Published online Jul 7, 2018. doi: 10.3748/wjg.v24.i25.2661
Peer-review started: April 3, 2018
First decision: May 29, 2018
Revised: June 4, 2018
Accepted: June 22, 2018
Article in press: June 22, 2018
Published online: July 7, 2018
Processing time: 93 Days and 4.1 Hours
The number of patients with nonalcoholic fatty liver diseases (NAFLD) including nonalcoholic steatohepatitis (NASH), has been increasing. NASH causes cirrhosis and hepatocellular carcinoma (HCC) and is one of the most serious health problems in the world. The mechanism through which NASH progresses is still largely unknown. Activation of caspases, Bcl-2 family proteins, and c-Jun N-terminal kinase-induced hepatocyte apoptosis plays a role in the activation of NAFLD/NASH. Apoptotic hepatocytes stimulate immune cells and hepatic stellate cells toward the progression of fibrosis in the liver through the production of inflammasomes and cytokines. Abnormalities in glucose and lipid metabolism as well as microbiota accelerate these processes. The production of reactive oxygen species, oxidative stress, and endoplasmic reticulum stress is also involved. Cell death, including apoptosis, seems very important in the progression of NAFLD and NASH. Recently, inhibitors of apoptosis have been developed as drugs for the treatment of NASH and may prevent cirrhosis and HCC. Increased hepatocyte apoptosis may distinguish NASH from NAFLD, and the improvement of apoptosis could play a role in controlling the development of NASH. In this review, the association between apoptosis and NAFLD/NASH are discussed. This review could provide their knowledge, which plays a role in seeing the patients with NAFLD/NASH in daily clinical practice.
Core tip: Nonalcoholic fatty liver diseases (NAFLD), including nonalcoholic steatohepatitis (NASH), are one of the most serious health issues. We searched articles written in English and listed on PubMed for the role of apoptosis in NASH. There are close association between apoptosis and NAFLD/NASH. Several inhibitors of apoptosis have been suggested as potential treatments for NASH, and some are now being tested in clinical trials. Therefore, we should focus on the role of apoptosis in the progression of NAFLD/NASH.
- Citation: Kanda T, Matsuoka S, Yamazaki M, Shibata T, Nirei K, Takahashi H, Kaneko T, Fujisawa M, Higuchi T, Nakamura H, Matsumoto N, Yamagami H, Ogawa M, Imazu H, Kuroda K, Moriyama M. Apoptosis and non-alcoholic fatty liver diseases. World J Gastroenterol 2018; 24(25): 2661-2672
- URL: https://www.wjgnet.com/1007-9327/full/v24/i25/2661.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i25.2661
The term nonalcoholic fatty liver disease (NAFLD) includes nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). The mechanism by which NASH progresses is still largely unknown. Liver biopsy is an important procedure for the diagnosis of NASH, with a typical case of NASH having hepatocellular steatosis and ballooning, mixed acute and chronic lobular inflammation, and zone 3 perisinusoidal and pericellular fibrosis[1]. These findings have also been observed in the liver of patients with alcoholic steatohepatitis. NAFLD cirrhosis and NAFLD-hepatocellular carcinoma (HCC) are the second leading cause of liver transplants in the USA[2]. Accordingly, there is increasing evidence that HCC can develop in the NASH[2].
In the liver of patients with alcoholic hepatitis, infiltrating polymorphonuclear leukocytes and apoptotic bodies derived from hepatocytes are observed[3]. A combination of environmental factors, host genetics, and gut microbiota can lead to an excess accumulation of fat in the hepatocytes, which can result in lipotoxicity and trigger hepatocyte cell death, liver inflammation, fibrosis, and pathological angiogenesis, resulting in NASH, cirrhosis, and HCC[4]. In this topical review, we will discuss apoptosis, which is involved in the development of NASH.
Feldstein et al[5] observed that terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive hepatocytes were significantly increased in the livers of NASH patients, compared to those from patients with alcoholic hepatitis or simple steatosis. Caspases have apoptotic functions as well as non-apoptotic functions. During apoptosis, the caspase cascade shapes the immunogenic properties of apoptosis[6]. Feldstein et al[5] found that active caspases 3 and 7 as well as the strong expression of Fas receptors in NASH specimens were strongly correlated with hepatocyte apoptosis and the progression of NASH. Caspase 3 activation and hepatocyte apoptosis are prominent features of different experimental models of NAFLD as well as human NAFLD and have been shown to be correlated with disease severity[5].
Caspase 3 is known to cleave several cellular substrates including cytokeratin-18 (CK-18), which is the major intermediate fragment protein in the liver[7]. Caspase 3 generated CK-18 fragments are an independent predictor of NASH in patients with suspected NAFLD[7,8]. Traffic-related air pollution has been shown to be associated with CK-18, a marker of hepatocellular apoptosis, in an overweight and obese pediatric population[9]. Mallory-Denk bodies (MDBs) are characteristic of both alcoholic and NASH and discriminate between the relatively benign simple steatosis and the more aggressive NASH. It has been shown that in genetically susceptible mice overexpressing CK-8, consumption of a high-fat diet (HFD) triggered hepatocellular injury, ballooning, apoptosis, inflammation, and MDB development[10].
Inhibition of hepatic apoptosis by pharmacological pan-caspase inhibitor VX-166 may reduce the development of fibrosis in mice with NASH[11,12]. Increases in active caspase 2, active caspase 3, and apoptosis were observed in the livers of patients with NASH[13]. Ballooned hepatocytes in NASH downregulate caspase 9, a pivotal caspase that executes the mitochondrial apoptosis pathway[14]. In rodents, a lack of caspase 8 expression in hepatocytes was shown to reduce the methionine-choline-deficient (MCD)-dependent increases in apoptosis, decreased the expression of pro-inflammatory cytokines, and reduced hepatic infiltration[15]. Caspase 8 may thus be critical for the pathogenesis of NASH.
Caspase 2 is an initiator caspase in lipid-induced cytotoxicity (lipoapoptosis), which plays a role in the pathogenesis of NASH[16]. Caspase 2 plays a role in lipid-induced hepatocyte apoptosis and is related to the production of apoptosis-associated fibrogenic factors[17]. Additionally, liver free coenzyme A content was shown to be reduced in mice with NASH. Decreased hepatic free coenzyme A content was associated with increased caspase 2 activity and correlated with more severe liver cell apoptosis, inflammation, and fibrosis[18].
It has been reported that Fas, Fas ligand (FasL), and caspase 8 mRNA activation are important contributing factors to NAFLD[19]. Another study showed that children with NASH had significantly higher levels of soluble Fas and soluble FasL than those in the “not NASH” group[20]. Fas apoptosis inhibitory molecule (FAIM), a ubiquitously expressed antiapoptotic protein, functions as a mediator of Akt signaling[21]. Loss of FAIM leads to spontaneous obesity and hepatic steatosis[21].
Hepatic cell apoptosis is associated with miR-34a/Sirtuin 1 (SIRT1)/p53 signaling in NASH[22]. p53 and its transcriptional target, miR34a, have been shown to be involved in the pathogenesis of fatty liver. The p53 inhibitor, pifithrin-α-p-nitro, was shown to attenuate steatosis, associated oxidative stress, and apoptosis in murine models of NAFLD[23]. The DNA damage checkpoint protein Ataxia telangiectasia mutated pathway plays a role in the response to hepatic fat accumulation and promotes hepatocellular apoptosis and fibrosis in mice models of NAFLD[24]. Massive hepatic progenitor cell expansion, especially in children with NASH, is associated with the degree of liver injury, hepatocyte apoptosis, and cell-cycle arrest[25].
Increased vimentin fragment levels are known to indicate the existence of substantial hepatocellular apoptosis in the progression of NASH[26]. Levels of the augmenter of liver regeneration (ALR) protein were lower in liver tissues from patients with advanced alcoholic liver disease and nonalcoholic steatohepatitis than in liver tissues from controls[27]. Levels of steatosis and apoptosis were reduced in mice with a liver-specific deletion of ALR[27]. Impairment of the formation of a newly discovered ubiquitin ligase complex called linear ubiquitin chain assembly complex, has been shown to result in insufficient NF-κB activation and may thus be one of the molecular mechanisms underlying the enhanced apoptotic response of hepatocytes in NASH mouse models[28].
IgM-free apoptosis inhibitor of macrophage serum levels appear to be a sensitive diagnostic marker for NASH-HCC[29]. Activation of apoptosis signal-regulating kinase 1 (ASK1) in hepatocytes is a key step in the progression of nonalcoholic steatohepatitis[30]. Additionally, tumor necrosis factor alpha-induced protein 3 directly interacts with and deubiquitinates ASK1 in hepatocytes[30].
Thus, the activation of caspases and other molecules that are involved in apoptosis are frequently observed in the livers of NASH patients and may be related to the progression of NAFLD and NASH.
Liver injury in NASH patients is associated with apoptosis and NF-κB activation even though anti-apoptotic B-cell lymphoma 2 (Bcl-2) is strongly expressed[31,32]. These changes are caspase-dependent. They are also associated with mitochondrial membrane depolarization and the release of cytochrome c, which activate the mitochondrial apoptosis pathways including activation of the proteins Bcl-2-associated X (Bax) and Bcl-2-interacting mediator of cell death (Bim)[33]. The upregulation of Bax and Bcl-2 expression may also be play an important role in apoptosis in NAFLD[19], although it has been reported that NASH patients had significantly lower levels of anti-apoptotic protein Bcl-2[34]. The degree of apoptosis was inversely correlated with the level of Bcl-2[34].
Activation of endoplasmic reticulum (ER) stress-associated c-Jun N-terminal kinase (JNK) promotes apoptosis by modifying the expression and function of pro-apoptotic members of the Bcl-2 family such as Bcl-2 homology 3 (BH3) only protein Bim and p53-upregulated modulator of apoptosis (PUMA)[35]. PUMA promotes the activation of Bax and thus mitochondrial outer membrane permeabilization, which leads to the relocation of these pro-apoptotic mediators into cytosol[35]. Cazanave et al[36] reported that miR-296-5p levels were inversely related to the BH3-only protein PUMA mRNA levels in human liver specimens, and that miR-296-5p regulates PUMA expression during hepatic lipoapoptosis.
Transglutaminase 2 (TG2), which is induced in the nuclei of ethanol-treated hepatocytes, crosslinks and inactivates the transcription factor, SpI, which results in hepatic apoptosis[37]. In NASH patients, nuclear TG2 and crosslinked SpI formation were elevated. Additionally, activation of apoptosis inducing factor and a release of cytochrome c were observed[37]. Hypoxia, oxidative stress, and lipoapoptosis could all influence the expression of mitochondrial-encoded NADH dehydrogenase (MT-ND3) in hepatocytes and MT-ND3 may play a role in the progression of hepatic steatosis[38]. Hepatocyte-specific c-Met deletion in hepatocytes was shown to trigger NASH progression. Increased apoptosis was a prominent feature in c-MetΔ(hepa) livers[39]. Intermittent high glucose levels under lipotoxicity could contribute to the development of NAFLD by increasing oxidative stress and hepatocyte apoptosis via changes in mitochondrial permeability and subsequent mitochondrial dysfunction[40].
Bid promotes liver fibrosis coupled with a reduction of inflammation in experimental NASH models. In these models, hepatocyte apoptosis triggered hepatic stellate cell activation as well as liver fibrosis[41]. Increased expression of hepatocellular carcinoma down-regulated mitochondrial carrier protein (HDMCP) was identified in NASH animal models and HFFA-72h cultured L02 cells. The miR-146-HDMCP-downstream effector pathway is involved in NASH[42]. Collectively, previous studies have demonstrated that apoptosis resulting from mitochondrial injury is associated with the progression of NAFLD and NASH.
Monounsaturated and saturated fatty acids have been shown to induce cellular steatosis, apoptosis, and JNK activation in hepatocytes[33]. Steatotic hepatocytes from a murine NAFLD model were sensitive to TNF-α-induced apoptosis via the ASK1–JNK signaling pathway[43]. Free fatty acids (FFA)-induced ER stress is associated with JNK activation, which has been well documented in human steatosis[35]. Mixed lineage kinase 3 (MLK) 3 is one of the mitogen-activated protein kinases (MAP3K) that mediate JNK activation in the liver. MLK3 is involved in human NASH through JNK activation[44].
The interplay of p-JNK with mitochondrial Sab (Sh3bp5) leads to impaired respiration, production of reactive oxygen species (ROS), sustained JNK activation, apoptosis in condition of lipotoxicity, and ultimately contributes to the pathogenesis of NASH[45]. Dramatically reduced expression of cellular repressor of E1A-stimulated genes (CREG) and hyperactivated JNK1 signaling has been observed in the livers of NAFLD patients[46]. CREG is a robust suppressor of hepatic steatosis and metabolic disorders through its direct interaction with ASK1 and the subsequent inactivation of ASK1-JNK1 signaling[46]. Thus, JNK signaling pathways play an important role in the apoptosis of NAFLD and NASH.
The complement cascade to clear apoptotic cells and promote liver regeneration is also involved in the progression of NAFLD and NASH[47]. Distant major histocompatibility complex class I-related chains A and B (MIC A/B) have been identified as ligands for the NK cell receptor G2D (NKG2D) in humans. Compared to controls, patients with NASH dysplayed increases in NKG2D and MIC A/B mRNA, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-death receptor 5 (DR5), CD95/Fas mRNA, and hepatocyte apoptosis[48]. These increases suggest that MIC A/B levels also affect the progression of NASH. TRAIL-producing natural killer (NK) cells actively promote a pro-inflammatory environment in the early stages of fatty liver disease, which suggests that this cell compartment may contribute to the progression of NASH[49].
Proliferation of hepatic macrophages, and the subsequent production of pro-inflammatory cytokines, initiate inflammatory cascades, orchestrate the activities of transcription factors involved in lipid metabolism/translocation, and modulate programmed cell death[50]. The macrophage activation marker-soluble CD163 was independently associated with the apoptosis marker CK-18 in Australian and Italian NAFLD patients[51]. Furthermore, down-modulation of NF-κB1 stimulates the progression of NASH in mice by promoting natural killer T (NKT)-cell-mediated responses[52]. Macrophage scavenger receptors), which play a role in the activation of signal transduction pathways that regulate inflammation, apoptotic cell clearance, chemoattraction and angiogenesis, are involved in both the early and advanced stages of NASH[53].
Wobser et al[54] has demonstrated that human hepatic stellate cells (HSCs) that were incubated with conditioned medium (CM) from steatotic hepatocytes and had fibrogenic activation and were resistant to apoptosis, which is important in the progression of fibrosis in chronic liver diseases[54]. Myeloperoxidase (MPO), a highly oxidative enzyme secreted by leukocytes, contributes to the activation of HSCs and is a part of a proapoptotic and profibrotic pathway of progression in NASH[55]. Thus, in patients with NAFLD and NASH, apoptotic hepatocytes stimulate immune cells and HSCs, which contributes to the progression of fibrosis in the liver.
Feeding tumor necrosis factor (TNF) receptors 1 and 2 double-knock out mice (TNFRDKO mice) with an MCD-diet for 8 weeks attenuated liver steatosis and fibrosis and also suppressed hepatic induction of TNF-α, vascular cell adhesion molecule 1, and intracellular adhesion molecule 1, compared to wild-type control mice[6]. These results suggest that blocking the signaling of TNF receptors 1 and 2 is a promising therapeutic target for patients with NASH[56]. The TNF receptor 1-signaling pathway plays a role in aggravating a state of “simple steatosis” towards a phenotype with “NASH”[57]. Cyclooxygenase (COX)-2 may promote hepatocellular apoptosis by interacting with TNF-α and IL6 in rats with NASH[58]. COX-2 is highly expressed in NASH.
Lipoapoptotic supernatants stimulated monocyte migration to a similar magnitude as monocyte chemoattractant protein, CCL2 (MCP-1)[59]. The release of pannexin1-dependent pathophysiological eATP in lipoapoptosis can stimulate the migration of human monocytes in NASH[59]. In cultured Kupffer cells, cholesterol induced the expression of chemotactic and inflammatory cytokines (CCL2 and CXCL2, and IL1β, TNF and oncostatin M, respectively) and rendered hepatocytes more susceptible to apoptosis[60]. Lipids, which stimulate DR5, have been shown to induce the release of hepatocyte extracellular vesicles, which contain TRAILs. Lipids also induced the expression of IL1β and IL6 messenger RNAs in bone marrow-derived macrophages in mice[61]. The C-X-C motif chemokine 10 (CXCL10), which is known to be a pro-inflammation chemokine, was recently shown to play a pivotal role in the pathogenesis of NASH. By binding to its specific receptor CXCR3, CXCL10 recruits activated CXCR3+ T lymphocytes and macrophages to the parenchyma and promotes inflammation, apoptosis, and fibrosis[62].
The dsRNA receptor Nod-like receptor X1 and NLRP3 inflammasomes may be important in the development of NASH[63]. The function of receptor interacting protein kinase-3 (RIP3)-dependent “necroptosis” in NASH and NASH-induced fibrosis is currently unknown[64]. RIP3-dependent necroptosis controls NASH-induced liver fibrosis[64]. The absence of RIP3, a key mediator of necroptosis, exacerbates HFD-induced liver injury. This exacerbation is associated with increased inflammation and hepatocyte apoptosis as well as early fibrotic responses. These findings indicate that shifts in the mode of hepatocellular death can influence disease progression. Therefore, they may have therapeutic implications because manipulation of hepatocyte cell death pathways is currently considered to be a target for treatment of nonalcoholic fatty liver disease[65]. Thus, inflammasomes and cytokines induce apoptosis and respond to hepatocyte apoptosis in NAFLD and NASH.
It has also been reported that oxidized phosphatidylcholine is localized in apoptotic hepatocytes in the livers of patients with the steatotic disorders, which indicates that oxidized phosphatidylcholine is formed in oxidatively damaged hepatocytes[66]. Transforming growth factor β (TGFβ) may regulate p53/p66Shc signaling in both the progression of human NASH and ROS levels and apoptosis[67]. NAFLD patients with reticuloendothelial system (RES) iron have increased TUNEL staining and cellular oxidative stress[68]. RES iron has been shown to be associated with NASH as well as more-severe histologic features[68].
TGFβ signaling activates Smad- and TGFβ-activated kinase 1-dependent signaling and plays a role in regulating cell survival, proliferation, fibrosis, and tumorigenesis. In hepatocytes, TGFβ signaling contributes to hepatocyte death and lipid accumulation through Smad signaling and ROS production, leading to the development of NASH[69]. NOX isoforms, including NOX1, NOX2 and NOX4, and NOX-derived ROS have all been implicated in regulating HSC activation and hepatocyte apoptosis. Both HSC activation and hepatocyte apoptosis are essential steps for the initiation of liver fibrosis and its progression[70]. Mainstream cigarette smoke has been shown to be associated with the degree of oxidative stress and hepatocellular apoptosis in NASH mice[71].
Oxidative stress is central to the pathogenesis of NASH. ROS are characterized by oxidative stress. ROS are generated in several cellular sites and their production is influenced by multi-organ interactions. For fatty liver diseases, mitochondrial dysfunction is the main source of ROS and is closely related to endoplasmic reticulum stress. Both are caused by lipotoxicity and together these three factors form a cycle of progressive organelle damage that results in sterile inflammation and apoptosis[72].
FFAs can increase ER stress, leading to nuclear NF-κB activation and TG2 induction through the pancreatic ER kinase (PERK)-dependent pathways[37,73]. CCAAT/enhancer-binding protein homologous protein (CHOP) deficiency has been found to attenuate apoptosis, inflammation, fibrosis, and tumorigenesis in mice who are exposed to fat-loading conditions. This finding indicates CHOP promotes hepatocarcinogenesis in NASH[74]. The overexpression of hypoxia-inducible factor 1α (HIF-1α) has also been shown to blunt upregulation of the ER stress markers, CHOP and chaperone immunoglobulin heavy chain binding protein (GRP78/Bip), while knocking down HIF-1α increases the level of CHOP. These finding indicate that hepatocyte lipotoxicity is associated with decreased HIF-1α expression[75].
MiR-615-3p regulates lipoapoptosis by inhibiting CHOP and may be associated with the pathogenesis of NASH[76]. After exposure to saturated FFA, CHOP has been shown to induce hepatocyte cell apoptosis and inflammatory responses by activating NF-κB through a pathway involving the expression of IL1 receptor associated kinase 2. This activation results in the direct secretion of the cytokines IL8 and TNFα from hepatocytes[77]. Glucagon-like peptide-1 was found to protect against NAFLD by inactivating the ER stress-associated apoptosis pathway[78].
Loss of the unfolded protein response of regulator X-box binding protein 1 enhances injury in both in vivo and in vitro models of fatty liver injury[79]. Hepatocytes in a lipotoxic state ultimately undergo apoptosis through the upregulation of proteins involved in various pathways including PERK, CHOP, JNK, BIM, PUMA, and eventually, caspases[80].
Expression of microtubule associated protein 1 light chain 3α (LC3)-II, a hallmark of autophagic flux, was founded to be markedly increased in liver specimens from patients with NASH. JNK1 promotes palmitic acid-induced lipoapoptosis, whereas JNK2 activates pro-survival autophagy and inhibits palmitic acid lipotoxicity[81]. Palmitate may induce autophagy by activating the PKCα pathway in hepatocytes. Autophagy plays a protective role in palmitate-induced apoptosis in hepatocytes[82]. Tumor protein p53 binding protein 2 (ASPP2) is a pro-apoptotic member of the p53 binding protein family that inhibits autophagy[83]. Xie et al[83] reported that ASPP2 may participate in the lipid metabolism of non-alcoholic steatohepatitis. Mitochondrial uncoupling protein 2 (UCP2) also plays a role in the development of NASH[84]. Increasing UCP2 expression in hepatoma cells may contribute to cell autophagy and may inhibit apoptosis as result of fatty acid injury[84]. Cellular degradation of Kelch-like ECH-associated protein 1 through the progress of sequestrosome (SQSTM)1/p62-dependent autophagy activates JNK, upregulates expression of Bim and PUMA, and contributes to hepatocyte apoptosis induced by saturated FFAs[85]. Parkin-mediated mitophagy may mitigate hepatocyte apoptosis, improve mitochondrial quality, and suppress steatosis (lipid accumulation) in animal models of alcoholic fatty liver disease[86]. In rats treated with ethanol-enhanced hepatic mitophagy was associated with Parkin mitochondrial translocation, which was triggered by oxidative mitochondrial DNA damage[86]. Rubicon is overexpressed and plays a pathogenic role in NAFLD by accelerating hepatocellular lipoapoptosis and lipid accumulation and inhibiting autophagy[87]. Sirtuin 3 (SIRT3) is a nicotinamide adenine dinucleotide-dependent deacetylase that is primarily located inside the mitochondria[88]. SIRT3 negatively regulates autophagy, thereby enhancing the susceptibility of hepatocytes to SFA-induced cytotoxicity[88].
Thus, ROS production, oxidative stress, and ER stress are all known to induce apoptosis. Autophagy modifies the progression of NAFLD and NASH and may have a protective role in hepatocyte apoptosis.
Hepatic insulin signaling is impaired in NASH patients, where downregulation of insulin-sensitive targets is associated with increased apoptosis and fibrogenesis[89]. Hyperinsulinemia has been shown to alter nuclear transcriptional regulators of cholesterol homeostasis. This leads hepatic accumulation of free cholesterol, hepatic injury, and apoptosis in NASH patients[90].
Fibroblast growth factor (FGF)-21 is highly expressed in the liver and regulates glucose and lipid metabolism in rodents. Concentration of FGF-21 were found to be significantly and independently correlated with hepatic fat content and markers of hepatic apoptosis in obese youth[91]. Another study found that FGF-21 mRNA expression in the human liver increased with steatosis grade and that its serum level is significantly elevated in adult NAFLD patients[92].
Intrahepatic expression of dipeptidyl peptidase-4 (DPP4) and circulating DPP4 (cDPP4) levels and its enzymatic activity are all increased in NAFLD[93]. Circulating DPP4 activity correlates with measures of hepatocyte apoptosis and fibrosis in NAFLD in patients with type 2 diabetes mellitus and/or obesity[93]. Senescence marker protein-30 is involved in both glucose metabolism disorder and NAFLD[94].
TRAIL receptor signaling was also found to be involved in the pathogenesis of NASH in mice with a genetic deletion of the TRAIL receptor[95]. Furthermore, patients with NASH had significantly reduced plasma TRAIL concentrations compared to controls, patients with simple steatosis, or obese individuals[96]. TRAIL protects against insulin resistance, NAFLD, and vascular inflammation. Increasing TRAIL levels may be an attractive therapeutic strategy for reducing symptoms of diabetes as well as liver and vascular injuries, which are commonly observed in individuals with NAFLD[96].
The serine/threonine kinases, glycogen synthase kinase GSK-3α and GSK-3β, can participate in pro-apoptotic signaling during FFA-induced lipoapoptosis[35]. More specifically, saturated fatty acids strongly induce hepatocyte apoptosis[97]. Saturated fatty acids up-regulate the inflammasome in hepatocytes and lead to sensitization to LPS-induced inflammasome activation and inflammatory injury[98,99]. Saturated fatty acids also induce hepatocyte apoptosis and the activation of caspase 8, which triggers the release of dangerous molecules[98].
Resistance to lipoapoptosis is, in part, due to an autocrine hedgehog signaling pathway[14]. Farnesoid X receptor is a member of the nuclear receptor superfamily that plays a crucial role in bile acid, cholesterol, lipid, and glucose metabolism as well as apoptosis[100]. It is also involved in the pathogenesis of NASH. The cellular inhibitor of apoptosis proteins 1 and 2 (cIAP-1 and cIAP-2) are potent inhibitors of death receptor-mediated apoptosis. Proteasomal degradation of cIAPs by FFA contributes to hepatocyte lipoapoptosis[101]. Palmitate-induced lipoapoptosis is dependent on calcium-stimulated mitochondrial activation, which induces oxidative stress and hepatic cell lipotoxicity[102].
Free cholesterol accumulates in NASH patients but not in simple steatosis. Mitochondrial free cholesterol deposition causes hepatocyte apoptosis and necrosis by activating JNK1[103]. High-mobility-group-box 1 and toll-like receptor 4 are both involved in this activation mechanism[103]. Cholesterol markedly promoted the apoptosis of steatosis HepG2 cells in vitro, likely through the up-regulation of expression of Bax and caspase 3[104]. Palmitate activation by fatty acid transport protein 4 triggers hepatocellular apoptosis via altered phospholipid composition and steatosis by acylation into complex lipids[105]. These complex lipids are involved in the development of NAFLD[105]. E2F transcription factors are known regulators of the cell cycle, proliferation, apoptosis, and differentiation. E2F1 regulates lipid synthesis and glycolysis and thus contributes to the development of NAFLD[106].
Androgen-dependent proapoptotic polycystic ovarian syndrome (PCOS) may directly contribute to NAFLD progression in PCOS patients[107]. A recent study gave a complementary fast food (FF) diet to a NASH mouse model, thus mimicking features of the metabolic syndrome. The study found that miR-21 levels increased in both the liver and muscle and expression of peroxisome proliferator-activated receptor α, a key miR-21 target, was decreased[108]. In a typical model of NASH-associated liver damage, miR-21 ablation results in a progressive decrease in steatosis, inflammation and lipoapoptosis, with a subsequent impairment of fibrosis[108].
Intestinal endotoxin [lipopolysaccharide (LPS)] augments liver injury in MCD mice[109]. In a recent study, a group of male C57BL/6 mice were fed with a MCD diet for 17 days, injected with LPS intraperitoneally, and sacrificed 6 h after LPS injection. The study found that LPS upregulated TNF-α production, which induce hepatocyte apoptosis[110]. Palmitate and lysophosphatidylcholine (LPC) induced upregulation of the p53-upregulated modulator of apoptosis and cell-surface expression of the death receptor TNF-related apoptosis-inducing ligand receptor 2[111]. In part, microbiota may be involved in the progression of NAFLD and NASH through hepatocyte apoptosis.
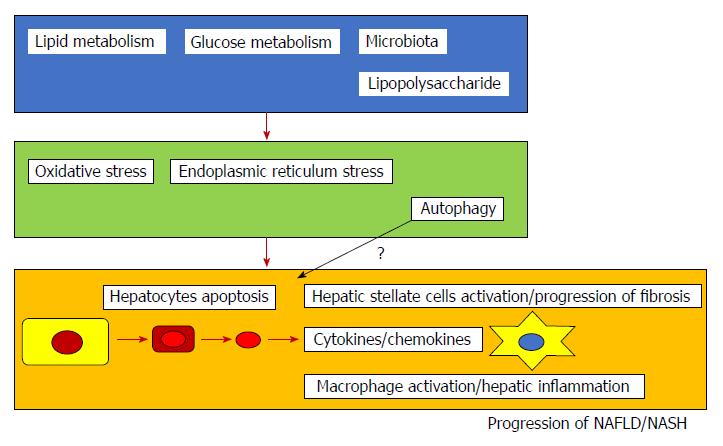
Cell death, including apoptosis, seems important in the progression of NAFLD and NASH (Figure 1). Recently, several inhibitors of apoptosis have been suggested as potential treatments for NASH (Table 1)[112-124]. Clinical trials for the treatment of NASH are currently being conducted[125] and some are targeting apoptosis in NASH patients. Increased hepatocyte apoptosis may distinguish NASH from NAFLD[126]. Repair responses may play an important role in controlling the disease severities of NASH[126]. We reviewed published articles related to this topic and discussed the importance of apoptosis in NAFLD and NASH.
| Drug and reagents | Targets | Mechanism of action | Ref. |
| Triacsin C | Intracellular long-chain acyl-CoA synthetases (ACSL) | Triacylglycerol (TAG) accumulation into lipid droplets | [112] |
| Ezetimibe | AMPK phosphorylation | TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition | [113] |
| Baicalin | Inhibition of NF-κB | NF-κB anti-inflammation signaling pathways | [114] |
| Granulocyte colony stimulating factor (G-CSF) | PI3K/Akt | Activation of PI3K and Akt pathway | [115] |
| Elafibranor (GFT505) | PPARα/δ | Agonist of PPARα/δ receptors | [116] |
| Isoquercitrin | Dipeptidyl peptidase-IV(DPP-IV) | Activation of glucagonlike peptide-1 (GLP-1) | [117] |
| Activated carbon N-acetylcysteine (ACNAC) microcapsules | Telomerase | Improved telomerase activity | [118] |
| 3-Acetyl-oleanolic acid (3Ac-OA) | Glucose transporter type 2 (GLUT-2), low-density lipoprotein receptor (LDLR) | AMPK-related pathways | [119] |
| Meretrix oligopeptides (MMO) | NF-κB | NF-κB anti-inflammation signaling pathways | [120] |
| Seladelpar (MBX-8025) | Proliferator-activated receptor- delta (PPAR-δ) | Selective PPAR-δ agonist | [121] |
| Resveratrol | Sirt1 | Antioxidant | [122] |
| TBE-31 | NF-E2 p45-related factor 2 (Nrf2) | Regulation of intracellular redox homeostasis | [124] |
| 1. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2928] [Article Influence: 108.4] [Reference Citation Analysis (3)] |
| 2. | Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37 Suppl 1:81-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 449] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 3. | Takahashi T, So-Wan T, Kamimura T, Asakura H. Infiltrating polymorphonuclear leukocytes and apoptotic bodies derived from hepatocytes but not from ballooning hepatocytes containing Mallory bodies show nuclear DNA fragmentation in alcoholic hepatitis. Alcohol Clin Exp Res. 2000;24:68S-73S. [PubMed] |
| 4. | Povero D, Feldstein AE. Novel Molecular Mechanisms in the Development of Non-Alcoholic Steatohepatitis. Diabetes Metab J. 2016;40:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437-443. [PubMed] |
| 6. | McArthur K, Kile BT. Apoptotic Caspases: Multiple or Mistaken Identities? Trends Cell Biol. 2018;28:475-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27-33. [PubMed] |
| 8. | Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 550] [Cited by in RCA: 533] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 9. | Hsieh S, Leaderer BP, Feldstein AE, Santoro N, McKay LA, Caprio S, McConnell R. Traffic-related air pollution associations with cytokeratin-18, a marker of hepatocellular apoptosis, in an overweight and obese paediatric population. Pediatr Obes. 2018;13:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Kucukoglu O, Guldiken N, Chen Y, Usachov V, El-Heliebi A, Haybaeck J, Denk H, Trautwein C, Strnad P. High-fat diet triggers Mallory-Denk body formation through misfolding and crosslinking of excess keratin 8. Hepatology. 2014;60:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Witek RP, Stone WC, Karaca FG, Syn WK, Pereira TA, Agboola KM, Omenetti A, Jung Y, Teaberry V, Choi SS. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50:1421-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 12. | Anstee QM, Concas D, Kudo H, Levene A, Pollard J, Charlton P, Thomas HC, Thursz MR, Goldin RD. Impact of pan-caspase inhibition in animal models of established steatosis and non-alcoholic steatohepatitis. J Hepatol. 2010;53:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Ferreira DM, Castro RE, Machado MV, Evangelista T, Silvestre A, Costa A, Coutinho J, Carepa F, Cortez-Pinto H, Rodrigues CM. Apoptosis and insulin resistance in liver and peripheral tissues of morbidly obese patients is associated with different stages of non-alcoholic fatty liver disease. Diabetologia. 2011;54:1788-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Kakisaka K, Cazanave SC, Werneburg NW, Razumilava N, Mertens JC, Bronk SF, Gores GJ. A hedgehog survival pathway in ‘undead’ lipotoxic hepatocytes. J Hepatol. 2012;57:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 15. | Hatting M, Zhao G, Schumacher F, Sellge G, Al Masaoudi M, Gaβler N, Boekschoten M, Müller M, Liedtke C, Cubero FJ. Hepatocyte caspase-8 is an essential modulator of steatohepatitis in rodents. Hepatology. 2013;57:2189-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Johnson ES, Lindblom KR, Robeson A, Stevens RD, Ilkayeva OR, Newgard CB, Kornbluth S, Andersen JL. Metabolomic profiling reveals a role for caspase-2 in lipoapoptosis. J Biol Chem. 2013;288:14463-14475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Machado MV, Michelotti GA, Pereira Tde A, Boursier J, Kruger L, Swiderska-Syn M, Karaca G, Xie G, Guy CD, Bohinc B. Reduced lipoapoptosis, hedgehog pathway activation and fibrosis in caspase-2 deficient mice with non-alcoholic steatohepatitis. Gut. 2015;64:1148-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Machado MV, Kruger L, Jewell ML, Michelotti GA, Pereira Tde A, Xie G, Moylan CA, Diehl AM. Vitamin B5 and N-Acetylcysteine in Nonalcoholic Steatohepatitis: A Preclinical Study in a Dietary Mouse Model. Dig Dis Sci. 2016;61:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Li CP, Li JH, He SY, Li P, Zhong XL. Roles of Fas/Fasl, Bcl-2/Bax, and Caspase-8 in rat nonalcoholic fatty liver disease pathogenesis. Genet Mol Res. 2014;13:3991-3999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Alkhouri N, Alisi A, Okwu V, Matloob A, Ferrari F, Crudele A, De Vito R, Lopez R, Feldstein AE, Nobili V. Circulating Soluble Fas and Fas Ligand Levels Are Elevated in Children with Nonalcoholic Steatohepatitis. Dig Dis Sci. 2015;60:2353-2359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Huo J, Ma Y, Liu JJ, Ho YS, Liu S, Soh LY, Chen S, Xu S, Han W, Hong A. Loss of Fas apoptosis inhibitory molecule leads to spontaneous obesity and hepatosteatosis. Cell Death Dis. 2016;7:e2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Castro RE, Ferreira DM, Afonso MB, Borralho PM, Machado MV, Cortez-Pinto H, Rodrigues CM. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J Hepatol. 2013;58:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 23. | Derdak Z, Villegas KA, Harb R, Wu AM, Sousa A, Wands JR. Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease. J Hepatol. 2013;58:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | Daugherity EK, Balmus G, Al Saei A, Moore ES, Abi Abdallah D, Rogers AB, Weiss RS, Maurer KJ. The DNA damage checkpoint protein ATM promotes hepatocellular apoptosis and fibrosis in a mouse model of non-alcoholic fatty liver disease. Cell Cycle. 2012;11:1918-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Nobili V, Carpino G, Alisi A, Franchitto A, Alpini G, De Vito R, Onori P, Alvaro D, Gaudio E. Hepatic progenitor cells activation, fibrosis, and adipokines production in pediatric nonalcoholic fatty liver disease. Hepatology. 2012;56:2142-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Lee SJ, Yoo JD, Choi SY, Kwon OS. The expression and secretion of vimentin in the progression of non-alcoholic steatohepatitis. BMB Rep. 2014;47:457-462. [PubMed] |
| 27. | Gandhi CR, Chaillet JR, Nalesnik MA, Kumar S, Dangi A, Demetris AJ, Ferrell R, Wu T, Divanovic S, Stankeiwicz T. Liver-specific deletion of augmenter of liver regeneration accelerates development of steatohepatitis and hepatocellular carcinoma in mice. Gastroenterology. 2015;148:379-391.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Matsunaga Y, Nakatsu Y, Fukushima T, Okubo H, Iwashita M, Sakoda H, Fujishiro M, Yamamotoya T, Kushiyama A, Takahashi S. LUBAC Formation Is Impaired in the Livers of Mice with MCD-Dependent Nonalcoholic Steatohepatitis. Mediators Inflamm. 2015;2015:125380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Koyama N, Yamazaki T, Kanetsuki Y, Hirota J, Asai T, Mitsumoto Y, Mizuno M, Shima T, Kanbara Y, Arai S. Activation of apoptosis inhibitor of macrophage is a sensitive diagnostic marker for NASH-associated hepatocellular carcinoma. J Gastroenterol. 2018;53:770-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Zhang P, Wang PX, Zhao LP, Zhang X, Ji YX, Zhang XJ, Fang C, Lu YX, Yang X, Gao MM. The deubiquitinating enzyme TNFAIP3 mediates inactivation of hepatic ASK1 and ameliorates nonalcoholic steatohepatitis. Nat Med. 2018;24:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 31. | Ramalho RM, Cortez-Pinto H, Castro RE, Solá S, Costa A, Moura MC, Camilo ME, Rodrigues CM. Apoptosis and Bcl-2 expression in the livers of patients with steatohepatitis. Eur J Gastroenterol Hepatol. 2006;18:21-29. [PubMed] |
| 32. | Ribeiro PS, Cortez-Pinto H, Solá S, Castro RE, Ramalho RM, Baptista A, Moura MC, Camilo ME, Rodrigues CM. Hepatocyte apoptosis, expression of death receptors, and activation of NF-kappaB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am J Gastroenterol. 2004;99:1708-1717. [PubMed] |
| 33. | Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093-12101. [PubMed] |
| 34. | El Bassat H, Ziada DH, Hasby EA, Nagy H, Abo Ryia MH. Apoptotic and anti-apoptotic seromarkers for assessment of disease severity of non-alcoholic steatohepatitis. Arab J Gastroenterol. 2014;15:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Ibrahim SH, Akazawa Y, Cazanave SC, Bronk SF, Elmi NA, Werneburg NW, Billadeau DD, Gores GJ. Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte lipoapoptosis. J Hepatol. 2011;54:765-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Cazanave SC, Mott JL, Elmi NA, Bronk SF, Masuoka HC, Charlton MR, Gores GJ. A role for miR-296 in the regulation of lipoapoptosis by targeting PUMA. J Lipid Res. 2011;52:1517-1525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Kuo TF, Tatsukawa H, Matsuura T, Nagatsuma K, Hirose S, Kojima S. Free fatty acids induce transglutaminase 2-dependent apoptosis in hepatocytes via ER stress-stimulated PERK pathways. J Cell Physiol. 2012;227:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Wang HN, Chen HD, Chen KY, Xiao JF, He K, Xiang GA, Xie X. Highly expressed MT-ND3 positively associated with histological severity of hepatic steatosis. APMIS. 2014;122:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Kroy DC, Schumacher F, Ramadori P, Hatting M, Bergheim I, Gassler N, Boekschoten MV, Müller M, Streetz KL, Trautwein C. Hepatocyte specific deletion of c-Met leads to the development of severe non-alcoholic steatohepatitis in mice. J Hepatol. 2014;61:883-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Yin X, Zheng F, Pan Q, Zhang S, Yu D, Xu Z, Li H. Glucose fluctuation increased hepatocyte apoptosis under lipotoxicity and the involvement of mitochondrial permeability transition opening. J Mol Endocrinol. 2015;55:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Eguchi A, De Mollerat Du Jeu X, Johnson CD, Nektaria A, Feldstein AE. Liver Bid suppression for treatment of fibrosis associated with non-alcoholic steatohepatitis. J Hepatol. 2016;64:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Jin X, Liu J, Chen YP, Xiang Z, Ding JX, Li YM. Effect of miR-146 targeted HDMCP up-regulation in the pathogenesis of nonalcoholic steatohepatitis. PLoS One. 2017;12:e0174218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Zhang W, Kudo H, Kawai K, Fujisaka S, Usui I, Sugiyama T, Tsukada K, Chen N, Takahara T. Tumor necrosis factor-alpha accelerates apoptosis of steatotic hepatocytes from a murine model of non-alcoholic fatty liver disease. Biochem Biophys Res Commun. 2010;391:1731-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 44. | Ibrahim SH, Gores GJ, Hirsova P, Kirby M, Miles L, Jaeschke A, Kohli R. Mixed lineage kinase 3 deficient mice are protected against the high fat high carbohydrate diet-induced steatohepatitis. Liver Int. 2014;34:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Win S, Than TA, Le BH, García-Ruiz C, Fernandez-Checa JC, Kaplowitz N. Sab (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. J Hepatol. 2015;62:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 46. | Zhang QY, Zhao LP, Tian XX, Yan CH, Li Y, Liu YX, Wang PX, Zhang XJ, Han YL. The novel intracellular protein CREG inhibits hepatic steatosis, obesity, and insulin resistance. Hepatology. 2017;66:834-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Rensen SS, Slaats Y, Driessen A, Peutz-Kootstra CJ, Nijhuis J, Steffensen R, Greve JW, Buurman WA. Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology. 2009;50:1809-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 48. | Kahraman A, Schlattjan M, Kocabayoglu P, Yildiz-Meziletoglu S, Schlensak M, Fingas CD, Wedemeyer I, Marquitan G, Gieseler RK, Baba HA. Major histocompatibility complex class I-related chains A and B (MIC A/B): a novel role in nonalcoholic steatohepatitis. Hepatology. 2010;51:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Gomez-Santos L, Luka Z, Wagner C, Fernandez-Alvarez S, Lu SC, Mato JM, Martinez-Chantar ML, Beraza N. Inhibition of natural killer cells protects the liver against acute injury in the absence of glycine N-methyltransferase. Hepatology. 2012;56:747-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Wu R, Nakatsu G, Zhang X, Yu J. Pathophysiological mechanisms and therapeutic potentials of macrophages in non-alcoholic steatohepatitis. Expert Opin Ther Targets. 2016;20:615-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Kazankov K, Barrera F, Møller HJ, Rosso C, Bugianesi E, David E, Ibrahim Kamal Jouness R, Esmaili S, Eslam M, McLeod D. The macrophage activation marker sCD163 is associated with morphological disease stages in patients with non-alcoholic fatty liver disease. Liver Int. 2016;36:1549-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 52. | Locatelli I, Sutti S, Vacchiano M, Bozzola C, Albano E. NF-κB1 deficiency stimulates the progression of non-alcoholic steatohepatitis (NASH) in mice by promoting NKT-cell-mediated responses. Clin Sci (Lond). 2013;124:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 53. | Bieghs V, Wouters K, van Gorp PJ, Gijbels MJ, de Winther MP, Binder CJ, Lütjohann D, Febbraio M, Moore KJ, van Bilsen M. Role of scavenger receptor A and CD36 in diet-induced nonalcoholic steatohepatitis in hyperlipidemic mice. Gastroenterology. 2010;138:2477-2486, 2486.e1-2486.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 54. | Wobser H, Dorn C, Weiss TS, Amann T, Bollheimer C, Büttner R, Schölmerich J, Hellerbrand C. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009;19:996-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 55. | Pulli B, Ali M, Iwamoto Y, Zeller MW, Schob S, Linnoila JJ, Chen JW. Myeloperoxidase-Hepatocyte-Stellate Cell Cross Talk Promotes Hepatocyte Injury and Fibrosis in Experimental Nonalcoholic Steatohepatitis. Antioxid Redox Signal. 2015;23:1255-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 56. | Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415-424. [PubMed] |
| 57. | Aparicio-Vergara M, Hommelberg PP, Schreurs M, Gruben N, Stienstra R, Shiri-Sverdlov R, Kloosterhuis NJ, de Bruin A, van de Sluis B, Koonen DP. Tumor necrosis factor receptor 1 gain-of-function mutation aggravates nonalcoholic fatty liver disease but does not cause insulin resistance in a murine model. Hepatology. 2013;57:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Cheng Q, Li N, Chen M, Zheng J, Qian Z, Wang X, Huang C, Xu S, Shi G. Cyclooxygenase-2 promotes hepatocellular apoptosis by interacting with TNF-α and IL-6 in the pathogenesis of nonalcoholic steatohepatitis in rats. Dig Dis Sci. 2013;58:2895-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Xiao F, Waldrop SL, Bronk SF, Gores GJ, Davis LS, Kilic G. Lipoapoptosis induced by saturated free fatty acids stimulates monocyte migration: a novel role for Pannexin1 in liver cells. Purinergic Signal. 2015;11:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Henkel J, Coleman CD, Schraplau A, Johrens K, Weber D, Castro JP, Hugo M, Schulz TJ, Krämer S, Schürmann A. Induction of steatohepatitis (NASH) with insulin resistance in wildtype B6 mice by a western-type diet containing soybean oil and cholesterol. Mol Med. 2017; 23: Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, Shah VH, Malhi H, Gores GJ. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology. 2016;150:956-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 419] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 62. | Xu Z, Zhang X, Lau J, Yu J. C-X-C motif chemokine 10 in non-alcoholic steatohepatitis: role as a pro-inflammatory factor and clinical implication. Expert Rev Mol Med. 2016;18:e16. [PubMed] |
| 63. | Wang YG, Fang WL, Wei J, Wang T, Wang N, Ma JL, Shi M. The involvement of NLRX1 and NLRP3 in the development of nonalcoholic steatohepatitis in mice. J Chin Med Assoc. 2013;76:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, Kreggenwinkel K, Schneider AT, Bartneck M, Neumann UP. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol Med. 2014;6:1062-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 353] [Article Influence: 32.1] [Reference Citation Analysis (1)] |
| 65. | Roychowdhury S, McCullough RL, Sanz-Garcia C, Saikia P, Alkhouri N, Matloob A, Pollard KA, McMullen MR, Croniger CM, Nagy LE. Receptor interacting protein 3 protects mice from high-fat diet-induced liver injury. Hepatology. 2016;64:1518-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 66. | Ikura Y, Ohsawa M, Suekane T, Fukushima H, Itabe H, Jomura H, Nishiguchi S, Inoue T, Naruko T, Ehara S. Localization of oxidized phosphatidylcholine in nonalcoholic fatty liver disease: impact on disease progression. Hepatology. 2006;43:506-514. [PubMed] |
| 67. | Tomita K, Teratani T, Suzuki T, Oshikawa T, Yokoyama H, Shimamura K, Nishiyama K, Mataki N, Irie R, Minamino T. p53/p66Shc-mediated signaling contributes to the progression of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2012;57:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Maliken BD, Nelson JE, Klintworth HM, Beauchamp M, Yeh MM, Kowdley KV. Hepatic reticuloendothelial system cell iron deposition is associated with increased apoptosis in nonalcoholic fatty liver disease. Hepatology. 2013;57:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 69. | Yang L, Roh YS, Song J, Zhang B, Liu C, Loomba R, Seki E. Transforming growth factor beta signaling in hepatocytes participates in steatohepatitis through regulation of cell death and lipid metabolism in mice. Hepatology. 2014;59:483-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 70. | Liang S, Kisseleva T, Brenner DA. The Role of NADPH Oxidases (NOXs) in Liver Fibrosis and the Activation of Myofibroblasts. Front Physiol. 2016;7:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 71. | Park S, Kim JW, Yun H, Choi SJ, Lee SH, Choi KC, Lim CW, Lee K, Kim B. Mainstream cigarette smoke accelerates the progression of nonalcoholic steatohepatitis by modulating Kupffer cell-mediated hepatocellular apoptosis in adolescent mice. Toxicol Lett. 2016;256:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 72. | Mann JP, Raponi M, Nobili V. Clinical implications of understanding the association between oxidative stress and pediatric NAFLD. Expert Rev Gastroenterol Hepatol. 2017;11:371-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Kuo TF, Tatsukawa H, Kojima S. New insights into the functions and localization of nuclear transglutaminase 2. FEBS J. 2011;278:4756-4767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Toriguchi K, Hatano E, Tanabe K, Takemoto K, Nakamura K, Koyama Y, Seo S, Taura K, Uemoto S. Attenuation of steatohepatitis, fibrosis, and carcinogenesis in mice fed a methionine-choline deficient diet by CCAAT/enhancer-binding protein homologous protein deficiency. J Gastroenterol Hepatol. 2014;29:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Yoo W, Noh KH, Ahn JH, Yu JH, Seo JA, Kim SG, Choi KM, Baik SH, Choi DS, Kim TW. HIF-1α expression as a protective strategy of HepG2 cells against fatty acid-induced toxicity. J Cell Biochem. 2014;115:1147-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Miyamoto Y, Mauer AS, Kumar S, Mott JL, Malhi H. Mmu-miR-615-3p regulates lipoapoptosis by inhibiting C/EBP homologous protein. PLoS One. 2014;9:e109637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 77. | Willy JA, Young SK, Stevens JL, Masuoka HC, Wek RC. CHOP links endoplasmic reticulum stress to NF-κB activation in the pathogenesis of nonalcoholic steatohepatitis. Mol Biol Cell. 2015;26:2190-2204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Ao N, Yang J, Wang X, Du J. Glucagon-like peptide-1 preserves non-alcoholic fatty liver disease through inhibition of the endoplasmic reticulum stress-associated pathway. Hepatol Res. 2016;46:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 79. | Liu X, Henkel AS, LeCuyer BE, Schipma MJ, Anderson KA, Green RM. Hepatocyte X-box binding protein 1 deficiency increases liver injury in mice fed a high-fat/sugar diet. Am J Physiol Gastrointest Liver Physiol. 2015;309:G965-G974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 80. | Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 448] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 81. | Tu QQ, Zheng RY, Li J, Hu L, Chang YX, Li L, Li MH, Wang RY, Huang DD, Wu MC. Palmitic acid induces autophagy in hepatocytes via JNK2 activation. Acta Pharmacol Sin. 2014;35:504-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Cai N, Zhao X, Jing Y, Sun K, Jiao S, Chen X, Yang H, Zhou Y, Wei L. Autophagy protects against palmitate-induced apoptosis in hepatocytes. Cell Biosci. 2014;4:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 83. | Xie F, Jia L, Lin M, Shi Y, Yin J, Liu Y, Chen D, Meng Q. ASPP2 attenuates triglycerides to protect against hepatocyte injury by reducing autophagy in a cell and mouse model of non-alcoholic fatty liver disease. J Cell Mol Med. 2015;19:155-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Lou J, Wang Y, Wang X, Jiang Y. Uncoupling protein 2 regulates palmitic acid-induced hepatoma cell autophagy. Biomed Res Int. 2014;2014:810401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Cazanave SC, Sanyal AJ. KEAP the balance between life and death. Mol Cell Oncol. 2014;2:e968065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 86. | Eid N, Ito Y, Otsuki Y. Triggering of Parkin Mitochondrial Translocation in Mitophagy: Implications for Liver Diseases. Front Pharmacol. 2016;7:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Tanaka S, Hikita H, Tatsumi T, Sakamori R, Nozaki Y, Sakane S, Shiode Y, Nakabori T, Saito Y, Hiramatsu N. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology. 2016;64:1994-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 292] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 88. | Li S, Dou X, Ning H, Song Q, Wei W, Zhang X, Shen C, Li J, Sun C, Song Z. Sirtuin 3 acts as a negative regulator of autophagy dictating hepatocyte susceptibility to lipotoxicity. Hepatology. 2017;66:936-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 89. | García-Monzón C, Lo Iacono O, Mayoral R, González-Rodríguez A, Miquilena-Colina ME, Lozano-Rodríguez T, García-Pozo L, Vargas-Castrillón J, Casado M, Boscá L. Hepatic insulin resistance is associated with increased apoptosis and fibrogenesis in nonalcoholic steatohepatitis and chronic hepatitis C. J Hepatol. 2011;54:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 90. | Van Rooyen DM, Larter CZ, Haigh WG, Yeh MM, Ioannou G, Kuver R, Lee SP, Teoh NC, Farrell GC. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 2011;141:1393-1403, 1403.e1-1403.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 278] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 91. | Giannini C, Feldstein AE, Santoro N, Kim G, Kursawe R, Pierpont B, Caprio S. Circulating levels of FGF-21 in obese youth: associations with liver fat content and markers of liver damage. J Clin Endocrinol Metab. 2013;98:2993-3000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 92. | Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, Zhang H, Pan X, Bao Y, Xiang K. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53:934-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 93. | Williams KH, Vieira De Ribeiro AJ, Prakoso E, Veillard AS, Shackel NA, Brooks B, Bu Y, Cavanagh E, Raleigh J, McLennan SV. Circulating dipeptidyl peptidase-4 activity correlates with measures of hepatocyte apoptosis and fibrosis in non-alcoholic fatty liver disease in type 2 diabetes mellitus and obesity: A dual cohort cross-sectional study. J Diabetes. 2015;7:809-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 94. | Kondo Y, Ishigami A. Involvement of senescence marker protein-30 in glucose metabolism disorder and non-alcoholic fatty liver disease. Geriatr Gerontol Int. 2016;16 Suppl 1:4-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 95. | Idrissova L, Malhi H, Werneburg NW, LeBrasseur NK, Bronk SF, Fingas C, Tchkonia T, Pirtskhalava T, White TA, Stout MB. TRAIL receptor deletion in mice suppresses the inflammation of nutrient excess. J Hepatol. 2015;62:1156-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 96. | Cartland SP, Harith HH, Genner SW, Dang L, Cogger VC, Vellozzi M, Di Bartolo BA, Thomas SR, Adams LA, Kavurma MM. Non-alcoholic fatty liver disease, vascular inflammation and insulin resistance are exacerbated by TRAIL deletion in mice. Sci Rep. 2017;7:1898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Machado MV, Cortez-Pinto H. Cell death and nonalcoholic steatohepatitis: where is ballooning relevant? Expert Rev Gastroenterol Hepatol. 2011;5:213-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 98. | Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 547] [Article Influence: 36.5] [Reference Citation Analysis (12)] |
| 99. | Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1620] [Cited by in RCA: 1937] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 100. | Koutsounas I, Giaginis C, Theocharis S. Farnesoid X Receptor (FXR) from normal to malignant state. Histol Histopathol. 2012;27:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 101. | Akazawa Y, Guicciardi ME, Cazanave SC, Bronk SF, Werneburg NW, Kakisaka K, Nakao K, Gores GJ. Degradation of cIAPs contributes to hepatocyte lipoapoptosis. Am J Physiol Gastrointest Liver Physiol. 2013;305:G611-G619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 102. | Egnatchik RA, Leamy AK, Jacobson DA, Shiota M, Young JD. ER calcium release promotes mitochondrial dysfunction and hepatic cell lipotoxicity in response to palmitate overload. Mol Metab. 2014;3:544-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 103. | Gan LT, Van Rooyen DM, Koina ME, McCuskey RS, Teoh NC, Farrell GC. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J Hepatol. 2014;61:1376-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 104. | Zhu C, Xie P, Zhao F, Zhang L, An W, Zhan Y. Mechanism of the promotion of steatotic HepG2 cell apoptosis by cholesterol. Int J Clin Exp Pathol. 2014;7:6807-6813. [PubMed] |
| 105. | Seeßle J, Liebisch G, Schmitz G, Stremmel W, Chamulitrat W. Palmitate activation by fatty acid transport protein 4 as a model system for hepatocellular apoptosis and steatosis. Biochim Biophys Acta. 2015;1851:549-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Denechaud PD, Lopez-Mejia IC, Giralt A, Lai Q, Blanchet E, Delacuisine B, Nicolay BN, Dyson NJ, Bonner C, Pattou F. E2F1 mediates sustained lipogenesis and contributes to hepatic steatosis. J Clin Invest. 2016;126:137-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 107. | Baranova A, Tran TP, Afendy A, Wang L, Shamsaddini A, Mehta R, Chandhoke V, Birerdinc A, Younossi ZM. Molecular signature of adipose tissue in patients with both non-alcoholic fatty liver disease (NAFLD) and polycystic ovarian syndrome (PCOS). J Transl Med. 2013;11:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 108. | Rodrigues PM, Afonso MB, Simão AL, Carvalho CC, Trindade A, Duarte A, Borralho PM, Machado MV, Cortez-Pinto H, Rodrigues CM. miR-21 ablation and obeticholic acid ameliorate nonalcoholic steatohepatitis in mice. Cell Death Dis. 2017;e2748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 109. | Kirsch R, Clarkson V, Verdonk RC, Marais AD, Shephard EG, Ryffel B, de la M Hall P. Rodent nutritional model of steatohepatitis: effects of endotoxin (lipopolysaccharide) and tumor necrosis factor alpha deficiency. J Gastroenterol Hepatol. 2006;21:174-182. [PubMed] |
| 110. | Kudo H, Takahara T, Yata Y, Kawai K, Zhang W, Sugiyama T. Lipopolysaccharide triggered TNF-alpha-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J Hepatol. 2009;51:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 111. | Hirsova P, Ibrahim SH, Gores GJ, Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758-1770. [PubMed] |
| 112. | Dechandt CRP, Zuccolotto-Dos-Reis FH, Teodoro BG, Fernandes AMAP, Eberlin MN, Kettelhut IC, Curti C, Alberici LC. Triacsin C reduces lipid droplet formation and induces mitochondrial biogenesis in primary rat hepatocytes. J Bioenerg Biomembr. 2017;49:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 113. | Kim SH, Kim G, Han DH, Lee M, Kim I, Kim B, Kim KH, Song YM, Yoo JE, Wang HJ. Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition. Autophagy. 2017;13:1767-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 114. | Zhang J, Zhang H, Deng X, Zhang N, Liu B, Xin S, Li G, Xu K. Baicalin attenuates non-alcoholic steatohepatitis by suppressing key regulators of lipid metabolism, inflammation and fibrosis in mice. Life Sci. 2018;192:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 115. | Nam HH, Jun DW, Jang K, Saeed WK, Lee JS, Kang HT, Chae YJ. Granulocyte colony stimulating factor treatment in non-alcoholic fatty liver disease: beyond marrow cell mobilization. Oncotarget. 2017;8:97965-97976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 116. | Issa D, Wattacheril J, Sanyal AJ. Treatment options for nonalcoholic steatohepatitis - a safety evaluation. Expert Opin Drug Saf. 2017;16:903-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 117. | Huang XL, He Y, Ji LL, Wang KY, Wang YL, Chen DF, Geng Y, OuYang P, Lai WM. Hepatoprotective potential of isoquercitrin against type 2 diabetes-induced hepatic injury in rats. Oncotarget. 2017;8:101545-101559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 118. | Shi T, Yang X, Zhou H, Xi J, Sun J, Ke Y, Zhang J, Shao Y, Jiang X, Pan X. Activated carbon N-acetylcysteine microcapsule protects against nonalcoholic fatty liver disease in young rats via activating telomerase and inhibiting apoptosis. PLoS One. 2018;13:e0189856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 119. | Ou-Yang Q, Xuan CX, Wang X, Luo HQ, Liu JE, Wang LL, Li TT, Chen YP, Liu J. 3-Acetyl-oleanolic acid ameliorates non-alcoholic fatty liver disease in high fat diet-treated rats by activating AMPK-related pathways. Acta Pharmacol Sin. 2018; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 120. | Huang F, Wang J, Yu F, Tang Y, Ding G, Yang Z, Sun Y. Protective Effect of Meretrix meretrix Oligopeptides on High-Fat-Diet-Induced Non-Alcoholic Fatty Liver Disease in Mice. Mar Drugs. 2018;16:pii: E39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 121. | Haczeyni F, Wang H, Barn V, Mridha AR, Yeh MM, Haigh WG, Ioannou GN, Choi YJ, McWherter CA, Teoh NC. The selective peroxisome proliferator-activated receptor-delta agonist seladelpar reverses nonalcoholic steatohepatitis pathology by abrogating lipotoxicity in diabetic obese mice. Hepatol Commun. 2017;1:663-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 122. | Hajighasem A, Farzanegi P, Mazaheri Z. Effects of combined therapy with resveratrol, continuous and interval exercises on apoptosis, oxidative stress, and inflammatory biomarkers in the liver of old rats with non-alcoholic fatty liver disease. Arch Physiol Biochem. 2018;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 123. | Sharma RS, Harrison DJ, Kisielewski D, Cassidy DM, McNeilly AD, Gallagher JR, Walsh SV, Honda T, McCrimmon RJ, Dinkova-Kostova AT. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2). Cell Mol Gastroenterol Hepatol. 2017;5:367-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 124. | Nakamura M, Kanda T, Sasaki R, Haga Y, Jiang X, Wu S, Nakamoto S, Yokosuka O. MicroRNA-122 Inhibits the Production of Inflammatory Cytokines by Targeting the PKR Activator PACT in Human Hepatic Stellate Cells. PLoS One. 2015;10:e0144295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 125. | Sumida Y, Yoneda M. Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol. 2018;53:362-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 455] [Cited by in RCA: 534] [Article Influence: 66.8] [Reference Citation Analysis (1)] |
| 126. | Jung Y, Diehl AM. Non-alcoholic steatohepatitis pathogenesis: role of repair in regulating the disease progression. Dig Dis. 2010;28:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Namisaki T, Shimizu Y, Takahashi T S- Editor: Wang JL L- Editor: A E- Editor: Huang Y