Published online Jun 7, 2018. doi: 10.3748/wjg.v24.i21.2300
Peer-review started: March 28, 2018
First decision: April 19, 2018
Revised: April 28, 2018
Accepted: May 6, 2018
Article in press: May 6, 2018
Published online: June 7, 2018
Processing time: 68 Days and 16.5 Hours
To evaluate the differences in acute kidney injury (AKI) between acute-on-chronic liver failure (ACLF) and decompensated cirrhosis (DC) patients.
During the period from December 2015 to July 2017, 280 patients with hepatitis B virus (HBV)-related ACLF (HBV-ACLF) and 132 patients with HBV-related DC (HBV-DC) who were admitted to our center were recruited consecutively into an observational study. Urine specimens were collected from all subjects and the levels of five urinary tubular injury biomarkers were detected,including neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), liver-type fatty acid binding protein (L-FABP), cystatin C (CysC), and kidney injury molecule-1 (KIM-1). Simultaneously, the patient demographics, occurrence and progression of AKI, and response to terlipressin therapy were recorded. All patients were followed up for 3 mo or until death after enrollment.
AKI occurred in 71 and 28 of HBV-ACLF and HBV-DC patients, respectively (25.4% vs 21.2%, P = 0.358). Among all patients, the levels of four urinary biomarkers (NGAL, CysC, L-FABP, IL-18) were significantly elevated in patients with HBV-ACLF and AKI (ACLF-AKI), compared with that in patients with HBV-DC and AKI (DC-AKI) or those without AKI. There was a higher proportion of patients with AKI progression in ACLF-AKI patients than in DC-AKI patients (49.3% vs 17.9%, P = 0.013). Forty-three patients with ACLF-AKI and 19 patients with DC-AKI were treated with terlipressin. The response rate of ACLF-AKI patients was significantly lower than that of patients with DC-AKI (32.6% vs 57.9%, P = 0.018). Furthermore, patients with ACLF-AKI had the lowest 90 d survival rates among all groups (P < 0.001).
AKI in ACLF patients is more likely associated with structural kidney injury, and is more progressive, with a poorer response to terlipressin treatment and a worse prognosis than that in DC patients.
Core tip: Acute kidney injury (AKI) is common in acute-on-chronic liver failure (ACLF) and decompensated cirrhosis (DC) patients. Though ACLF and DC have been identified as two different diseases, the difference in AKI between these two diseases is rarely studied, and whether AKI should be handled in the same way in both diseases is still uncertain. This study combined multiple tubular injury biomarkers and has shown that AKI in patients with ACLF is distinctly different from in DC patients. AKI in ACLF patients is more likely to be caused by structural damage, and tends to be more progressive, with poorer response to terlipressin treatment and a worse prognosis.
- Citation: Jiang QQ, Han MF, Ma K, Chen G, Wan XY, Kilonzo SB, Wu WY, Wang YL, You J, Ning Q. Acute kidney injury in acute-on-chronic liver failure is different from in decompensated cirrhosis. World J Gastroenterol 2018; 24(21): 2300-2310
- URL: https://www.wjgnet.com/1007-9327/full/v24/i21/2300.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i21.2300
Acute kidney injury (AKI), including hepatorenal syndrome (HRS), is a common complication of patients with acute-on-chronic liver failure (ACLF) or decompensated cirrhosis (DC) and is always associated with poor outcome[1-3]. Previous studies have clearly demonstrated that acute-on-chronic liver failure and decompensated cirrhosis are two different diseases[4,5]. In patients with decompensated cirrhosis, the liver and extrahepatic organ failure usually occurs gradually over several weeks to several months on the basis of cirrhosis, and patients often have severe circulatory dysfunction. For acute-on-chronic liver failure, the liver failure often happens suddenly within 4 wk, in patients with either previously diagnosed or undiagnosed chronic liver disease and is usually associated with a precipitating even, and the systemic inflammatory response play an important role in the pathogenesis of organ failure[4,5]. However, the differences in acute kidney injury between patients with these two diseases are rarely studied, and it is uncertain whether AKI should be treated in the same way in these two diseases. A clear clarification on the differences in AKI between ACLF and DC patients will promote timely and more appropriate management of the patients.
Clinically, AKI can be divided into structural and functional kidney injury, prerenal azotemia and HRS are the most common causes of functional kidney injury, and acute tubular necrosis is the most common cause of structural renal impairment[6-8]. Accurate distinguishing the etiologies of AKI is critical as their treatments differ markedly[6-8]. In recent years, studies on kidney tubular injury biomarkers for early detection of AKI have garnered broad interest, several studies demonstrated that some of these biomarkers in urine are significantly increased in patients with structural kidney injury and have the potential to distinguish structural from functional AKI, the combination of these biomarkers can improve the accuracy of diagnosis[7-10]. Terlipressin is a vasoconstrictor and is widely used in the treatment of HRS. Previous studies have shown that it can improve renal function in most patients with HRS.However, it is ineffective in patients with structural kidney injury[11,12].
Furthermore, due to the high incidence of hepatitis B virus (HBV) infection, patients with HBV-ACLF account for over 80% of all ACLF patients in China[1]. Therefore, in this prospective study, we assessed the levels of five extensively studied urinary biomarkers of tubular damage, including neutrophil gelatinase-associated lipocalin (NGAL), interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1), liver-type fatty acid binding protein (L-FABP), and cystatin C (CysC), to explore the etiological differences of AKI between HBV-ACLF and HBV-DC patients. Simultaneously, differences in the natural course of AKI, patient’s response to terlipressin treatment and patient outcomes were also evaluated, aimed to clarify the differences in AKI between ACLF and DC patients.
Consecutive patients with HBV-ACLF or HBV-DC who were admitted to Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology between December 2015 and July 2017 were enrolled in this observational study. This study was approved by the Ethics Committee of Tongji Hospital (TJ-C20151108), and written informed consents were obtained from all participants or their legal representatives. Two hundred and eighty patients with HBV-ACLF and 132 patients with HBV-DC were recruited and were divided into four groups according to the presence of ACLF, DC, and AKI, as follows: (1) Patients with DC without AKI (DC-non-AKI) group; (2) patients with ACLF without AKI (ACLF-non-AKI) group; (3) patients with both DC and AKI (DC-AKI) group; and (4) patients with both ACLF and AKI (ACLF-AKI) group. Patients with HBV-ACLF were diagnosed according to the definition of the Asian-Pacific Association for the Study of the Liver (APASL) 2014[5], this includes patients with previous HBV infection who had developed jaundice (total bilirubin ≥ 5 mg/dL) and coagulopathy (prothrombin activity (PTA) < 40% or INR ≥ 1.5) within 4 wk, and complicated by ascites and/or encephalopathy. HBV-DC patients were those with HBV-related cirrhosis, which were confirmed by a combination of clinical, imaging (computed tomography, magnetic resonance imaging, or ultrasonography) and endoscopic findings, presenting with significant signs of decompensation, such as ascites, hepatic encephalopathy, variceal bleeding, spontaneous bacterial peritonitis (SBP), or hepatorenal syndrome, but have not yet reached the ACLF diagnostic criteria, or have a history of liver function decompensation[13].
AKI was diagnosed according to the International Club of Ascites (ICA)-AKI criteria[3], as follows: an increase in serum creatinine by more than 0.3 mg/dL (≥ 26.5 μmol/L) within 48 h or to more than 1.5 times the baseline value. The most recent serum creatinine result within the previous three months, or the serum creatinine result upon hospital admission, was considered as the baseline serum creatinine. AKI was categorized into three stages according to the ICA-AKI staging standard[3]: Stage 1 (AKI-1), an increase in serum creatinine to more than 0.3 mg/dL (26.5 μmol/L) or by 1.5 to 2 fold from baseline value; stage 2 (AKI-2), an increase in serum creatinine by 2 to 3 fold from baseline value; stage 3 (AKI-3), an increase in serum creatinine to more than 3 fold from baseline or need renal replacement therapy. The recovery or progression of AKI was evaluated at discharge and the patients were classified as no-change (if there was no change of AKI stage), recovery (if the patient reached a lower stage from the first recorded or acquired a normal renal function), or progression (if there was AKI stage deterioration to a higher stage or if the patient needed dialysis).
Twenty-four patients with mild chronic hepatitis B (CHB) and 20 health controls (HC) during the same period were also included as control groups. Our exclusion criteria included those patients with chronic kidney disease, obstructive uropathy, urinary tract infection, hepatocellular carcinoma or other malignancies, cirrhosis or liver failure without HBV infection, acute liver failure, previous kidney or liver transplantation, pregnancy, age < 18 or > 80 years.
All study participants were hospitalized and received anti-HBV therapy along with standard supportive treatment according to their individual indications. Patients with stage 2 or 3 AKI who do not respond to the diuretic withdrawal and plasma volume expansion with albumin and without apparent structural kidney injury had received terlipressin treatment according to the International Club of Ascites (ICA)-AKI recommendations[3]. Among them, 10 patients with ACLF-AKI and 6 patients with DC-AKI were treated with octreotide at the same time due to gastrointestinal bleeding or acute pancreatitis. Patient’s response to terlipressin was assessed at the end of treatment, as follows: (1) No response, no regression of AKI; (2) partial response, AKI regression to a lower stage with serum creatinine decreased to ≥ 0.3 mg/dL (26.5 μmol/L) above the baseline value; or (3) full response, serum creatinine decreased to a value within 0.3 mg/dL (26.5 μmol/L) of the baseline value.
Patient demographics, clinical and laboratory data,and the natural course of AKI were recorded after enrollment, all patients were followed up for at least 3 mo or until death.
Ten milliliter of fresh urine samples were collected on the day of enrollment and/or after AKI was confirmed. The samples were immediately centrifuged at 3000 rpm for 15 min at -4 °C and the supernatants were subsequently stored at -80 °C for future biomarker and creatinine measurements. Five urine samples were could not be collected due to either the patients’ inability to cooperate or the presence of anuria. Samples from 24 CHB patients and 20 healthy controls (HC) were also collected.
The biomarkers of kidney tubular damage were measured using corresponding enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturers’ instructions: NGAL (BioPorto, Gentofte, Denmark), L-FABP (Hycultbiotech, Uden, The Netherlands), IL-18 (Medical and Biological Laboratories, Nagoya, Japan), CysC (R&D Systems, Minneapolis, MN), KIM-1 (R&D Systems, Minneapolis, MN).The ELISA methods and detection ranges for these biomarkers were as previously described[14,15]. All intra-assay and inter-assay variabilities were less than 10%. Urine creatinine was measured by enzyme colorimetry using an automatic biochemical analyzer (cobas8000, Roche Diagnostics, Mannheim, Germany).The concentrations of all urinary biomarkers were normalized to urinary creatinine to adjust for variations of urine concentration.
In this study, categorical variables were expressed as frequencies and percentages, and were compared using Fisher’s exact test or the chi-square test. Continuous variables were reported as mean ± SD for normally distributed variables and were compared using the Student’s t test or one-way ANOVA testing. Continuous variables with non-normal distributions were presented as medians with interquartile ranges (IQR) and were compared using the Mann-Whitney U test or the Kruskall-Wallis test. The cumulative survival rates at 90 d were estimated using the Kaplan-Meier method and were compared by the Log-rank test. A Cox proportional-hazards model, adjusted for potential confounders, was used to estimate the effects of DC, ACLF and AKI on 90-day mortality. All analyses in this study were conducted using IBM SPSS Statistics 23.0 and P < 0.05 (two-sided) was considered statistically significant.
A total of 280 patients with HBV-ACLF and 132 with HBV-DC were enrolled. During admission or hospitalization, 71 and 28 patients developed AKI in HBV-ACLF and HBV-DC groups, respectively (25.4% vs 21.2%, P = 0.358). Baseline and hospitalization characteristics of patients with HBV-ACLF or HBV-DC are shown in Tables 1 and 2.
| Characteristics | HBV-DC | HBV-ACLF | P valuea | P valueb | ||
| DC-non-AKI (n = 104) | DC-AKI (n = 28) | ACLF-non-AKI (n = 209) | ACLF-AKI (n = 71) | |||
| Age (yr)1 | 51.4 ± 1.0 | 58.1 ± 2.2 | 44.2 ± 0.8 | 49.3 ± 1.3 | 0.002 | < 0.001 |
| Male (%)3 | 89 (85.6) | 17 (60.7) | 189 (90.4) | 65 (91.5) | 0.002 | < 0.001 |
| Cirrhosis (%)3 | 104 (100) | 28 (100) | 87 (41.6) | 34 (47.9) | < 0.001 | < 0.001 |
| Complications | ||||||
| Ascites (%)3 | 73 (70.2) | 27 (96.4) | 127 (60.8) | 58 (81.7) | 0.105 | < 0.001 |
| HE (%)3 | 6 (5.8) | 1 (3.6) | 13 (6.2) | 14 (19.7) | 0.06 | 0.006 |
| GI bleeding (%)3 | 8 (7.7) | 4 (14.3) | 2 (1) | 3 (4.2) | 0.097 | 0.001 |
| SBP (%)3 | 13 (12.5) | 17 (60.7) | 28 (13.4) | 41 (57.7) | 0.787 | < 0.001 |
| Pulmonary infection (%)3 | 11 (10.6) | 8 (28.6) | 14 (6.7) | 23 (32.4) | 0.638 | < 0.001 |
| Diabetes (%)3 | 10 (9.6) | 3 (10.7) | 17 (8.1) | 10 (14.1) | 1 | 0.492 |
| Hypertension (%)3 | 6 (5.7) | 4 (14.3) | 13 (6.2) | 8 (11.3) | 0.736 | < 0.212 |
| Clinical and laboratory data | ||||||
| ALT (U/L)2 | 40.5 (22-82) | 33.5 (21-59.5) | 134 (70.5-302) | 136 (60.5-253.5) | < 0.001 | < 0.001 |
| AST (U/L)2 | 56 (39.3-88.7) | 61 (39.5-104) | 119 (78.5-207) | 146 (62-277.5) | < 0.001 | < 0.001 |
| ALP (U/L)2 | 103 (82.3-137.8) | 97 (70.8-120) | 132 (110-162) | 129 (101.5-155) | 0.001 | < 0.001 |
| Serum bilirubin (mg/dL)2 | 2.8 (1.3-5.3) | 4.1 (1.7-8.0) | 17.5 (11.2-25) | 25.7 (18.4-34) | < 0.001 | < 0.001 |
| Serum albumin (g/L)2 | 31.3 (27.05-34.4) | 28.6 (24.1-33.7) | 31.8 (29.2-34.4) | 31.5 (28.7-34.6) | 0.023 | < 0.057 |
| Serum creatinine (mg/dL)2 | 0.78 (0.68-0.87) | 0.97 (0.81-1.23) | 0.68 (0.6-0.81) | 0.94 (0.74-1.26) | 0.665 | < 0.001 |
| BUN (mmol/L)2 | 4.0 (3.3-5.2) | 12.8 (8.0-17.8) | 3.5 (2.8-4.3) | 11.2 (8.2-18) | 0.905 | < 0.001 |
| eGFR (mL/min/1.73 m2)2 | 104 (92.8-115.1) | 45.9 (40-59.5) | 113.9 (102.8-124.7) | 42.7 (27.4-58.5) | 0.164 | < 0.001 |
| Serum sodium (mmol/L)2 | 138.5 (134.7-141) | 135.4 (133.2-138.4) | 137.3 (134.7-139.4) | 130 (126.4-133.9) | 0.001 | < 0.001 |
| Serum potassium (mmol/L)2 | 4.0 (3.6-4.3) | 3.9 (3.4-4.3) | 4.1 (3.6-4.4) | 3.6 (3.1-4.5) | 0.487 | < 0.001 |
| INR2 | 1.45 (1.28-1.81) | 1.65 (1.48-2.14) | 1.89 (1.6-2.65) | 2.81 (1.98-3.86) | < 0.001 | < 0.001 |
| Leukocyte count (× 109/L)2 | 3.6 (2.5-5.0) | 4.1 (3.1-6.6) | 5.9 (4.4-8.4) | 10.0 (6.0-13.3) | < 0.001 | < 0.001 |
| PLT (× 109/L)2 | 61.3 (45.3-104.8) | 67.5 (39.3-89.3) | 95.2 (64.5-140.5) | 79 (47-115.5) | 0.058 | < 0.001 |
| Hemoglobin (g/L)2 | 114 (94.5-126) | 95.5 (75.75-112) | 123 (107.5-136) | 115 (100.5-131.5) | < 0.001 | < 0.001 |
| MAP (mmHg)1 | 82.9 ± 1.1 | 75.9 ± 1.5 | 86.7 ± 0.7 | 76.7 ± 1.1 | 0.921 | < 0.001 |
| HBV-DNA (log10)2 | 4.5 (2.7-6.3) | 4.1 (2.8-6.1) | 5.4 (3.7-7.1) | 5.3 (3.5-7.2) | 0.043 | 0.013 |
| Child-Pugh score2 | 9 (7-11) | 11 (8-12) | 11 (9-12) | 12 (11-13) | 0.061 | < 0.001 |
| MELD score2 | 13 (8.1-16) | 19.7 (16.2-25.3) | 21.2 (19-25) | 34.5 (29.2-41.6) | < 0.001 | < 0.001 |
| Characteristics | HBV-DC | HBV-ACLF | P valuea | P valueb | ||
| DC-non-AKI (n = 104) | DC-AKI (n = 28) | ACLF-non-AKI (n = 209) | ACLF-AKI (n = 71) | |||
| Hospitalization (d)1 | 13 (8-20) | 12.5 (9-18.3) | 26 (17-43) | 16 (10.5-33) | 0.144 | < 0.001 |
| Complications | ||||||
| Ascites (%)2 | 80 (76.9) | 28 (100) | 141 (67.5) | 67 (94.4) | 0.570 | < 0.001 |
| HE (%)2 | 8 (7.7) | 3 (10.7) | 41 (19.6) | 31 (43.7) | < 0.001 | < 0.001 |
| GI bleeding (%)2 | 11 (10.6) | 5 (17.9) | 5 (2.4) | 6 (8.5) | 0.151 | 0.002 |
| SBP (%)2 | 22 (21.2) | 19 (67.9) | 68 (32.5) | 47 (66.2) | 0.872 | < 0.001 |
| Pulmonary infection (%)2 | 20 (19.2) | 8 (28.6) | 43 (20.6) | 23 (32.4) | 0.944 | 0.134 |
| Serum creatinine (mg/dL)1 | ||||||
| Baseline | 0.78 (0.68-0.87) | 0.97 (0.81-1.23) | 0.68 (0.6-0.81) | 0.94 (0.74-1.26) | 0.665 | < 0.001 |
| Peak | 0.84 (0.74-0.96) | 1.69 (1.44-2.07) | 0.83 (0.7-0.94) | 1.99 (1.63-2.57) | 0.028 | < 0.001 |
| Final | 0.76 (0.66-0.87) | 1.05 (0.77-1.48) | 0.74 (0.64-0.85) | 1.48 (0.98-2.32) | 0.014 | < 0.001 |
| Treated with terlipressin (%)2 | - | 19 (67.9) | - | 43 (60.6) | 0.499 | - |
| Treatment time1 | - | 5 (3-9) | - | 6 (3-9) | 0.023 | - |
| 30-d mortality2 | 7 (6.7) | 9 (32.1) | 38 (18.2) | 42 (59.2) | 0.015 | < 0.001 |
| 90-d mortality2 | 10 (9.6) | 14 (50) | 69 (33) | 51 (71.8) | 0.039 | < 0.001 |
Patients in the ACLF-AKI group had the highest Model for End-stage Liver Disease (MELD) score, serum bilirubin levels, INR, and leukocyte counts and the lowest serum sodium levels. In contrast, patients with DC-AKI had the lowest serum albumin and hemoglobin levels. Prevalences of ascites, SBP, and pulmonary infection was noted to be higher among AKI patients compared to those without AKI, but there were no differences between the ACLF-AKI and DC-AKI groups. Hepatic encephalopathy (HE) was more common in ACLF-AKI patients than in DC-AKI patients.
The concentrations of NGAL, CysC, L-FABP, IL-18 in urine were found to be significantly elevated in patients with ACLF-AKI, which were markedly higher than those in the DC-AKI group and the groups without AKI,but there was no significant difference in the levels of these biomarkers between DC-AKI and non-AKI patients. The level of urinary KIM-1 was significantly higher in ACLF-AKI patients than in those without AKI, while no difference was observed between ACLF-AKI and DC-AKI groups (Figure 1).
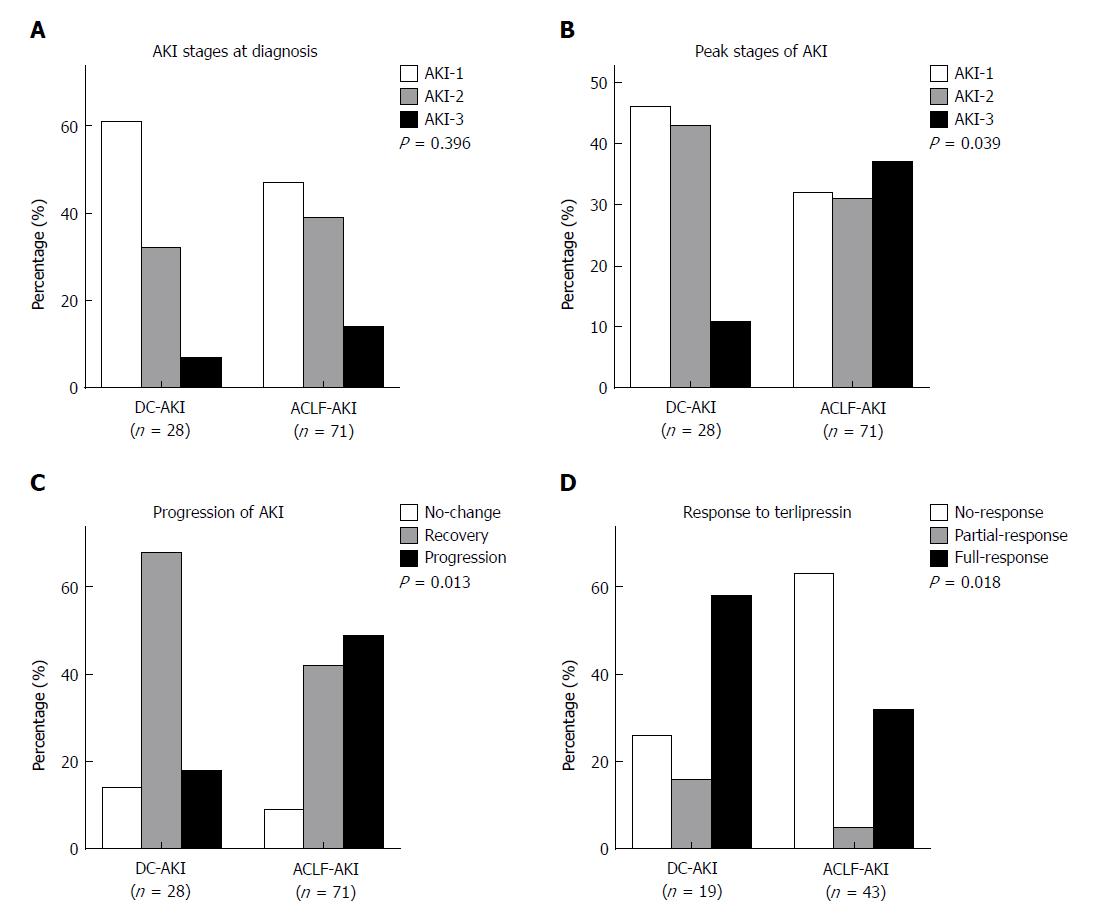
At the time of AKI diagnosis, there were 33 (46.5%) AKI-1, 28 (39.4%) AKI-2, and 10 (14.1%) AKI-3 patients in the ACLF-AKI group and 17 (60.7%) AKI-1, 9 (32.1%) AKI-2, and 2 (7.2%) AKI-3 patients in the DC-AKI group (P = 0.396) (Figure 2A). However, for the peak stages of AKI, these proportions were significantly different among ACLF-AKI and DC-AKI patients: there were 23 (32.4%) AKI-1, 22 (31%) AKI-2, and 26 (36.6%) AKI-3 patients in the ACLF-AKI group and 13 (46.4%) AKI-1, 12 (42.9%) AKI-2, and 3 (10.7%) AKI-3 patients in the DC-AKI group (P = 0.039) (Figure 2B). Next, we assessed the progression of AKI at discharge and found a higher proportion of patients with AKI progression in the ACLF-AKI group than in the DC-AKI group (49.3% vs 17.9%, P = 0.013) (Figure 2C).
There were 43 and 19 patients treated with terlipressin in the ACLF-AKI and DC-AKI groups, respectively (60.6% vs 67.9%, P = 0.499). At the end of treatment, there were 27 (62.8%) non-responders, 2 (4.7%) partial responders, and 14 (32.6%) full responders in the ACLF-AKI group and 5 (26.3%) non-responders, 3 (15.8%) partial responders, and 11 (57.9%) full responders in the DC-AKI group. The response rate in the ACLF-AKI group was significantly lower than that in the DC-AKI group (P = 0.018) (Figure 2D).
Next, we used logistic regression analysis to determine factors associated with the response to terlipressin treatment. A univariate analysis showed that DC patients with lower leukocyte count, serum creatinine, INR, total bilirubin (TBIL) and MELD scores, without the occurrence of HE had a good response to terlipressin. The levels of TBIL, INR, serum creatinine and MELD scores were closely related to the patient’s grouping, therefore were excluded from multivariate analysis. Among the parameters for multivariate analysis including patient’s grouping (DC or ACLF), HE, and leukocyte count, patient’s grouping (DC or ACLF) was independently associated with treatment response. Patients with ACLF-AKI were the poorest responders of terlipressin treatment (Table 3).
| Variables | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age | 1.024 (0.975-1.075) | 0.344 | ||
| Gender | 0.35 (0.090-1.357) | 0.129 | ||
| Grouping (DC/ACLF) | 0.282 (0.087-0.913) | 0.035 | 0.282 (0.087-0.913) | 0.035 |
| Baseline serum creatinine | 1.074 (0.417-2.77) | 0.882 | ||
| Peak serum creatinine | 0.499 (0.268-0.930) | 0.029 | ||
| Cirrhosis | 1.50 (0.513-4.385) | 0.459 | ||
| HE | 0.318 (0.103-0.981) | 0.046 | - | 0.148 |
| GI bleeding | 1.091 (0.262-4.537) | 0.905 | ||
| Ascites | 0.735 (0.044-12.330) | 0.831 | ||
| SBP | 0.452 (0.125-1.633) | 0.226 | ||
| Pulmonary infection | 0.970 (0.324-2.904) | 0.956 | ||
| ALT | 0.997 (0.993-1.001) | 0.153 | ||
| AST | 0.997 (0.993-1.002) | 0.095 | ||
| Serum albumin | 0.986 (0.895-1.1087) | 0.782 | ||
| Serumbilirubin | 0.956 (0.917-0.996) | 0.032 | ||
| Serum sodium | 1.071 (0.986-1.163) | 0.103 | ||
| INR | 0.462 (0.260-0.823) | 0.009 | ||
| Leukocyte count | 0.903 (0.816-0.999) | 0.048 | - | 0.180 |
| MAP | 0.998 (0.937-1.062) | 0.944 | ||
| Child-Pugh score | 0.809 (0.608-1.076) | 0.146 | ||
| MELD | 0.921 (0.870-0.975) | 0.004 | ||
| Treatment time | 1.020 (0.978-1.065) | 0.352 | ||
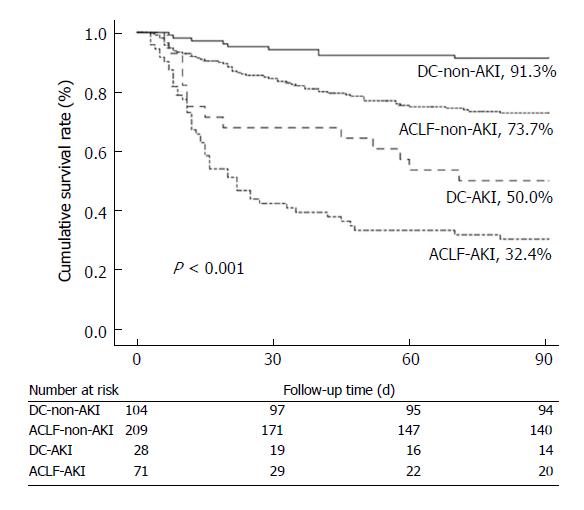
Survival rates at 90 d were significantly decreased in patients with AKI in comparison with those without. Patients with ACLF-AKI had the lowest survival rates among all groups (P < 0.001) (Figure 3). A total of 14 patients received liver transplantation. One of the fourteen patients had AKI before transplantation and this patient survived until a 90 d follow-up. Five patients (2 patients with DC and 3 patients with ACLF) were lost to follow-up. All patients with mild CHB survived at 90 d follow-up.
To further assess the effects of AKI, ACLF and DC on 90-day mortality, several factors (age, presence of ascites, HE, SBP, and leukocyte count) that were associated with mortality in the univariate analysis were adjusted in a Cox proportional hazards model (Table 4). ACLF-AKI patients had a highest death risk [HR 7.986 (3.823-16.683)], markedly higher than that in other groups. The risk of death was also higher in DC-AKI patients [HR 4.674 (1.977-10.943)] than those in ACLF-non-AKI and DC-non-AKI individuals. In addition, older age and the presence of HE and ascites were also associated with 90 d mortality.
This study was conducted to explore the etiology, natural course and prognostic differences of AKI between patients with HBV-ACLF and HBV-DC. The response to terlipressin was also assessed between the two groups.We have demonstrated that the structural tubular damage is the dominant pathophysiological mechanism of AKI during the course of ACLF-AKI. We have also showed that AKI in HBV-ACLF patients were more progressive and have a lower response rate to terlipressin treatment as well as a worse prognosis compared with that in HBV-DC patients.
To the best of our knowledge, there is only one published study by Maiwall et al[16] that reported differences in AKI between ACLF and DC patients. In that study, patients with ACLF-AKI were found to be more likely to have structural kidney injury, which had a greater possibility to resolve despite of the faster progression and poorer prognosis compared to patients with DC. However, the majority of patients in that study were caused by alcoholic cirrhosis and AKI were classified based on microscopic urinalysis[16], which cannot accurately distinguish the type of renal injury in some cases[17,18]. Current study is the first one to investigate differences in AKI between HBV-ACLF and HBV-DC patients by evaluating of the levels of novel tubular damage biomarkers and comparing the patients’ response to terlipressin treatment in different groups.
Accumulating evidences has shown that biomarkers of renal tubular injury in urine can distinguish between structural and functional renal impairment, though the specific biomarkers for differential diagnosis and their effect size remain controversial[7,8]. Fagundes et al[10] have previously shown that NGAL levels in urine could distinguish structural and functional kidney injury effectively. Ariza et al[19] also found that urinary NGAL is a good biomarker for differential diagnosis, followed by IL-18, but CysC and KIM-1 were found less useful for this purpose. Belcher et al[7] studied five biomarkers (NGAL, IL-18, L-FABP, KIM-1 and albumin) in their research and concluded that a combination of all those biomarkers significantly improved accuracy in the differentiation of structural and functional kidney injury compared with any single biomarker alone.
In the current study, five of the most extensively studied biomarkers (NGAL, CysC, L-FABP, IL-18, and KIM-1) were evaluated. Four (NGAL, CysC, L-FABP, and IL-18) of these biomarkers levels in urine were markedly elevated in ACLF-AKI patients, but not in DC-AKI patients and those without AKI. According to the findings of previous studies, the results of current study drove us to the hypothesis that AKI in HBV-ACLF patients is more likely to be caused by structural kidney injury than in HBV-DC patients, and our findings are consistent with that of Maiwall et al[16]. In addition to Maiwall’s findings, we have further revealed that AKI is not only more progressive in HBV-ACLF patients but also associated with poor recovery.
In patients with DC, organ hypoperfusion due to progressive hemodynamic dysfunction caused by serious splanchnic vasodilation is considered a major cause of AKI. Patients with AKI usually have a lower mean arterial pressure (MAP)[2,20]. Similarly, we found that MAP was significantly lower in the DC-AKI group than in patients without AKI. There was no significant difference in MAP levels between the ACLF-AKI and DC-AKI groups, which was expected because of the similar but severe hemodynamic changes in ACLF and DC[20,21]. Previous studies have reported that the systemic inflammatory response plays a more important role than hemodynamic dysfunction in the pathogenesis of ACLF and organ failure, and these patients usually have elevated levels of pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs)[20,21]. These inflammatory mediators can directly or indirectly lead to microcirculation dysfunction, oxidative stress, mitochondrial energy metabolism disorders, and eventually renal tubular cell apoptosis and necrosis[22,23]. IL-18 is not only a biomarker of kidney injury but also an inflammatory mediator, and the levels of IL-18 in urine were significantly higher in patients with ACLF-AKI in this study. We also found significantly higher leukocyte counts in patients with ACLF, especially in those with ACLF-AKI. The different pathogeneses of ACLF and DC may explained the hypothesis that there is a difference in the etiology and natural course of AKI between these two disease states. In addition, previous studies have found that hyperbilirubinemia is one of the causes of structural renal injury in patients with liver disease[24,25]. The level of serum bilirubin in patients with ACLF was significantly higher than that in DC patients, this may also contribute to the differences in AKI between these two diseases.
Terlipressin is a vasoactive agent and has been widely used for the treatment of HRS[11,26]. Several previous studies have demonstrated that the use of terlipressin significantly improves renal function and survival in patients with decompensated cirrhosis[11,26]. However, research on the use of terlipressin to treat AKI in ACLF patients is limited. Jindal et al[27] reported that only 35% of patients with ACLF-AKI responded to terlipressin, which is lower than 40%-60% responders in DC-AKI as reported by other investigators. In this study, we also found that the response rate of the ACLF-AKI group was significantly lower than that of the DC-AKI group, and having HBV-related ACLF was an independent predictor of poor response to terlipressin. As terlipressin is ineffective in patients with structural renal impairment, and our study found that the levels of biomarkers that represent structural renal impairment in patients with ACLF-AKI was significantly higher than that in patients with DC-AKI, we considered the low response rate of terlipressin treatment in ACLF-AKI patients is associated with a higher proportion of structural kidney damage in these patients. In addition, previous studies have shown that high serum bilirubin levels are associated with a low response to terlipressin treatment, and elevated serum bilirubin levels are associated with the development of structural kidney injury[24,25,28,29]. Serum bilirubin levels were significantly higher in patients with ACLF-AKI than in DC-AKI patients in this study, further explaining our results. Although some of patients recieved octreotide, there was no significant difference in the proportion of patients receiving octreotide between the two groups.
There is persuasive evidence that AKI is associated with high mortality in patients with liver disease[30,31]. Similarly, we also found that survival rates were significantly lower in patients with AKI than those without. Moreover, it is interesting that survival rates in the ACLF-AKI group were significantly lower than those in the DC-AKI group. Many studies had demonstrated that the mortality of patients with AKI is stage-dependent and closely related to the etiologies of AKI[1,32,33]. Singer et al[34] reported that patients with structural kidney injury were usually associated with poor prognosis. Nadim et al[35] also showed that the presence of structural kidney injury was associated with higher mortality. A higher proportion of stage 2 or 3 AKI in HBV-ACLF patients was observed in this current study and which is more likely to be caused by structural kidney injury. This may explain the lower survival rates in ACLF-AKI pateints.
Although this is a prospective observational study with a large series of patients, there are still limitations. First, our findings cannot be further verified,as it is impractical to obtain kidney biopsies from most of the AKI patients in this serious condition. In addition, all patients in our study were enrolled from a single-center in China,there may be a certain selection bias. A multi-center prospective study needed for further investigation. Finally, this sutdy mainly focuses on HBV-related ACLF and DC patients. One should consider the definitions and etiology differences when interpret these results into western patients , where alcoholism constitutes the major etiology of ACLF (type A non-cirrhosis, type B with compensated cirrhosis, type C with decompensated cirrhosis) and DC[4].
In conclusion, this study demonstrated that AKI in patients with HBV-ACLF is distinctly different from that in HBV-DC patients. In patients with HBV-ACLF, AKI was more likely to be due to structural kidney injury, tended to be more progressive, with a lower response rate to terlipressin therapy and a poorer prognosis compared with those in DC-AKI patients. Accurate differentiating the causes of AKI is critical, and AKI in patients with HBV-ACLF or HBV-DC should be managed in different ways. Further studies are required to validate these findings.
Acute kidney injury (AKI) is a common and serious complication of acute-on-chronic liver failure (ACLF) and decompensated cirrhosis (DC). Previous studies have been clearly established that the acute-on-chronic liver failure and decompensated liver cirrhosis are two different diseases.However, the differences in acute kidney injury among patients with these two diseases are rarely studied and whether AKI should be managed in the same way in patients with these two diseases is still uncertain.
Clinically, the treatment of patients with different types of renal impairment is significantly different. A clear clarification on the differences in AKI between ACLF and DC patients will promote timely and more appropriate management of the patients.
This study was conducted to clarify the differences in AKI between hepatitis B virus (HBV)-ACLF and HBV-DC patients, including the differences in the etiology of AKI, natural course, patient’s response to terlipressin and prognosis.
This study is a prospective observational study, patients with HBV-ACLF and HBV-DC who were admitted to our hospital between 2015.12 and 2017.7 were consecutively recruited. Urine specimens of all patients were collected at the time of admission and when AKI was diagnosed, and the levels of five tubular injury biomarkers in urine were detected. Simultaneously, the demographic data, natural course of AKI, patient’s response to terlipressin treatment and patient outcomes were recorded.
Patients with ACLF-AKI have significantly higher urinary biomarker levels than those with DC-AKI or without AKI. There was a higher proportion of patients with AKI progression in ACLF-AKI patients than in DC-AKI patients (49.3% vs 17.9%, P = 0.013). Forty-three patients with ACLF-AKI and 19 patients with DC-AKI were treated with terlipressin, the response rate to terlipressin was significantly lower in patients with ACLF-AKI than in patients with DC-AKI (32.6% vs 57.9%, P = 0.018). In addition, patients in the ACLF-AKI group had the lowest survival rate at 90 d among all groups (P < 0.001).
Our study demonstrated that AKI in patients with HBV-ACLF is distinct different from in HBV-DC patients.In HBV-ACLF patients, AKI is more likely to be caused by structural damages and tends to be more progressive, with a poorer response to terlipressin and a worse prognosis than in HBV-DC patients.
Our results suggest that AKI occurring in patients with HBV-ACLF or HBV-DC should be managed in different ways. Large-scale multi-center studies are required to validate these findings, and the differences in AKI between patients with ACLF and DC caused by other etiologies still need to be further studied.
| 1. | Shi X, Zhu P, Yan G, Liu C, Zhang C, Huang G, Zhang Y, Yan Z, Wang Y. Clinical characteristics and long-term outcome of acute kidney injury in patients with HBV-related acute-on-chronic liver failure. J Viral Hepat. 2016;23:920-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013;62:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 3. | Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (3)] |
| 4. | Bernal W, Jalan R, Quaglia A, Simpson K, Wendon J, Burroughs A. Acute-on-chronic liver failure. Lancet. 2015;386:1576-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 245] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 5. | Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 511] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 6. | Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, Angeli P, Moreau R, Davenport A, Jalan R. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 289] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 7. | Belcher JM, Sanyal AJ, Peixoto AJ, Perazella MA, Lim J, Thiessen-Philbrook H, Ansari N, Coca SG, Garcia-Tsao G, Parikh CR; TRIBE-AKI Consortium. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol. 2016;65:809-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 334] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Fagundes C, Pépin MN, Guevara M, Barreto R, Casals G, Solà E, Pereira G, Rodríguez E, Garcia E, Prado V. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Moreau R, Lebrec D. The use of vasoconstrictors in patients with cirrhosis: type 1 HRS and beyond. Hepatology. 2006;43:385-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Ginès P. Management of Hepatorenal Syndrome in the Era of Acute-on-Chronic Liver Failure: Terlipressin and Beyond. Gastroenterology. 2016;150:1525-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Jang JW, Choi JY, Kim YS, Woo HY, Choi SK, Lee CH, Kim TY, Sohn JH, Tak WY, Han KH. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology. 2015;61:1809-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Parikh CR, Butrymowicz I, Yu A, Chinchilli VM, Park M, Hsu CY, Reeves WB, Devarajan P, Kimmel PL, Siew ED. Urine stability studies for novel biomarkers of acute kidney injury. Am J Kidney Dis. 2014;63:567-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Zeng XF, Li JM, Tan Y, Wang ZF, He Y, Chang J, Zhang H, Zhao H, Bai X, Xie F. Performance of urinary NGAL and L-FABP in predicting acute kidney injury and subsequent renal recovery: a cohort study based on major surgeries. Clin Chem Lab Med. 2014;52:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Maiwall R, Kumar S, Chandel SS, Kumar G, Rastogi A, Bihari C, Sharma MK, Thakur B, Jamwal K, Nayak S. AKI in patients with acute on chronic liver failure is different from acute decompensation of cirrhosis. Hepatol Int. 2015;9:627-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Tsai MH, Chen YC, Yang CW, Jenq CC, Fang JT, Lien JM, Hung CC, Weng HH, Wu CS, Peng YS. Acute renal failure in cirrhotic patients with severe sepsis: value of urinary interleukin-18. J Gastroenterol Hepatol. 2013;28:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3:1615-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Ariza X, Solà E, Elia C, Barreto R, Moreira R, Morales-Ruiz M, Graupera I, Rodríguez E, Huelin P, Solé C. Analysis of a urinary biomarker panel for clinical outcomes assessment in cirrhosis. PLoS One. 2015;10:e0128145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 454] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 21. | Clària J, Stauber RE, Coenraad MJ, Moreau R, Jalan R, Pavesi M, Amorós À, Titos E, Alcaraz-Quiles J, Oettl K, Morales-Ruiz M, Angeli P, Domenicali M, Alessandria C, Gerbes A, Wendon J, Nevens F, Trebicka J, Laleman W, Saliba F, Welzel TM, Albillos A, Gustot T, Benten D, Durand F, Ginès P, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL-CLIF Consortium and the European Foundation for the Study of Chronic Liver Failure (EF-CLIF). Systemic inflammation in decompensated cirrhosis: Characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 587] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 22. | Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, Kellum JA. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 585] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 23. | Umbro I, Gentile G, Tinti F, Muiesan P, Mitterhofer AP. Recent advances in pathophysiology and biomarkers of sepsis-induced acute kidney injury. J Infect. 2016;72:131-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | van Slambrouck CM, Salem F, Meehan SM, Chang A. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 25. | Nayak SL, Kumar M, Bihari C, Rastogi A. Bile Cast Nephropathy in Patients with Acute Kidney Injury Due to Hepatorenal Syndrome: A Postmortem Kidney Biopsy Study. J Clin Transl Hepatol. 2017;5:92-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 26. | Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O’Leary JG, Ganger D, Jamil K, Pappas SC; REVERSE Study Investigators. Terlipressin Plus Albumin Is More Effective Than Albumin Alone in Improving Renal Function in Patients With Cirrhosis and Hepatorenal Syndrome Type 1. Gastroenterology. 2016;150:1579-1589.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 27. | Jindal A, Bhadoria AS, Maiwall R, Sarin SK. Evaluation of acute kidney injury and its response to terlipressin in patients with acute-on-chronic liver failure. Liver Int. 2016;36:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Boyer TD, Sanyal AJ, Garcia-Tsao G, Blei A, Carl D, Bexon AS, Teuber P; Terlipressin Study Group. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Nazar A, Pereira GH, Guevara M, Martín-Llahi M, Pepin MN, Marinelli M, Solá E, Baccaro ME, Terra C, Arroyo V. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (6)] |
| 30. | Doyle JF, Forni LG. Acute kidney injury: short-term and long-term effects. Crit Care. 2016;20:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 31. | Belcher JM, Garcia-Tsao G, Sanyal AJ, Bhogal H, Lim JK, Ansari N, Coca SG, Parikh CR; TRIBE-AKI Consortium. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 32. | Montoliu S, Ballesté B, Planas R, Alvarez MA, Rivera M, Miquel M, Masnou H, Cirera I, Morillas RM, Coll S. Incidence and prognosis of different types of functional renal failure in cirrhotic patients with ascites. Clin Gastroenterol Hepatol. 2010;8:616-622; quiz e80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Martín-Llahí M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, Solá E, Pereira G, Marinelli M, Pavesi M. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140:488-496.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 34. | Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J, Luft FC, Schmidt-Ott KM. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Nadim MK, Genyk YS, Tokin C, Fieber J, Ananthapanyasut W, Ye W, Selby R. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: de Mattos AZ, Moini M, Kahraman A, Staufer K S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y