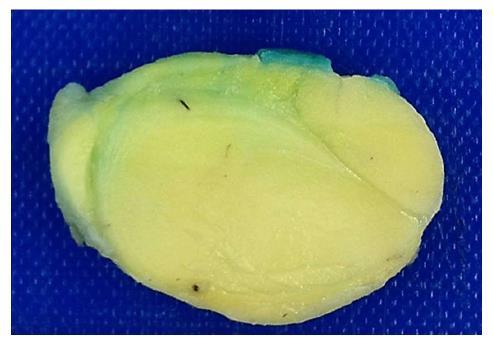
The histopathological diagnosis was a well-differentiated liposarcoma (Figures 6 and 7). FISH testing demonstrated amplification of the MDM2 gene (Figure 8).
Liposarcoma, a kind of malignant tumor of mesenchymal origin, is one of the most common soft tissue sarcomas[1]. However, liposarcoma of the stomach is extremely rare. The first case was reported by Abrama and Tuberville in 1941, and until now only 37 cases (with a mean age of 57.0 years) have been reported worldwide (Table 1).
| Year | Author | Age | Sex | Treatment | Size (cm) | Histologic subtype | Outcome | |
| 1 | 1941 | Abrams et al[23] | 52 | M | Exploratory laparotomy | Entire length of the stomach minor curvature | Unknown | DOD in 4 mo |
| 2 | 1955 | Hohf et al[20] | 77 | M | S + radiation | 15 × 8 × 6 antrum | Unknown | WR in 8 mo |
| 3 | 1965 | Hawkins et al[21] | 86 | M | S | 10 × 10 fundus | MY | WR in 24 mo |
| 4 | 1968 | Orita et al[22] | 42 | M | S | 1.2 × 1.0 × 1.0 body | Unknown | WR 60 mo |
| 5 | 1969 | Souhei Suzuki et al[23] | 42 | F | S | 13 × 11 × 9.5 | Mixed | WR |
| 6 | 1983 | Hiroaki et al[24] | 41 | M | S | 4.0 × 3.5 × 1.5 | MY | DOD in 18 mo |
| 7 | 1984 | Lopez et al[28] | 24 | M | S | 10 in diameter | MY | WR |
| 8 | 1986 | Kiyoshi Kagawa et al[23] | 64 | F | Tumor resection | About hen-egg | WD | WR |
| 9 | 1986 | Shokouh-Amiri et al[29] | 15 | M | S | 30 × 20 Greater curvature | MY | WR 8 mo |
| 10 | 1986 | Laky et al[9] | 67 | F | P | 5 × 2 × 1.5 antrum | Mixed | WR 12 mo |
| 11 | 1988 | Toshiki Hirose et al[23] | 66 | M | T | 10 × 8 × 3 | My | Dissemination |
| 12 | 1990 | Sacchiko Matsusaki[23] | 42 | F | S | about 600 g | MY | WR |
| 13 | 1992 | Costa et al[30] | 70 | F | S | 9 in diameter | WD | WR |
| 14 | 1993 | Ryou Mochituki et al[23] | 56 | F | P | Child's head 1300 g | WD | Unknown |
| 15 | 1993 | Yoshiaki Suzuki et al[23] | 59 | M | T | / | Dedifferentiated | Unknown |
| 16 | 1993 | Ferrozzi et al[25] | 58 | M | Tumor resection | 25 × 20 × 8 antrum | Pleomorphic | Unknown |
| 17 | 1995 | Shigeharu Suziki et al[23] | 57 | M | S + chemotherapy | 4.8 in diameter | WD | WR |
| 18 | 1995 | Yamamoto et al[26] | 58 | M | P + endoscopic resection | 1.3 × 0.5 Greater curvature | Mixed | WR 12 mo |
| 19 | 1996 | Mitsuyoshi Sakayani[23] | 72 | F | T | 17.5 × 7.5 × 1.3; 1700 g | Pleomorphic | Unknown |
| 20 | 1997 | Tsutomu Andou et al[23] | 68 | F | T | 10.6 × 4 | WD | Unknown |
| 21 | 1997 | Masahiro Matsuzawa[23] | 34 | F | Distal gastrectomy | 3.5 × 3 × 3 | MY | Unknown |
| 22 | 1997 | Lopez-Negrete[15] | 74 | F | T | 15 in diameter minor curvature | Mixed | Sudden death |
| 23 | 1998 | Seki et al[11] | 68 | F | T | 10.5 × 5.5 × 4 body | WD | WR 13 mo |
| 24 | 2000 | Philipps et al[27] | 74 | F | S | 3.4 × 1.3 × 0.5 antrum | MY | WR 15 d |
| 25 | 2002 | Hisanobu Saegusa et al[23] | 34 | F | S | 4 × 2.8 minor curvature | WD | WR in 36 mo |
| 26 | 2005 | Noushin et al[15] | 62 | M | S | 7 × 6 minor curvature | WD | Unknown |
| 27 | 2007 | Konstantinos et al[6] | 68 | M | T | 9 × 4 fundus | WD | WR in 24 mo |
| 28 | 2007 | Michiels et al[7] | 27 | F | Subtotal gastrectomy, liver, diaphragm, pancreas, spleen, pericardium; adjuvant chemotherapy | 30 × 20 (5 kg) minor curvature | Pleomorphic | DOD in 16 mo |
| 29 | 2012 | Mohamed et al[11] | 51 | M | T | 9 × 7.5 × 5 antrum | WD | WR in 12 mo |
| 30 | 2013 | Akin et al[1] | 59 | F | Distal gastrectomy | 4 × 3 × 2.5 antrum | WD | WR in 12 mo |
| 31 | 2014 | Kim et al[18] | 46 | F | Laparoscopic, distal gastrectomy; adjuvant treatment | 7 in diameter body | WD | Unknown |
| 32 | 2015 | Abderrahman et al[3] | 70 | M | Antrectomy + adjuvant therapy | 36 in diameter antrum | MY | DOD in 11 mo |
| 33 | 2016 | Matone et al[4] | 76 | M | Laparoscopic +P | 7.5-7.0 in diameter antrum | WD | WR in 6 mo |
| 34 | 2017 | Jiang et al[14] | 55 | F | P + tail of pancreas and spleen was resected | 1.5 in diameter fundus | WD | WR in 48 mo |
| 35 | 2017 | Hisata et al[13] | 79 | F | Surgery for the cardiac tumor | 0.5-1.0 in diameter greater curvature | Dedifferentiated | DOD in 55 d |
| 36 | 2017 | Tomofuji et al[31] | 61 | F | Laparoscopic total gastrectomy | 5 in diameter fundus | WD | WR in 14 mo |
| 37 | 2018 | Girardot-Miglierina et al[32] | / | / | / | Gastro-esophageal junction | Unknown | Unknown |
| 38 | 2016 | Our case | 70 | M | Endoscopic resection | 6 × 3.5 × 2 minor curvature | WD | WR in 20 mo |
| 39 | 2009 | Our case | 45 | F | Tumor resection | 6 × 5 × 4 body | WD | WR in 9 yr |
According to the 2013 WHO classification of soft tissue tumors, liposarcoma is a malignant fat cell tumor that can be histologically subdivided into the following five types: atypical lipomatous tumor/well differentiated liposarcoma, dedifferentiated liposarcoma, myxoid liposarcoma, pleomorphic liposarcoma, and liposarcoma, not otherwise specified[2].
Both of our cases had well-differentiated liposarcomas. Well-differentiated liposarcoma (including atypical lipomas) is the most common subtype, accounting for about 40%-45% of all liposarcomas[3]. Under the microscope, the tumor cells look like normal fat cells. This kind of liposarcoma is usually a low-grade tumor in the early stages and grows slowly[4]. There is a risk of local recurrence but a low potential for metastasis[5]. Dedifferentiated liposarcomas and well-differentiated liposarcomas are related[1]. Approximately 10% of dedifferentiated liposarcomas are recurrences of well-differentiated liposarcomas[3]. The second most common subtype is myxoid liposarcoma, which represents about a third of all liposarcomas[3]. Myxoid liposarcoma is characterized by a myxoid matrix[6]. Under the microscope, its cells look less normal and may have a high grade component. Tumor cells infiltrate blood vessels in the fibromyxoid stroma that form characteristic clusters or branches. Therefore, we usually categorize myxoid liposarcomas as intermediate to high grade tumors. Pleomorphic liposarcoma is considered the least common subtype and has been properly characterized only recently. It accounts for approximately 5% of liposarcomas and is a highly malignant lesion[7]. Pleomorphic liposarcoma is characterized by increased mitotic activity and hemorrhage as well as necrosis[8]. Microscopically, the tumor cells consist of varying amounts of pleomorphic lipoblasts. Pleomorphic sarcoma consists of highly shaped spindle cells, round cells, polygonal cells, or giant tumor cells. Several grading systems have been developed to classify the tumors and to differentiate between low-grade and high-grade tumors.
These tumors can appear anywhere in the body but often occur in the limbs and retroperitoneal space, and the rest occur in the head and neck, abdominal wall, chest wall, and other areas. Liposarcomas hardly ever occurs in organs[6] and are mainly found in adults, with a peak incidence between the age of 50 and 65 years[4]. The origin of gastric liposarcomas is likely the proliferation of undifferentiated mesenchymal cells within the submucosa and the tunica muscularis layer of the stomach[9]. Gastric liposarcomas are characterized by an exophytic growth that adheres to the gastric wall, and the typical location of gastric liposarcomas is the antrum. According our statistics, approximately 30.8% (8/26) of gastric liposarcomas are located in the antrum, and they are usually of submucosal origin. In addition, 23.1% (6/26) of gastric liposarcomas are located in the minor curvature, 15.4% (4/26) in the fundus, 15.4% (4/26) in the body, 11.5% (3/26) in the greater curvature, and 3.8% (1/26) in the gastro-esophageal junction. The diameter of the tumors described in the literature varies from 0.5 to 36 cm.
The etiology of liposarcoma is not yet clear; environmental factors, radiation, genetic variation, and immune defects are potential risk factors[10]. Some patients have a familial history of soft tissue tumors[4], which suggests genetic factors may play an important role in the occurrence of gastric liposarcoma.
Because the tumor develops within the gastric wall, it presents an extra-luminal growth, and the patient can remain asymptomatic for a long time. The symptoms of gastric liposarcoma range from dyspepsia, nausea, vomiting, anorexia, abnormal bowel movements, asthenia, and epigastric abdominal pain to upper gastrointestinal tract bleeding. The type of symptom that appears depends on the location and size of the tumor and the presence of ulcerations[3]. Space-occupying lesions of the stomach or abdominal cavity contribute to the appearance of clinical symptoms[1]. When the submucosal mass extrudes into the lumen, it can cause traumatic and inflammatory changes and result in necrosis, ulceration, and hemorrhage[11]. For patients with giant tumors, the main clinical sign may be the presence of a large abdominal mass of unknown origin[3]. In our cases, the main clinical sign in both patients was epigastric abdominal pain that continued for longer than 6 mo. In both cases, the typical exophytic growth explains the lack of specific gastrointestinal symptoms and the delayed diagnosis[12]. Some cases of gastric liposarcoma can involve other organs synchronously, and unique symptoms may be present[13,14].
Unfortunately, because of the lack of specific symptoms, it is difficult to achieve an early diagnosis[7]. The diagnosis of gastric liposarcoma mainly relies on pathological examination. Cytogenetics and molecular biology provide effective tools for differentiating among types of lipomatous tumors[11]. Macroscopically, liposarcoma present intricate myxomatous zones, which include round cells, pleomorphous clearly differentiated lipoblastic aspects, and hemorrhagic areas[15]. Because endoscopic biopsies do not penetrate the submucosa, the diagnostic value of the endoscopy is unclear, and it is difficult to make a precise judgment on the basis of biopsy findings. Endoscopic biopsies may be useful when the tumor presents endoluminal development. With the guidance of EUS or abdominal ultrasound, biopsy may be possible, and a histological examination, immunohistochemistry, and a cytogenetic study can be performed[3]. Detection of MDM2 is probably important in diagnosis. In terms of imaging, computed tomography is considered the most informative examination. The presence of fat density areas is pathognomonic for fatty tumors, and an association with enhanced areas is highly suggestive of the diagnosis[16]. CT scans can also show secondary lesions in the liver, lung, peritoneum, or other places.
Currently, the main therapy for gastric liposarcoma is surgical removal. The type of gastrectomy chosen depends on the location of the tumor. According to the rules of sarcoma resection, surgeons should resect the tumor with a wide margin of healthy tissue around it and make sure there is no remaining tumor tissue[3]. Lymph node dissection may be unnecessary[17]. One of our patients underwent a ESD. Due to the lack of sufficient data, the advantages and disadvantages of this method are unknown. In consideration of the successful application of ESD in early gastric cancer, we believe this method is available for a low-grade tumor in the early stages. Chemotherapy and radiotherapy combined with surgery have been successful in most malignant tumors; however, we still cannot develop a guideline for chemotherapy and radiotherapy in patients with gastric liposarcoma. There is very little information in the literature about the use of chemotherapy for gastric liposarcoma. Because of a high local recurrence rate of 70%-90% for high-grade soft-tissue sarcomas[18], adjuvant therapy may be necessary. On the contrary, Matone et al[4] hold the opinion that there is currently no evidence that chemotherapy or radiotherapy improves survival rates. Three drugs, ifosfamide, doxo/epirubicin, and dacarbazine are active in the therapy of adult soft tissue sarcoma[7]; they provide a potential therapeutic pathway for gastric liposarcoma. Radiation therapy may be beneficial by killing tumor cells and reducing the chance of the tumor returning in the same location[3] and may be widely used in the treatment of sarcoma. Only six cases reported in the literature received adjuvant treatment. In our cases, patients did not undergo any adjuvant or neoadjuvant therapy. Both patients are free from recurrence after sarcoma resection.
The main prognostic factor for the primary tumor is histological type, and other factors include the scope and location of the tumor[7]. Kim et al[19] believe the main prognostic factor for the primary tumor is anatomical location. According to our statistics, mortality associated with gastric liposarcoma is usually found in cases of dedifferentiated liposarcoma, myxoid liposarcoma, pleomorphic liposarcoma, and mixed type liposarcoma. Of the 37 cases described, six patients died of the disease, while the outcome for nine patients is not known. Their survival time ranged from sudden death to 18 mo (specifically, the times were immediate, 55 d, 4 mo, 11 mo, 16 mo, and 18 mo). Some studies reported that 30% of well-differentiated liposarcomas present with local recurrence; however, metastasis is hardly ever seen[1,4]. Pleiomorphic liposarcoma is considered a highly malignant lesion and may indicate a poor outcome[7]. Due to the lack of sufficient data, we still cannot clearly determine the relationship between histological type and disease prognosis. The outcome of gastric liposarcoma is still unclear, and further study is needed.
From the reported cases and literature review[20-32], we conclude that liposarcoma is rarely seen in the viscera, especially the stomach. Diagnosis of this tumor mainly depends on histopathological examination. Gastric liposarcomas are extremely rare tumors for which there is no therapeutic consensus. Although medications and devices have improved in recent years, surgery may be the most reasonable treatment, and the role of adjuvant treatment is not clearly defined. The prognosis is still unclear, and more research is needed. However, we believe that if the tumor is diagnosed early and treated effectively, the postoperative outcome may be positive.
Epigastric abdominal pain that continued for longer than 6 mo.
Gastrointestinal stromal tumor (GIST) and gastric lipoma.
Differential diagnosis: GIST and gastric lipoma. Definitive diagnosis is reached only by histopathological examination.
Gastric liposarcoma.
Computed tomography: Gastric lipoma.
Gastric liposarcoma.
Partial gastrectomy and endoscopic submucosal dissection.
The first case was reported by Abrama and Tuberville in 1941, and until now only 37 cases have been reported worldwide (Table 1).
Two cases of gastric liposarcoma are reported and a review of the literature.
Diagnosis of gastric liposarcoma mainly depends on histopathological examination, and surgery may be the most reasonable treatment.
| 1. | Bostanoğlu A, Yıldız B, Kulaçoğlu S, Avşar F. Primary liposarcoma of the stomach. Turk J Gastroenterol. 2013;24:167-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Fletcher CDM, Bridge JA, Hogendoom PCW. World Health Organization classification of tumous. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press 2013; 10-238. |
| 3. | Elhjouji A, Jaiteh L, Mahfoud T, Belhamidi S, Bounaim A, AitAli A, Sair K, Zentar A. Giant Gastric Liposarcoma: A Fatal Exceptional Location. J Gastrointest Cancer. 2016;47:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Matone J, Okazaki S, Maccapani GN, Amancio TT, Filippi RZ, Macedo AL. Giant gastric lipossarcoma: case report and review of the literature. Einstein (Sao Paulo). 2016;14:557-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Wu JM, Montgomery E. Classification and pathology. Surg Clin North Am. 2008;88:483-520, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Tepetes K, Christodoulidis G, Spyridakis ME, Nakou M, Koukoulis G, Hatzitheofilou K. Liposarcoma of the stomach: a rare case report. World J Gastroenterol. 2007;13:4154-4155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Michiels A, Hubens G, Ruppert M, Balliu L, Vaneerdeweg W. Giant liposarcoma of the stomach involving the mediastinum. Acta Chir Belg. 2007;107:468-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Einarsdóttir H, Skoog L, Söderlund V, Bauer HC. Accuracy of cytology for diagnosis of lipomatous tumors: comparison with magnetic resonance and computed tomography findings in 175 cases. Acta Radiol. 2004;45:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Laky D, Stoica T. Gastric liposarcoma. A case report. Pathol Res Pract. 1986;181:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 424] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 11. | Hamdane MM, Brahim EB, Salah MB, Haouas N, Bouhafa A, Chedly-Debbiche A. Giant gastric lipoma mimicking well-differentiated liposarcoma. Gastroenterol Hepatol Bed Bench. 2012;5:60-63. [PubMed] |
| 12. | Seki K, Hasegawa T, Konegawa R, Hizawa K, Sano T. Primary liposarcoma of the stomach: a case report and a review of the literature. Jpn J Clin Oncol. 1998;28:284-288. [PubMed] |
| 13. | Hisata Y, Tasaki Y, Kozaki S, Yamada T. A case of dedifferentiated liposarcoma of the heart and stomach. Int J Surg Case Rep. 2017;41:36-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Jiang HF, Ying XJ. Synchronous liposarcoma of pancreas and stomach: a case report and literature review. Int Surg J 2017, 4: 780-783. . [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Noushin AM, Safaei M. Primary Liposarcoma of the Stomach: A Rare Mesenchymal Tumor. Medical J Islamic Republic of lran 2005, 19: 275-278. . |
| 16. | López-Negrete L, Luyando L, Sala J, López C, Menéndez de Llano R, Gomez JL. Liposarcoma of the stomach. Abdom Imaging. 1997;22:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Mutter D, Marescaux J. Gastrectomies pour lesions benignes. Techniques chirurgicales-Appareil digestif 2001, 16: 40-320. . [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 18. | Bramwell VH. Adjuvant chemotherapy for adult soft tissue sarcoma: Is there a standard of care? J Clin Oncol. 2001;19:1235-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 57] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Kim MJ, Gu MJ, Choi JH, Kim SW, Kim KO. Gastric liposarcoma presenting as a huge pedunculated polyp. Endoscopy. 2014;46 Suppl 1 UCTN:E441-E442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Hawkins PE, Terrell GK. Liposarcoma of the stomach: a case report. JAMA. 1965;191:758-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Orita K, Kokumai Y, Kawada K, Takagi S, Kawahara T. Liposarcoma of stomach: report of a case. Acta Med Okayama. 1968;22:167-173. [PubMed] |
| 23. | Hisanobu S, Toshiki S, Yasuhide O, Tetsuya I, Kouichi M, Ryugo O, Kenji S, Taiji A. Primary Gastric Liposarcoma, Report of a Case and Review of the Literature. Gastroenterol Endosc. 2002;44:1168-1174. [DOI] [Full Text] |
| 24. | Seki H, Kataba Y, Okamura R, Namatame K, M Hagiwara M. Metastatic Liposarcoma of the Stomach Report of a Case. Gastroenterological Endoscopy. 1983;4:628-635. [DOI] [Full Text] |
| 25. | Ferrozzi F, Bova D, Garlaschi G. Gastric liposarcoma: CT appearance. Abdom Imaging. 1993;18:232-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Yamamoto K, Teramae N, Uehira H, Wakabayashi N, Fukuda S, Kodama T, Kashina K, Tsuchihashi Y. Primary liposarcoma of the stomach resected endoscopically. Endoscopy. 1995;27:711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Philipps B, Lörken M, Manegold E, Kasperk R, Schumpelick V. [Primary liposarcoma of the stomach wall--a rare mesenchymal tumor]. Chirurg. 2000;71:334-336. [PubMed] |
| 28. | López de la Riva M, Urquijo Tomatti H. [Gastric liposarcoma. Presentation of a case]. Rev Esp Enferm Apar Dig. 1984;66:335-338. [PubMed] |
| 29. | Shokouh-Amiri MH, Hansen CP, Moesgaard F. Liposarcoma of the stomach. A case report. Acta Chir Scand. 1986;152:389-391. [PubMed] |
| 30. | Costa e Silva N, Melo CM, Naves EB, Dias MA. [Gastric liposarcoma: report of a case]. Rev Hosp Clin Fac Med Sao Paulo. 1992;47:89-91. [PubMed] |
| 31. | Tomofuji K, Watanabe J, Ishida N, Kajiwara S. Gastric liposarcoma resected by laparoscopic total gastrectomy to achieve a wide surgical margin. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Girardot-Miglierina A, Clerc D, Suter M. Gastric liposarcoma in a patient with severe obesity. Ann R Coll Surg Engl. 2018;100:e88-e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
CARE Checklist (2013) statement: The authors have read the CARE Checklist (2013), and the manuscript was prepared and revised according to the CARE Checklist (2013).
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Lim SC, Odes S, Soriano-Ursua MA S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y