Published online Jul 7, 2018. doi: 10.3748/wjg.v24.i25.2733
Peer-review started: March 22, 2018
First decision: April 10, 2018
Revised: April 14, 2018
Accepted: June 2, 2018
Article in press: June 2, 2018
Published online: July 7, 2018
Processing time: 104 Days and 23.9 Hours
To understand the cellular and molecular changes in peripheral blood that can lead to the development of hepatocellular carcinoma (HCC) and provide new methods for its diagnosis and treatment.
Peripheral blood mononuclear cells were isolated from the peripheral blood of HCC patients and normal controls and then analyzed by flow cytometry. The percentage of transforming growth factor-β (TGF-β)+ regulatory cells (Tregs) in the peripheral blood was measured, and the expression of TGF-β was also determined. Then, the relationship between the changes and the 5-year survival of patients was analyzed. In addition, recombinant human TGF-β (rhTGF-β) and recombinant human interleukin-6 were added to stimulate the cultured cells, and their effects on HCC were evaluated.
The expression of TGF-β and the percentage of TGF-β+ Tregs in the peripheral blood of HCC patients increased significantly compared with normal controls. Compared with the low TGF-β expression group, the high TGF-β expression group had a significantly lower 5-year survival rate, and the same result was found in the two TGF-β+ Treg groups, suggesting that TGF-β and TGF-β+ Tregs were negatively correlated with the overall survival of the patients. In addition, rhTGF-β promoted the growth of tumor cells and induced high expression levels of IL-6, which further promoted tumor proliferation.
The results showed that TGF-β may promote tumor growth and proliferation by inducing the production of IL-6, and TGF-β and TGF-β+ Tregs may serve as new markers for predicting a poor prognosis in HCC.
Core tip: We aimed to understand the cellular and molecular changes in peripheral blood of hepatocellular carcinoma (HCC) and provide new methods for its diagnosis and treatment. The results showed that transforming growth factor-β (TGF-β) may promote tumor growth and proliferation by inducing the production of interleukin-6, and TGF-β and TGF-β+ regulatory cells may serve as new markers for predicting poor prognosis of HCC.
- Citation: An Y, Gao S, Zhao WC, Qiu BA, Xia NX, Zhang PJ, Fan ZP. Transforming growth factor-β and peripheral regulatory cells are negatively correlated with the overall survival of hepatocellular carcinoma. World J Gastroenterol 2018; 24(25): 2733-2740
- URL: https://www.wjgnet.com/1007-9327/full/v24/i25/2733.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i25.2733
Hepatocellular carcinoma (HCC) is the second leading cause of cancer deaths in the word[1]. According to the World Health Organization (WHO), approximately 788000 people die from primary liver cancer each year. It has been reported that the morbidity and mortality of HCC in China are very high[2,3]. Improvements have been made in HCC treatment, including surgery, chemotherapy, radiation therapy, and ablation; however, HCC still has a poor prognosis because of recurrence and tumor metastasis[4].
Studies have shown that tumors can establish the tumor microenvironment by regulating lymphocytes, which play an important role in tumor development through inflammatory immunity[5,6]. Tumor cells can inhibit the function of tumor infiltrating lymphocytes (TILs) in the tumor microenvironment through inhibitory signaling pathways in the immune system; this process results in the immunosuppression of tumors[7,8]. Tumors can also accumulate infiltrated immune cells to inhibit anti-tumor effects by secreting immunosuppressive factors, such as interleukin-2 (IL-2), transforming growth factor-β (TGF-β), matrix metalloproteinase (MMP), vascular endothelial growth factor (VEGF), and interleukin-10 (IL-10), into the microenvironment[9]. It has also been reported that tumors can affect the proliferation, migration, and survival of cancer cells through the inflammatory effects of immune cells[10,11]. It is well known that regulatory cells (Tregs) can promote the development of tumors by inhibiting immune surveillance[12]. The knockdown of forkhead box P3 (Foxp3), which is specifically expressed in Tregs, can reduce the immunosuppression of Tregs, which can suppress tumor growth in mice with HCC[13]. Therefore, understanding the immune status, especially the inflammatory state of cancer, may provide a new method of immunomodulation for the treatment of cancer.
Studies have shown that TGF-β is one of the key products of Tregs[14], and it maintains the internal stability of stem cells and promotes fibrosis, embryonic development, tissue repair, cell proliferation and differentiation, and immune regulation[15]. Tumor-associated TGF-β negatively regulates the host immune response through the following mechanisms[16,17]. First, tumor-associated TGF-β mediates the differentiation of Th1 cells to Th2 cells via IL-10. Second, tumor-associated TGF-β directly inhibits M1 giant macrophages and the antitumor immune responses mediated by Th1. Third, tumor-associated TGF-β inhibits the functions of CD8+ T lymphocytes, natural killer (NK) cells, and dendritic cells, which have cytotoxic effects. Fourth, tumor-associated TGF-β produces CD4+ CD25+ Tregs to inhibit the function of other lymphocyte groups[18]. Finally, tumor-associated TGF-β promotes M2 macrophages, which can produce reactive oxygen species to promote tumor development through secreting immunosuppressive cytokines (TGF-β and IL-10), angiogenic factors (CXC chemokines, MMP-9, and VEGF), proinflammatory cytokines (IL-1, TNF-α, and IL-6) and tumor growth factors. TGF-β has a dual role in the development of tumors, and it can promote the invasion and metastasis of tumor cells mainly by regulating the immune system and tumor microenvironment[16].
We included 100 patients with HCC who were diagnosed at Beijing Cancer Hospital between 2010 and 2014, and we included 36 healthy subjects without any signs of disease. Blood samples were collected before any treatment. According to the 2002 American Joint Committee on Cancer (AJCC) staging criteria, the samples were divided into two groups: Early stage (stages I and II) and late stage (stages III and IV). All patient information is shown in Table 1. Of the HCC patients, 63 were followed up until death or until July 31, 2017. The median follow-up time was 40.3 mo (mean ± SD: 40.3 ± 11.6 mo). All participants provided informed consent, and the study was approved by the Ethics Committee of Beijing Cancer Hospital.
| Variables | HCC (n = 100) | Normal controls (n = 36) | P value |
| Average age (yr) | 53.33 | 50.36 | 0.16 |
| Age (yr) | 0.61 | ||
| ≤ 50 | 45 (45.0) | 18 (50.0) | |
| > 50 | 55 (55.0) | 18 (50.0) | |
| Sex | 0.68 | ||
| Male | 65 (65.0) | 22 (61.1) | |
| Female | 35 (35.0) | 14 (38.9) | |
| T classification | |||
| T1 | 12 (12.0) | ||
| T2 | 36 (36.0) | ||
| T3 | 42 (42.0) | ||
| T4 | 10 (10.0) |
Whole blood samples from all patients and normal controls were centrifuged at 3000 g for 10 min to collect the serum; then, the concentrations of TGF-β and IL-6 were measured by enzyme-linked immunosorbent assay (ELISA) kits (eBioscience, Waltham, MA, United States) and human inflammation CBA kits (BD Biosciences, San Jose, CA, United States), respectively.
Density centrifugation of 5 mL of heparinized peripheral blood from patients with HCC and normal controls was performed to obtain PBMCs. There were two steps of the horizontal centrifugation: 2000 r/min for 20 min and 15000 r/min for 10 min[19]. The final cell pellet was resuspended and cultured in the appropriate amount of 1640 medium containing 10% fetal bovine serum. The cells were counted using a microscope, and the cell concentration was adjusted to 2 × 106/mL. A total of 200 μL of each cell suspension was pipetted into a 96-well flat bottom cell culture plate, and 1 μL of 10 μg/mL PMA, 2 μL of 100 μg/mL IO, and 2 μL of 300 μg/mL BFA were added. The cells were incubated at 37 °C with 5% CO2 for 4-5 h and then subjected to flow cytometry and analysis.
CD4 and CD3 antibodies were added to label the cells. A Foxp3 antibody was also added to detect the Tregs. A constant volume of 100 μL of phosphate buffered saline (PBS) was used for the flow cytometry analyses, which were performed on a BD FACSCalibur system.
HepG2, PLHC-1, and LMH cell lines were selected for cell culture, and all three cell lines were cultured in an incubator at 37 °C and 5% CO2. rhTGF-β or rhIL-6 (PeproTech, Rocky Hill, NJ, United States) was added to three 6-well plates containing the three HCC cell lines to stimulate the tumor cells for 24-48 h according to the manufacturer’s instructions. To analyze the proliferation rates, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays and cell counting were performed.
Statistical analyses were performed using GraphPad Prism 5.0 (San Diego, CA, United States). The data are expressed as the mean ± SD. T-tests were used to assess the differences between groups. The overall survival rates of HCC were analyzed with Kaplan-Meier curves. All tests were two-tailed, and P < 0.05 was considered to be statistically significant.
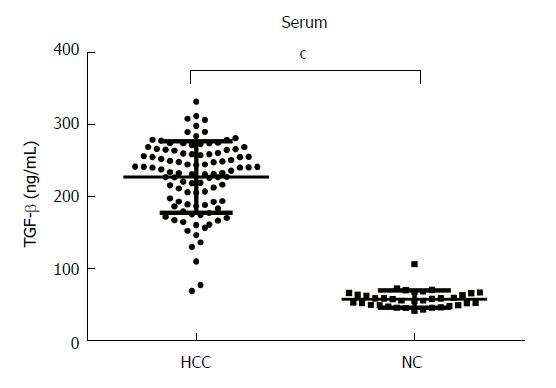
We measured the levels of TGF-β in the serum of 100 patients with HCC and 36 normal controls using ELISAs. The results showed that the serum levels of TGF-β were significantly higher in HCC patients (225.82 ± 48.93 ng/mL) than in normal controls (57.29 ± 11.70 ng/mL, P < 0.0001, Figure 1).
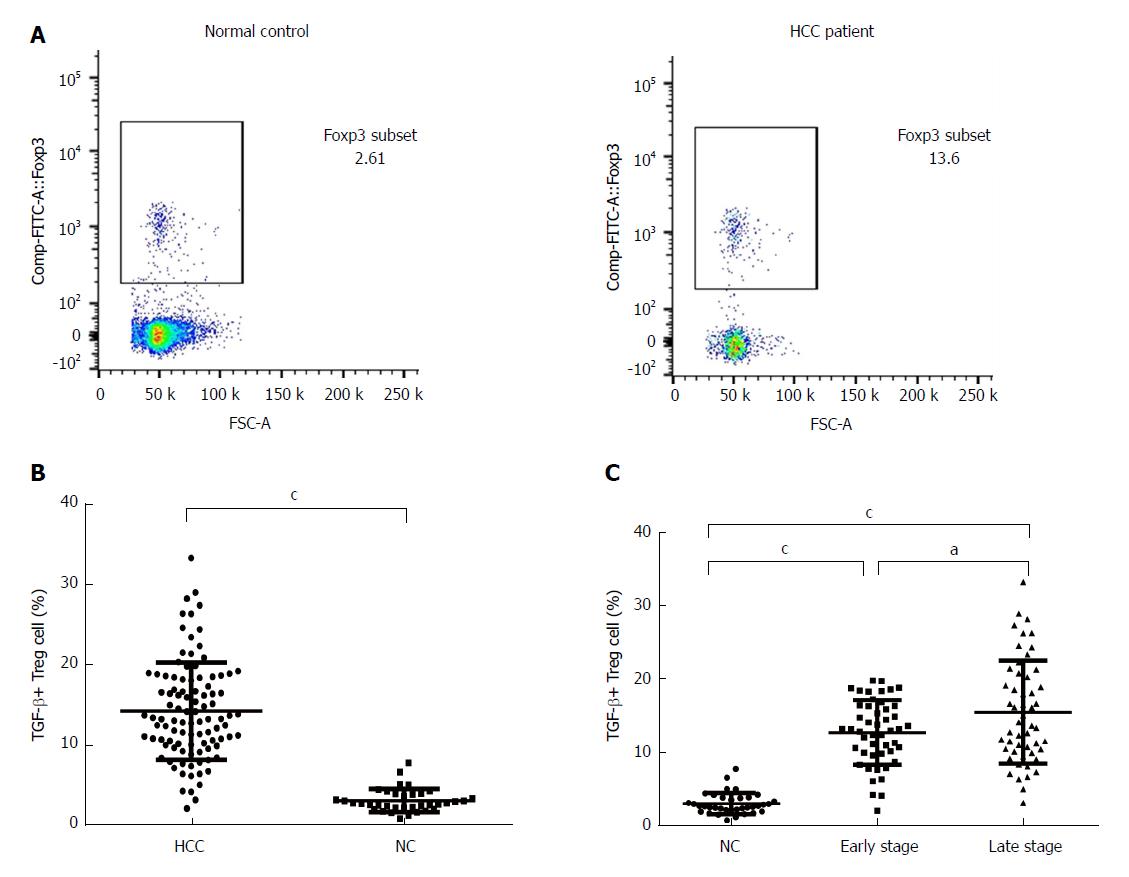
Studies have shown that TGF-β is one of the key products of Tregs[14]. We performed flow cytometry analyses of 100 HCC samples and 36 normal controls, and the results clearly indicated TGF-β-expressing cell populations in the PBMCs and Tregs. The population of TGF-β-expressing cells in the Tregs was analyzed by flow cytometry, and Figure 2A is representative of all the data figures. A statistical analysis showed that the proportion of TGF-β+ Tregs was significantly higher in HCC patients (14.14 ± 6.02%) than in normal controls (3.00 ± 1.43%, P <0.001, Figure 2B). In fact, the percentage of TGF-β+ Tregs was significantly higher in HCC patients in advanced stages (15.47 ± 6.95%) than in HCC patients in early stages (12.70 ± 4.37%) and normal controls (Figure 2C), suggesting a possible positive correlation between the presence of TGF-β+ Tregs and tumor progression.
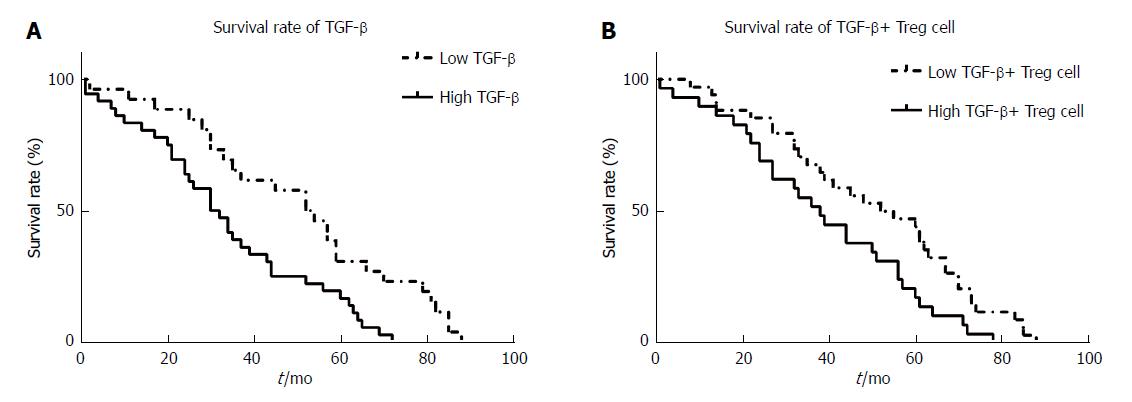
In this study, we analyzed the effects of high and low levels of peripheral blood TGF-β and TGF-β+ Tregs on the five-year survival rate of 63 patients with HCC who were followed up. The results showed that compared to the low TGF production group, the high TGF-β production group had a significantly lower overall survival rate (Figure 3A, 31.0% vs 53.0%, P = 0.010). Compared with the low TGF-β+ Tregs group, the high TGF-β+ Tregs group had a significantly higher death hazard rate (Figure 3B, 38.0% vs 53.5%, P = 0.047). The results suggest that the overall survival of the patients with HCC is negatively correlated with the levels of TGF and Tregs.
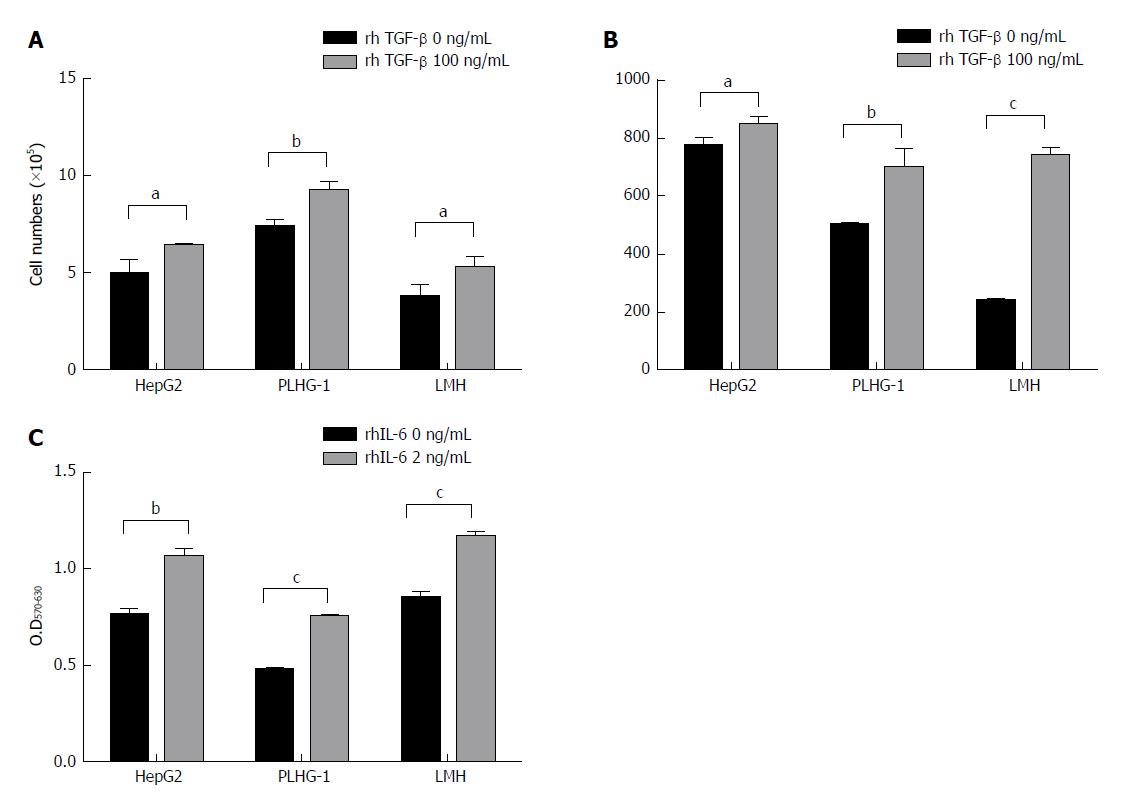
Three human HCC cell lines, namely, HepG2, PLHC-1, and LMH, were selected for this study. We added rhTGF-β to stimulate the three HCC cell lines and then detected changes in their biological function. Compared with the untreated group, all three HCC cell lines treated with 100 ng/mL rhTGF-β for 48 h showed significant cell proliferation afterwards (Figure 4A). We also examined IL-6 levels in the cell supernatants and found that IL-6 levels were significantly higher in the rhTGF-β-treated cell supernatants than in the untreated cell supernatants (Figure 4B). Furthermore, the three HCC cell lines were treated with rhIL-6, and significantly higher cell proliferation rates were found in all three HCC cell lines treated with rhIL-6 than in the untreated cells (Figure 4C). These results indicate that TGF-β has the potential to promote HCC cell proliferation, and it can also mediate IL-6 production to promote further tumor cell proliferation.
This study demonstrated that the levels of TGF-β and TGF-β+ Tregs in the peripheral blood were significantly higher in HCC patients than in normal controls, and these levels were negatively associated with the 5-year survival of HCC patients. At the same time, TGF may promote the development of tumors by inducing IL-16 expression, suggesting that peripheral TGF-β and TGF-β+ Tregs can be used as potential markers to predict a poor prognosis in HCC patients.
Understanding the immune status, especially the inflammatory state of cancer, may provide a new method of immunomodulation for the treatment of cancer. In the early stages of cancer, or the stages of hepatocellular degeneration and injury, abnormally high expression levels of IGF-II have been observed, which can be an indicator for the early diagnosis of liver cancer. Liver injury may be caused by the abnormal activation of the IGF-II gene by the cancer-causing factor diethylnitrosamine[20,21]. It was also reported that high expression levels of IGF-II in the serum and livers of rats are events in the early and advanced stages of the occurrence and development of liver cancer[21]. Some studies have shown that VEGF is more sensitive for the prediction of liver cancer in patients with cirrhosis related to hepatitis C virus (HCV), and VEGF expression is related to tumor vascular invasion[22]. VEGF expression has been associated with tumor diameter and lymph node metastasis, and its positive rate is 72.0% in HCC. Therefore, VEGF may be applied for evaluating hematopoietic liver cancer metastasis and predicting HCC with HCV. It has been reported that the expression levels of Golgi protein 73 (GP73) are significantly higher in HCC serum samples than in control serum samples. GP73 has high specificity and sensitivity for diagnosing HCC and is expected to be validated as another liver cancer marker[23]. The expression levels of des-gamma-carboxy prothrombin (DCP) are affected by the pathological type and stage of tumors and the type of hepatic lesions in HCC. Its ability to diagnose HCC is better than that of alpha fetoprotein (AFP) and AFP-L3, which are first-line clinical tumor markers, and it can be used as an early indicator for diagnosing liver cancer[24,25].
Tregs are a subpopulation of T lymphocytes that have unique in vivo functions; Tregs can secrete IL-4, IL-10, and TGF-β and inhibit effector T cells, and they are involved in autoimmune diseases, transplantation immunity, and tumor immunity[26]. According to the biological function of Th cells and Tregs, there is a dynamic balance between the two types of immune cells. The disruption of the balance between proinflammatory Th17 cells and suppressor Tregs is a key factor in the development of inflammation, autoimmune diseases, and tumors, and their expression levels in different immune response types are not the same. Recent studies have shown that tumor cells secrete multiple factors that can induce the production of Tregs[27,28]. Thl7 cells are negatively correlated with tumor progression because of the reduction in their number and proportion, whereas Tregs are positively correlated since their quantity and proportion are increased[29]; these data are consistent with the findings of our study. Tregs can suppress immune surveillance during tumor development in a mouse model of carcinogen-induced sarcoma[30]. Tregs can also be considered as the main component of tumor evasion of the host immune system and, thus, can serve as an indicator of poor prognosis and even as a target for immunotherapy[31-33].
In summary, our study demonstrated that the levels of expression of TGF-β and TGF-β+ Tregs in the peripheral blood of HCC patients were significantly increased and were related to the progression of HCC and the 5-year survival of patients. This suggests that peripheral blood TGF-β levels can serve as a potential indicator of poor prognosis for HCC and provide a more effective method for the diagnosis and treatment of HCC.
Although the treatment of hepatocellular carcinoma (HCC) has been improved, including surgery, chemotherapy, radiation therapy, and ablation, prognosis remains poor because of the recurrence and tumor metastasis.
Understanding the immune status, especially the inflammatory state, of cancer may provide insight into the development of new immunomodulation methods for the treatment of cancer and may potentially provide novel prognostic predictors of HCC.
To understand the cellular and molecular changes in peripheral blood that can lead to the development of HCC and provide new methods for its diagnosis and treatment.
Peripheral blood mononuclear cells were isolated from peripheral blood of HCC patients and normal controls and then analyzed by flow cytometry. The percentage of transforming growth factor-β (TGF-β)+ Treg cells in peripheral blood was measured, and the expression of TGF-β was also detected. The relationship between the changes and the 5-year survival of patients was analyzed. In addition, recombinant human TGR-β and recombinant human IL-6 were added respectively to stimulate the cultured cells to evaluate its effect on HCC.
The expression of TGF-β and the percentage of TGF-β+ Treg cells in peripheral blood in HCC patients increased significantly compared with normal controls. Compared with the low TGF-β expression group, the 5-year survival rate was significantly lower in the high TGF-β expression group, and the same result was found in the two TGF-β+ Treg groups, suggesting that TGF-β and TGF-β+ Treg cells were negatively correlated with the overall survival of the patients. In addition, recombinant human TGF-β promoted the growth of tumor cells and induced high expression of IL-6 which can further promote tumor proliferation.
The results showed that TGF-β may promote tumor growth and proliferation by inducing the production of IL-6, and TGF-β and TGF-β+ Treg cells may serve as new markers for predicting poor prognosis of HCC.
Our study provided a novel insight to understand the cellular and molecular changes in peripheral blood of HCC and provide new methods for its diagnosis and treatment.
| 1. | Llovet JM, Villanueva A, Lachenmayer A, Finn RS. Advances in targeted therapies for hepatocellular carcinoma in the genomic era. Nat Rev Clin Oncol. 2015;12:436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 2. | Han KH, Kudo M, Ye SL, Choi JY, Poon RT, Seong J, Park JW, Ichida T, Chung JW, Chow P. Asian consensus workshop report: expert consensus guideline for the management of intermediate and advanced hepatocellular carcinoma in Asia. Oncology. 2011;81 Suppl 1:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (2)] |
| 3. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25606] [Article Influence: 1707.1] [Reference Citation Analysis (11)] |
| 4. | Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 703] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 5. | Ganss R, Hanahan D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 1998;58:4673-4681. [PubMed] |
| 6. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11513] [Article Influence: 479.7] [Reference Citation Analysis (2)] |
| 7. | Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492-499. [PubMed] |
| 8. | Chen X, Liang H, Guan D, Wang C, Hu X, Cui L, Chen S, Zhang C, Zhang J, Zen K. A combination of Let-7d, Let-7g and Let-7i serves as a stable reference for normalization of serum microRNAs. PLoS One. 2013;8:e79652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Beatty GL, Gladney WL. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 825] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 10. | Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1465] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 11. | Douglas WG, Tracy E, Tan D, Yu J, Hicks WL Jr, Rigual NR, Loree TR, Wang Y, Baumann H. Development of head and neck squamous cell carcinoma is associated with altered cytokine responsiveness. Mol Cancer Res. 2004;2:585-593. [PubMed] |
| 12. | Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunol Res. 2005;32:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Shi C, Zhang Y, Yang H, Dong T, Chen Y, Xu Y, Yang X, Liu P. Ultrasound-targeted microbubble destruction-mediated Foxp3 knockdown may suppress the tumor growth of HCC mice by relieving immunosuppressive Tregs function. Exp Ther Med. 2018;15:31-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Glebezdina NS, Olina AA, Nekrasova IV, Kuklina EM. Role of Endogenous Melatonin in the Regulation of Th17/Treg Balance during Pregnancy. Bull Exp Biol Med. 2018;164:462-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Patman G. Liver cancer: TGF-β and cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2016;13:2-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Achyut BR, Yang L. Transforming growth factor-β in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141:1167-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3797] [Cited by in RCA: 4399] [Article Influence: 191.3] [Reference Citation Analysis (8)] |
| 18. | Shen Y, Wei Y, Wang Z, Jing Y, He H, Yuan J, Li R, Zhao Q, Wei L, Yang T. TGF-β regulates hepatocellular carcinoma progression by inducing Treg cell polarization. Cell Physiol Biochem. 2015;35:1623-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 634] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 20. | Ikeda Y, Kajiyama K, Yamashita Y, Ikegami T, Uchiyama H, Soejima Y, Kawanaka H, Ikeda T, Morita M, Oki E. Differential expression of insulin-like growth factor 1 in human primary liver cancer. Fukuoka Igaku Zasshi. 2013;104:334-338. [PubMed] |
| 21. | Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 22. | Mukozu T, Nagai H, Matsui D, Kanekawa T, Sumino Y. Serum VEGF as a tumor marker in patients with HCV-related liver cirrhosis and hepatocellular carcinoma. Anticancer Res. 2013;33:1013-1021. [PubMed] |
| 23. | Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, Zhao H, Chen W, Xu Y, Chi T. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut. 2010;59:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 24. | Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 294] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark. 2007;3:79-87. [PubMed] |
| 26. | Ernerudh J, Berg G, Mjösberg J. Regulatory T helper cells in pregnancy and their roles in systemic versus local immune tolerance. Am J Reprod Immunol. 2011;66 Suppl 1:31-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Maturu P, Jones D, Ruteshouser EC, Hu Q, Reynolds JM, Hicks J, Putluri N, Ekmekcioglu S, Grimm EA, Dong C. Role of Cyclooxygenase-2 Pathway in Creating an Immunosuppressive Microenvironment and in Initiation and Progression of Wilms’ Tumor. Neoplasia. 2017;19:237-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Holmgaard RB, Zamarin D, Li Y, Gasmi B, Munn DH, Allison JP, Merghoub T, Wolchok JD. Tumor-Expressed IDO Recruits and Activates MDSCs in a Treg-Dependent Manner. Cell Rep. 2015;13:412-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 394] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 29. | Chen X, Du Y, Lin X, Qian Y, Zhou T, Huang Z. CD4+CD25+ regulatory T cells in tumor immunity. Int Immunopharmacol. 2016;34:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 30. | Betts G, Twohig J, Van den Broek M, Sierro S, Godkin A, Gallimore A. The impact of regulatory T cells on carcinogen-induced sarcogenesis. Br J Cancer. 2007;96:1849-1854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Shou J, Zhang Z, Lai Y, Chen Z, Huang J. Worse outcome in breast cancer with higher tumor-infiltrating FOXP3+ Tregs : a systematic review and meta-analysis. BMC Cancer. 2016;16:687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 32. | Xu W, Liu H, Song J, Fu HX, Qiu L, Zhang BF, Li HZ, Bai J, Zheng JN. The appearance of Tregs in cancer nest is a promising independent risk factor in colon cancer. J Cancer Res Clin Oncol. 2013;139:1845-1852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H. Localisation pattern of Foxp3+ regulatory T cells is associated with clinical behaviour in gastric cancer. Br J Cancer. 2008;98:148-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Czubkowski P, Gobejishvili L, Tatsuya O S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y