Published online May 21, 2018. doi: 10.3748/wjg.v24.i19.2120
Peer-review started: March 7, 2018
First decision: March 21, 2018
Revised: April 3, 2018
Accepted: April 16, 2018
Article in press: April 15, 2018
Published online: May 21, 2018
Processing time: 71 Days and 18.5 Hours
To clarify the previous discrepant conclusions, we performed a meta-analysis to evaluate the prognostic value of red cell distribution width (RDW) in esophageal cancer (EC).
We searched the PubMed, EMBASE, Web of Science and Cochrane Library databases to identify clinical studies, followed by using STATA version 12.0 for statistical analysis. Studies that met the following criteria were considered eligible: (1) Studies including EC patients who underwent radical esophagectomy; (2) studies including patients with localized disease without distant metastasis; (3) studies including patients without preoperative neoadjuvant therapy; (4) studies including patients without previous antiinflammatory therapies and with available preoperative laboratory outcomes; (5) studies reporting association between the preoperative RDW and overall survival (OS)/disease-free survival (DFS)/cancer-specific survival (CSS); and (6) studies published in English.
A total of six articles, published between 2015 and 2017, fulfilled the selection criteria in the end. Statistical analysis showed that RDW was not associated with the prognosis of EC patients, irrespective of OS/CSS [hazard ratio (HR) = 1.27, 95% confidence interval (CI): 0.97-1.57, P = 0.000] or DFS (HR = 1.42, 95%CI: 0.96-1.88, P = 0.000). Subgroup analysis indicated that elevated RDW was significantly associated with worse OS/CSS of EC patients when RDW > 13% (HR = 1.45, 95%CI: 1.13-1.76, P = 0.000), when the patient number ≤ 400 (HR = 1.45, 95%CI: 1.13-1.76, P = 0.000) and when the study type was retrospective (HR = 1.42, 95%CI : 1.16-1.69, P = 0.000).
Contrary to our general understanding, this meta-analysis revealed that RDW cannot serve as an indicator of poor prognosis in patients with EC. However, it may still be a useful predictor of unfavorable prognosis using an appropriate cut-off value.
Core tip: Red cell distribution width (RDW) has been established as a prognostic factor for cancer patients. In consideration of esophageal cancer (EC), many articles have concluded that RDW is correlated with poor prognosis. However, recent studies have indicated that elevated RDW harbors no prognostic value for EC, which might, instead, be a favorable prognostic factor for EC patients. No consensus is available in the previous literature concerning whether elevated RDW is a negative or favorable prognostic factor for EC patients. To this end, for the first time, this systematic review and meta-analysis was performed to evaluate the prognostic value of RDW in EC.
- Citation: Xu WY, Yang XB, Wang WQ, Bai Y, Long JY, Lin JZ, Xiong JP, Zheng YC, He XD, Zhao HT, Sang XT. Prognostic impact of the red cell distribution width in esophageal cancer patients: A systematic review and meta-analysis. World J Gastroenterol 2018; 24(19): 2120-2129
- URL: https://www.wjgnet.com/1007-9327/full/v24/i19/2120.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i19.2120
Esophageal cancer (EC) is one of the most common digestive malignancies worldwide[1,2], ranking as the fourth leading cause of cancer-related death[3] and leading to approximately 400000 deaths in 2012[4]. The incidence of EC varies widely across different countries and regions[5]. According to the latest national statistics, EC is the fourth most common malignant tumor type in China[1], with cancer-related morbidity and mortality rates of 11.1% and 13.3%, respectively, in 2015, which has emerged as a severe public health problem, particularly in some high-risk rural areas[6]. However, in 2012, the cancer-related morbidity and mortality rates of EC (3.41% and 2.98%, respectively) were lower in North America and Europe than those in China[4].
EC is a highly aggressive digestive malignancy characterized by rapid growth and early metastasis. The rate of distant metastasis in EC patients is as high as 20%-30% at the time of initial diagnosis[7]. Despite the progress in radical resection and adjuvant therapy (radiation and chemotherapy), the 5-year overall survival (OS) rate of patients with EC remains approximately 20% in China[8], emphasizing an urgent need to detect effective prognostic biomarkers which could guide personalized therapeutic strategy for EC patients[9,10].
In recent years, accumulating evidence has shown that systemic inflammatory responses are closely associated with tumor initiation, progression and invasion[11-14]. Therefore, a variety of inflammatory indicators have been explored to assess their potential prognostic roles in various cancers. One such marker is the red blood cell distribution width (RDW), which is defined as the coefficient of variation in the red blood cell size. An elevated RDW indicates anisocytosis, which is considered as the basis for the clinical diagnosis of iron deficiency anemia[15]. However, fluctuations in the RDW have recently been reported as involved in many other pathophysiological conditions. For example, an elevated RDW is strongly associated with chronic inflammation, poor nutritional status, and age-associated diseases, which is indicative of changes in erythropoiesis. In addition, a number of studies have demonstrated a significant correlation between RDW and conventional inflammatory parameters, such as the C-reactive protein, interleukin-6 and tumor necrosis factor-α levels and erythrocyte sedimentation rate. Cancer has been revealed to be associated with chronic inflammation, and the latter is a key determinant of disease progression and survival in various cancers[16,17]. In 2007, Felker et al[18] found that RDW could serve as an independent predictor of morbidity and mortality in heart failure. Recent studies have revealed that RDW is associated with prognosis in several types of cancer, such as lung cancer[19], prostate cancer[20] and EC[21].
RDW is an important complete blood count parameter that is routinely monitored in cancer patients. Several studies conducted in recent years[21-26] have investigated the relationship between EC prognosis and RDW due to the easy accessibility of obtaining blood samples and the low cost of analyzing RDW. However, the results of these studies show some discrepancies, which could be attributed to differences in the study design and relatively small sample sizes. To this end, in the present study, a meta-analysis was performed to identify the correlation between RDW and survival in EC patients.
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
A systematic review of studies that evaluated the prognostic value of RDW in EC patients was performed. Four databases were electronically searched: Medline (host: OVID) from 1946 to April 2017; EMBASE (host: OVID) from 1974 to April 2017; and Web of Science and Cochrane Database of Systematic Reviews from 2005 to June 2017. The search terms used in our study were “RDW”, “red cell distribution width with esophageal neoplasm”, “esophagus neoplasm”, “esophagus neoplasms”, “cancer of esophagus”, “cancer of the esophagus”, “esophagus cancer”, “esophagus cancers”, “esophageal cancer”, “esophageal cancers”, “esophageal squamous cell cancer” (ESCC), and “esophageal adenocarcinoma”. Both free text and MeSH terms were used as keywords. The search strategy used for the PubMed database is shown in Table 1, and the presented search strategy was also used for the other electronic databases.
| Number | Search items |
| #1 | Esophageal Neoplasm.ti,ab |
| #2 | esophagus neoplasm.ti,ab |
| #3 | esophagus neoplasms.ti,ab |
| #4 | cancer of esophagus.ti,ab |
| #5 | cancer of the esophagus.ti,ab |
| #6 | esophagus cancer.ti,ab |
| #7 | esophagus cancers.ti,ab |
| #8 | esophageal cancer.ti,ab |
| #9 | esophageal cancers.ti,ab |
| #10 | esophageal squamous cell cancer.ti,ab |
| #11 | ESCC.ti,ab |
| #12 | esophageal adenocarcinoma.ti,ab |
| #13 | or #1- #12 |
| #14 | red cell distribution. ti,ab |
| #15 | RDW.ti,ab |
| #16 | or #14- #15 |
| #17 | #13 and #16 |
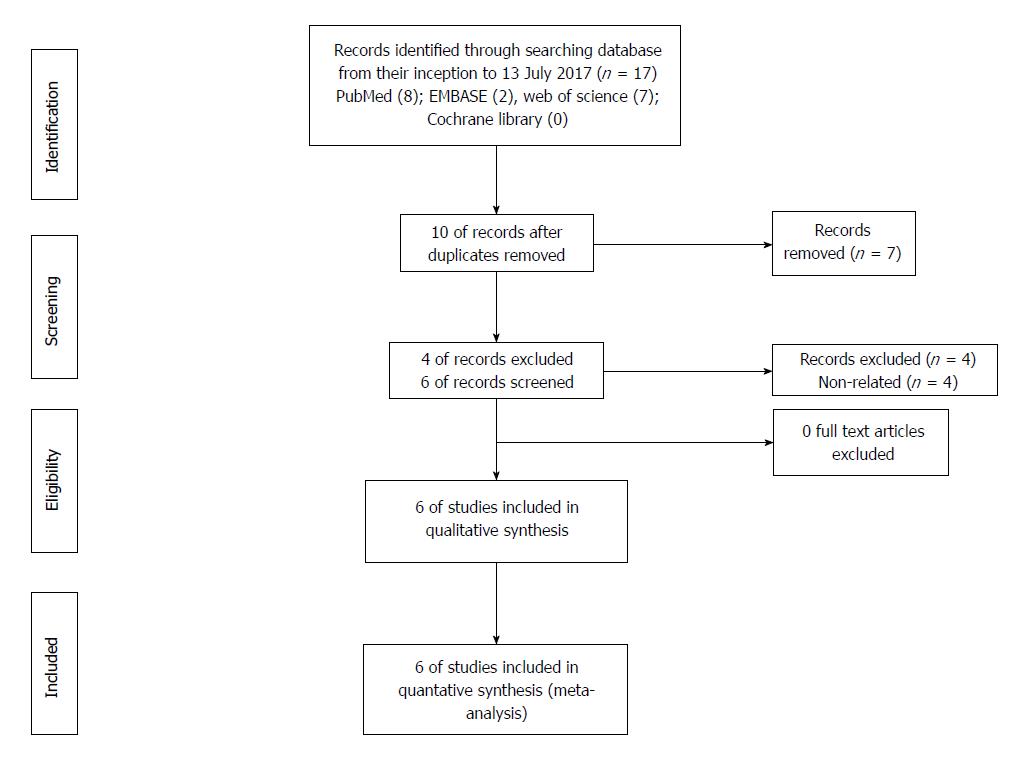
The search was performed by two investigators (Xu and Wang) who also evaluated the titles and abstracts of all candidate articles. Full-text was reviewed when the articles could not be categorized based on the title and abstract. Articles were included and excluded in accordance with the corresponding criteria defined in this study. Any disputes during the selection period were discussed with and resolved by a third investigator (Xiong). A flowchart demonstrating the details of the study selection according to the PRISMA guidelines is shown in Figure 1.
Studies that met the following criteria were considered eligible: (1) A study of EC patients who underwent radical esophagectomy; (2) a study of patients with localized disease without distant metastasis; (3) a study of patients without preoperative neoadjuvant therapy; (4) a study of patients without previous antiinflammatory therapies and with available preoperative laboratory outcomes; (5) a study of the association between the preoperative RDW and OS/disease-free survival (DFS)/cancer-specific survival (CSS); and (6) a complete paper published in English. Studies that met the following criteria were excluded: (1) Letters, case reports, reviews or preclinical studies; (2) studies describing a repeated analysis or duplicate data; (3) studies lacking key information for further analysis; and (4) nonhuman studies.
We used predesigned extraction forms for data collection. The following information was extracted from each study: first author’s name, year of publication, country of the patients, research type, number of male/female patients included in the study, pathological types, RDW cut-off value, hazard ratio (HR) of elevated RDW for OS, CSS and DFS with 95% confidence interval (CI) and P-value. Assuming that most of the deaths were related to cancer, in the case of unavailability of OS information, data for CSS were extracted. HRs from multivariable analyses were extracted when available; otherwise, HRs from univariable analyses were extracted or estimated from Kaplan-Meier survival curves as described by Parmar and colleagues[27]. HRs for subgroups were compared defined by different markers.
HRs and the corresponding 95%CIs were directly obtained from each publication. If the values were not directly reported, the values were calculated according to the method described by Parmar and colleagues[27]. The meta-analysis was performed with STATA software version 12.0 (STATA Corporation, College Station, TX, United States) to combine the HRs with 95%CIs for categorical data and the weighted mean difference or standardized mean difference with 95%CIs for continuous data. All statistical tests were bilateral, and a P value < 0.05 was considered as statistical significance. If the data were not suitable for pooling, a systematic narrative synthesis of the information was performed, which was presented in the text to understand and to summarize the findings as well as characteristics of the included studies.
The heterogeneity of the pooled results was assessed through Cochran’s Q test and Higgins I-squared statistic. Significant heterogeneity was identified by P < 0.05 and/or I2 > 50%, and the random-effects model (DerSimonian-Laird method) was used to combine the data. Otherwise, the fixed-effects model (Mantel-Haenszel method) was employed. To explore the potential source of heterogeneity among studies, subgroup analyses were performed according to various variables, such as the RDW cut-off value, the patient number in each study, and the study type and quality.
The quality of the included studies was assessed by using the Newcastle-Ottawa quality scale (NOS)[28]. Three aspects, namely, selection, comparability and outcomes, were assessed on this scale, which had a maximum score of 9. Studies with scores ≥ 7 were considered to be of high quality.
If significant heterogeneity was observed, a sensitivity analysis was performed after data extraction and subgroup analyses. This sensitivity analysis, which included the sequential omission of each study using the “metaninf” STATA command, aimed to validate the findings of this meta-analysis.
Begg’s funnel plot and Egger’s linear regression test were performed to evaluate publication bias, and a P value of < 0.05 was considered statistically significant.
Initially, 17 studies were selected from the electronic databases, and 10 studies remained after the removal of duplicates. After reading the titles and/or abstracts, four unrelated studies were excluded, and six full-text articles[21-26] were further assessed. None of these studies were excluded after thorough review of full-text. These six studies[21-26], which included 3826 patients, were included in this meta-analysis. The detailed search method and a flowchart representing the selection process are shown in Figure 1. These studies included five retrospective studies and one prospective study. The sample sizes varied from 144 to 2396, with a median value of 638. All six studies were conducted in Asian countries. The cut-off values for the RDW ranged from 12.2% to 15.3%. All six studies reported a correlation between RDW and OS/CSS, and two of the studies also investigated the association between RDW and DFS. With the exception of one study, the NOS scores of all the other studies were > 5. The general characteristics of the six included studies are summarized in Table 2.
| Order number | Author | Year of publication | Country | Research type | Patients number | Male | Female | Pathological types | RDW, cut-off value, % | CSS/OS/PFS/DFS | HR, U | LCI, U | UCI, U | P-value, U | HR, M | LCI, M | UCI, M | P-value, M | NOS score |
| 1 | Chen et al | 2015 | China | Retrospective | 277 | 240 | 37 | ESCC | 14.5 | CSS | 1.719 | 1.268 | 2.331 | < 0.001 | 1.396 | 1.022 | 1.908 | 0.036 | 6 |
| 2 | Wan et al | 2016 | China | Retrospective | 179 | 150 | 29 | ESCC (133), EAC (46) | 15 | OS | 3.087 | 1.85 | 5.152 | < 0.001 | 1.895 | 1.023 | 3.508 | 0.042 | 7 |
| 2 | Wan et al | 2016 | China | Retrospective | 179 | 150 | 29 | ESCC (133), EAC (46) | 15 | DFS | 3.208 | 1.922 | 5.353 | < 0.001 | 1.907 | 1.02 | 3.565 | 0.043 | 7 |
| 3 | Hirahara et al | 2016 | Japan | Retrospective | 144 | 129 | 15 | ESCC | 15.3 | CSS | 2.332 | 1.304 | 4.19 | 0.005 | 1.684 | 0.929 | 3.071 | 0.03 | 6 |
| 4 | Sun et al | 2016 | China | Retrospective | 362 | 268 | 94 | ESCC | 13.6 | OS | 1.381 | 0.946 | 2.016 | 0.094 | 5 | ||||
| 5 | Zhang et al | 2016 | China | Retrospective | 468 | 376 | 92 | ESCC | 12.2 | OS | 1.505 | 1.068 | 2.122 | 0.02 | 1.356 | 0.948 | 1.94 | 0.095 | 7 |
| 5 | Zhang et al | 2016 | China | Retrospective | 468 | 376 | 92 | ESCC | 12.2 | DFS | 1.474 | 1.046 | 2.077 | 0.027 | 1.349 | 0.943 | 1.929 | 0.101 | 7 |
| 6 | Hu et al | 2017 | China | Prospective | 2396 | 1822 | 574 | ESCC | 12.90 (men) | OS | 0.85 | 0.76 | 0.94 | 0.002 | 0.84 | 0.75 | 0.93 | 0.001 | 8 |
| 6 | Hu et al | 2017 | China | Prospective | 2396 | 1822 | 574 | ESCC | 12.70 (women) | OS | 1.02 | 0.89 | 1.18 | 0.73 | 1.01 | 0.88 | 1.17 | 0.996 | 8 |
| 6 | Hu et al | 2017 | China | Prospective | 2396 | 1822 | 574 | ESCC | OS | 0.92 | 0.75 | 1.08 | 0.051 | 8 |
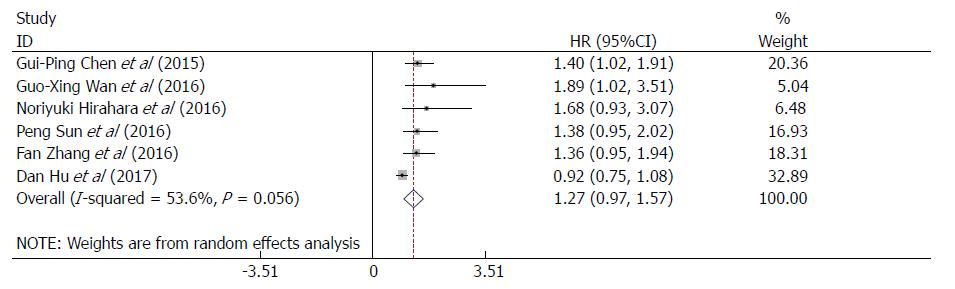
The HRs and 95%CIs from the six studies involving 3826 patients were extracted and then pooled. The pooled results showed that the RDW was not associated with OS or CSS (HR = 1.27, 95%CI: 0.97-1.57, P = 0.000; Figure 2), with significant heterogeneity among the six studies (I2 = 53.6%, P = 0.056; Figure 2); thus, the random-effects model was adopted for further analyses. The correlation between the RDW and DFS in EC patients were further investigated based on the pooled HRs and 95% CIs from two studies comprising 647 patients. As a result, RDW was not associated with DFS (HR = 1.42, 95%CI: 0.96-1.88, P = 0.000; Supplementary Figure 1), and no heterogeneity was observed (I2 = 0.0%, P = 0.423; Supplementary Figure 1). In consideration of the significant heterogeneity of the pooled results regarding the effect of the RDW on OS/CSS, subgroup analyses, sensitivity analyses and Begg’s funnel plot and Egger’s linear regression analyses were conducted to further identify the heterogeneity source.
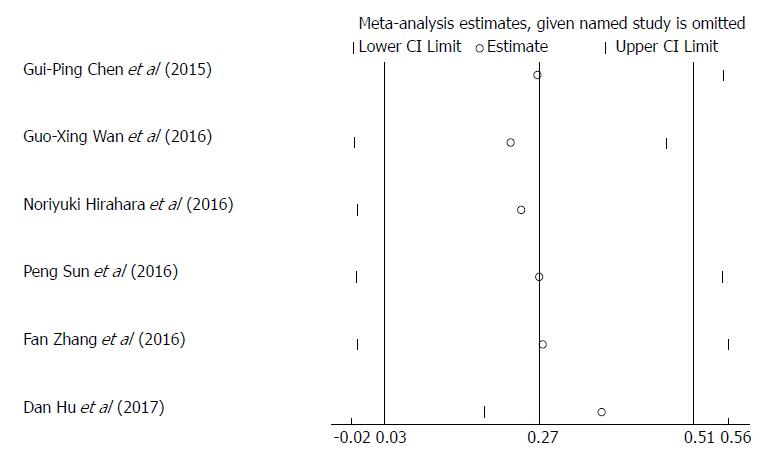
Sensitivity analyses was carried out by sequentially omitting each study to investigate its influence on results, indicating that the study conducted by Hu et al[26] was the primary source of heterogeneity (Figure 3). After exclusion of this study, the heterogeneity was effectively reduced or eliminated (I2 = 0.0%, P = 0.926; Supplementary Figure 2). Surprisingly, the corresponding pooled HR varied with the inclusion (HR = 1.27, 95%CI: 0.97-1.57, P = 0.000; Figure 2) and omission of this study (HR = 1.42, 95%CI: 1.16-1.69, P = 0.000; Supplementary Figure 2). After reviewing the six studies included in our meta-analysis, we found that the study conducted by Hu et al[26] was the only prospective study, whereas the other five studies were retrospective ones. The sensitivity analyses indicated that the study type might be a source of heterogeneity. Therefore, we performed a subgroup analysis based on the study type (Supplementary Figure 3) and found that a high RDW was significantly associated with poor OS/CSS in patients with EC in the subgroup of retrospective studies.
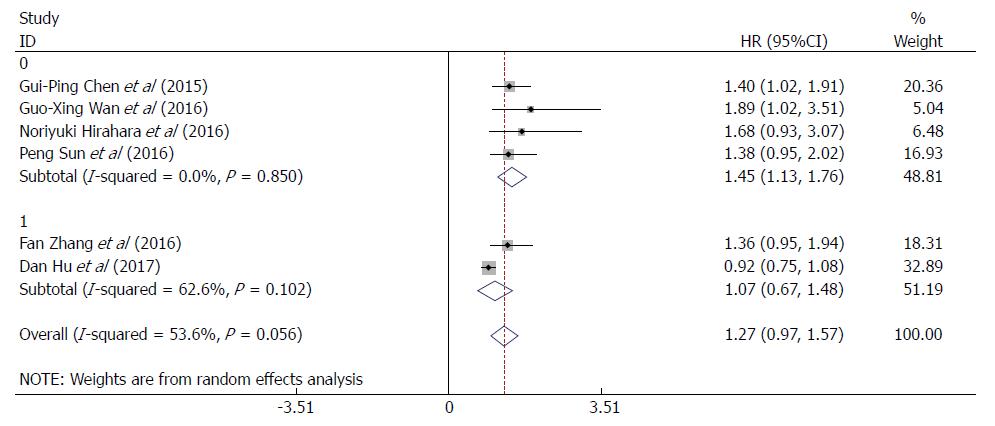
To further explore other sources of heterogeneity, we performed a subgroup analysis based on the RDW cut-off values (≤ 13% or > 13%). For RDW cut-off value > 13%, heterogeneity was effectively reduced or eliminated after exclusion of the study in which the cut-off value was ≤ 13%, (I2 = 0.0%, P = 0.850; Figure 4), and the corresponding pooled HR was increased (HR = 1.45, 95%CI: 1.13-1.76, P = 0.000; Figure 4). This finding indicated that the RDW cut-off value might be another source of heterogeneity. Thus, at an RDW cut-off value > 13%, a high RDW is significantly associated with poor OS/CSS in patients with EC.
Furthermore, when the subgroups were stratified by patient number (≤ 400 or > 400), the heterogeneity was effectively reduced or eliminated after excluding the studies with > 400 patients (I2 = 0.0%, P = 0.850; Supplementary Figure 4), and the corresponding pooled HR was increased when the patient number was ≤ 400 (HR = 1.45, 95%CI: 1.13-1.76, P = 0.000; Supplementary Figure 4). Hence, the subgroup analyses indicated that the patient number might also be a source of heterogeneity. For studies with patient number ≤ 400, a high RDW was significantly associated with poor OS/CSS in patients with EC.
Finally, when the subgroups were stratified by NOS scores (≤ 6 or > 6), heterogeneity was effectively reduced or eliminated after omitting the studies with NOS scores > 6 (I2 = 0.0%, P = 0.876; Supplementary Figure 5), and the corresponding pooled HR was increased (HR = 1.42, 95%CI: 1.09-1.74, P = 0.000; Supplementary Figure 5). Thus, the study quality might be another source of heterogeneity.
Begg’s funnel plot and Egger’s linear regression analyses were performed to estimate the potential publication bias in the present meta-analysis. The P values regarding OS/CSS were 0.133 (Begg’s test; Supplementary Figure 6) and 0.005 (Egger’s test; Supplementary Figure 7). Due to the small sample sizes of the included studies, there was significant publication bias in our study, which was also demonstrated by the funnel plot (Funnel plot; Supplementary Figure 8). Therefore, publication bias might be another source of heterogeneity.
To the best of our knowledge, this study constitutes the first meta-analysis investigating the prognostic value of the RDW in EC. The most notable finding is that RDW is not associated with the prognosis of EC patients, including both OS/CSS and DFS. This novel finding is inconsistent with previous conclusions regarding the prognostic value of RDW in EC. Significant heterogeneity was observed across the included studies. After investigating the source of this heterogeneity by subgroup and sensitivity analyses, we derived four major conclusions. (1) In the retrospective studies, an elevated RDW was associated with poor OS/CSS, which did not affect DFS. (2) In the included prospective study, an elevated RDW was associated with a favorable prognosis in male ESCC patients, which is contradictory to the conclusions of most previous studies on cancers[20,29-32]. Additionally, RDW was not associated with prognosis in female patients. (3) When the patient number was ≤ 400, an elevated RDW was associated with poor OS/CSS, but this prognostic correlation was not observed when the number of patients was > 400. And, finally, (4) an elevated RDW was significantly associated with poor OS/CSS when the RDW cut-off value was > 13%, but this association was not observed when the cut-off value was ≤ 13%.
The role of the RDW is being increasingly appreciated due to its close correlation with the risks of cardiovascular diseases and systematic inflammation[33,34]. Previous studies have identified RDW as an accurate predictor of inflammation in hepatitis B infection, mortality due to acute pancreatitis, and activity of inflammatory bowel disease[34-36]. Moreover, an elevated RDW has been found to be a risk factor and progression indicator in multiple malignancies[19,31,37,38].
In the last 2 to 3 years, studies concerning the correlation between RDW and EC prognosis have become increasingly prominent. However, the conclusions of these studies are varied and sometimes even conflicting. Four small-scale retrospective studies[21-24] included in this meta-analysis concluded that an elevated RDW was significantly associated with worse OS in EC patients. In addition, one intermediate-scale retrospective study[25] concluded that the RDW was not associated with OS at all. Moreover, one large-scale prospective study[26] concluded that an elevated RDW was associated with better OS in male but not female EC patients. Considering that men are three to four times more likely to suffer from EC than women[7], this finding harbors important clinical implications in guiding therapeutic strategies for EC.
There are several reasons for the discrepant conclusions from diverse studies. Four small-scale retrospective studies[21-24] demonstrated that an elevated RDW was a predictor of unfavorable prognosis in EC patients, and the underlying mechanism might be one of the following. First, since Rudolf Virchow noted the presence of leucocytes within tumor tissues approximately 150 years ago and suggested that cancer might be initiated as a result of chronic inflammation[11,39], numerous preclinical and population-based studies have verified his observation. Inflammation might contribute to increased RDW levels by not only impairing iron metabolism but also inhibiting the production of or response to erythropoietin or by reducing red blood cell survival[40,41]. Second, chronic inflammation has also been associated with poor response to chemotherapy[42]. Third, RDW has been found to be correlated with malnutrition, which is an independent risk factor for nosocomial infections associated with poor therapeutic response, an increased rate of treatment-related toxicity, reduced survival rates, and poor quality of life[43,44].
In contrast, one large-scale prospective study[26] concluded that an elevated RDW was a positive predictor of prognosis in male EC patients, however the actual mechanism remains largely undefined. This finding is consistent with the results of another cohort study[45] with data from 26709 nondiabetic adults with more than 14 years of follow-up, which indicated that a low RDW was significantly associated with an increased incidence of diabetes mellitus independent of traditional risk factors. The underlying mechanism might be as follows. Aerobic glycolysis has been proposed as a hallmark of cancer, and the acidic environment caused by aerobic glycolysis is a necessary component of carcinogenesis[46]. Due to the significant association between a low RDW and an increased incidence of diabetes mellitus, it is reasonable to speculate that an elevated RDW might be a surrogate indicator of improved glucose metabolism, which is a key factor for prolonged survival in EC patients. Nevertheless, further clinical evidence and preclinical experiments are warranted to support and verify the accurate mechanism and to identify the real prognostic significance of the RDW in EC.
However, when the data from female patients were included in the present study, the RDW was not found to be associated with OS (HR = 0.92, 95%CI: 0.75-1.08, P = 0.000; Supplementary Figure 9), which is consistent with the conclusions of an intermediate-scale retrospective study[25] included in this meta-analysis. Further analyses revealed that the two above studies have some common characteristics-the sample size was relative large (468 vs 2396) and the RDW cut-off values were 13%. This finding is consistent with the results of a study[47] conducted in 2012, which revealed that the RDW was elevated (> 14.8%) in 31.6% of benign biliary obstruction cases and 68.4% of malignant biliary stricture cases, whereas the RDW was reduced (< 14.8%) in 72.9% of benign cases and 27.1% of malignant cases (these differences were statistically significant, P < 0.001). Therefore, an RDW cut-off of 14.8% was the most suitable for predicting a malignant biliary stricture, with a sensitivity of 72% and a specificity of 69% (area under the curve = 0.755, 95%CI: 0.649-0.810). The conclusions of the two studies might be partly attributed to the lower RDW cut-off value. Thus, more large-scale studies exploring the actual relationship between RDW and the prognosis of EC patients are urgently needed in the future. Furthermore, it is necessary to establish a reasonable method for identifying the appropriate RDW cut-off value for predicting the prognosis of EC patients.
However, in contrast to the findings obtained from male EC patients, an elevated RDW was not associated with the prognosis of female EC patients[26]. Despite the prospective nature of the study demonstrating these results, we cannot neglect a potential correlation between the RDW and sex due to the large sample size in that study. However, more studies are needed to investigate and confirm this correlation and to explore the underlying mechanisms.
There are certain limitations that should be acknowledged in this meta-analysis. First, most of the studies included in this meta-analysis were retrospective in nature, and the numbers of patients in these retrospective studies were relatively small. Only one study was prospective, which might prevent generalization of the results. Second, this meta-analysis was performed based on the pooled HRs and 95%CIs from eligible studies, rather than detailed individual information. We were unable to exclude uncontrolled or unmeasured risk factors from the original studies, which might have confounded the true association, resulting in potential bias. Third, the cut-off values for the RDW varied across the included studies due to differences in the study populations and experimental methods. Although the patients were divided into RDW-high and RDW-low populations, the stratification might change depending on the cut-off values. Therefore, a standard and uniform cut-off value is needed to accurately define high versus low RDW. Fourth, all of the included articles were in English, most of the included studies included a small number of patients, and potential publication bias cannot be neglected. Thus, more large-scale, well-designed and high-quality prospective studies are required to elucidate the precise mechanisms linking the RDW to survival in EC patients.
In conclusion, an elevated RDW is not associated with the prognosis of EC patients, including both OS/CSS and DFS. This finding is contrary to previous knowledge regarding the prognostic value of the RDW in malignant tumors, particularly in EC. However, when the RDW cut-off value is > 13%, the patient number is ≤ 400, and the study type is retrospective, an elevated RDW is indeed significantly associated with worse OS/CSS in EC patients.
Esophageal cancer (EC) was the eighth most common cancer globally, with about half of all cases occurring in China. Prominent symptoms usually do not appear until the cancer has infiltrated over 60% of the circumference of the esophageal tube, by which time the tumor is already in an advanced stage and the prognosis generally tends to be fairly poor. Therefore, finding a simple and effective prognostic indicator is particularly urgent for individualized treatment of EC patients. Recently, red blood cell distribution width (RDW), as an important complete blood count parameter which has a close correlation with cancer-related inflammation, has been investigated as an important prognostic factor for EC patients in more and more studies, but the conclusions of these studies have not been consistent. Therefore, we conducted this meta-analysis to explore and verify the real role of RDW in the prognosis of patients with EC.
We systematically reviewed the existing studies regarding the role of RDW in the prognosis of EC patients and performed a meta-analysis with the extracted data to clarify the real impact of RDW on the outcomes of the EC patients. Identifying the real role of RDW in the prognosis of patients with EC and the defects existing in the previous and current studies can guide the future researchers to conduct more well-designed related studies on this topic and the upstream or downstream research related to RDW.
The main objectives of this article were to perform a meta-analysis of the data provided in these studies with inconsistent conclusions about the prognostic effect of RDW on EC patients, and to verify the real impact of RDW on the prognosis of EC patients by increasing the sample size. In the end, we could determine whether we need to conduct further studies on this topic according to the conclusion of this systematic review and meta-analysis.
First, we searched four related electronic databases (PubMed, EMBASE, Web of Science and Cochrane Library) using the identified MESH terms, and finally identified six studies which met the standards based on the inclusion and exclusion criteria of the selected literature, then we assessed the quality of the included studies according to Newcastle-Ottawa quality scale. Second, we used the electronic EXCEL table to collect the data from the included studies that we needed and utilized statistical software (STATA version 12.0) to conduct statistical analysis of the related data. Third, we performed the sensitivity analysis, subgroup analysis, Begg’s funnel plot and Egger’s linear regression test to explore the potential source of heterogeneity among studies, to find the influencing factors that affect the role of RDW in the prognosis of EC patients and point out the directions for further related research in the future.
Different from the traditional review, we used meta-analysis methods to synthesize data and perform statistical analysis to the relevant literature and quantify the effect of RDW on the prognosis of EC patients. Moreover, in addition to sensitivity analysis and subgroup analyses to find sources of heterogeneity, we also used the Begg’s funnel plot and Egger’s linear regression test to quantify publication bias rather than just using the traditional funnel plot for qualitative analysis. These were the characteristics and indicate the novelty of the research methods used in our study.
This systematic review and meta-analysis indicated that elevated RDW was not an independent risk factor for the worse outcome of EC patients overall, whether it’s for overall survival/cancer-specific survival [hazard ratio (HR) = 1.27, 95% confidence interval (CI): 0.97-1.57, P = 0.000] or disease-free survival (HR = 1.42, 95% CI: 0.96-1.88, P = 0.000). The prognostic value of RDW in patients with EC is only reflected in the retrospective study (HR = 1.42, 95%CI: 1.16-1.69, P = 0.000) of small samples (sample size ≤ 400, HR = 1.45, 95% CI = 1.13-1.76, P = 0.000) currently, and there is a need to choose the appropriate RDW cutoff value (RDW > 13%, HR = 1.45, 95%CI: 1.13-1.76, P = 0.000) as a prerequisite. Therefore, the actual effect of RDW on the prognosis of EC patients needs further prospective multicenter large-sample studies to be validated in the future.
Different from the traditional viewpoints, our systematic and meta-analysis demonstrated that RDW had no correlation with the prognosis of EC patients, no matter favorable or unfavorable. Therefore, such traditional theories and assumptions, that cancer-related inflammation leads to an increased RDW in the blood, and elevated RDW in turn suggests the occurrence of cancer, were challenged and questioned by the results of our meta-analysis. At the same time, it also suggests that we could perform the meta-analysis to statistically analyze the inconsistent result data of different types of small-sample studies and achieve a conclusion that is completely different from our previous understanding. This leads to the emergence of new theories and assumptions and provides direction for our future research design and potential mechanism research. Our systematic reviews and meta-analysis suggest that we should be more cautious and rational to see the impact of increased RDW on the prognosis of EC patients in our future clinical work.
From our study, we could learn that we can’t blindly believe in traditional ideas that already exist. When the opinions of previous studies are inconsistent and chaotic, we should use statistical methods to perform statistical clustering analysis on various data, and draw a scientific conclusion to guide our clinical work and indicate the future research direction. Moreover, through the systematic analysis of the previous research, we should carry out more multicenter, large-sample prospective studies in the future to overcome the defects of the current research in the study design to further verify the role of RDW in the prognosis of EC patients. In addition, we also need to conduct further basic experiments based on the results of such above-mentioned optimized research to uncover its underlying mechanisms.
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13326] [Article Influence: 1332.6] [Reference Citation Analysis (4)] |
| 2. | Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31:281-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (2)] |
| 3. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9876] [Article Influence: 759.7] [Reference Citation Analysis (5)] |
| 4. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20723] [Article Influence: 1883.9] [Reference Citation Analysis (23)] |
| 5. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11889] [Article Influence: 792.6] [Reference Citation Analysis (6)] |
| 6. | Zhang SW, Min Z, Guang-Lin LI. An Analysis of Incidence and Mortality of Esophageal Cancer in China, 2003-2007. China Cancer. 2012;. |
| 7. | Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 499] [Cited by in RCA: 604] [Article Influence: 50.3] [Reference Citation Analysis (14)] |
| 8. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2233] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 9. | Zeng H, Zheng R, Guo Y, Zhang S, Zou X, Wang N, Zhang L, Tang J, Chen J, Wei K. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer. 2015;136:1921-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 508] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 10. | Tseng RC, Chang JM, Chen JH, Huang WR, Tang YA, Kuo IY, Yan JJ, Lai WW, Wang YC. Deregulation of SLIT2-mediated Cdc42 activity is associated with esophageal cancer metastasis and poor prognosis. J Thorac Oncol. 2015;10:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5854] [Article Influence: 234.2] [Reference Citation Analysis (1)] |
| 12. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2125] [Article Influence: 125.0] [Reference Citation Analysis (2)] |
| 13. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11515] [Article Influence: 479.8] [Reference Citation Analysis (2)] |
| 14. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8431] [Article Influence: 526.9] [Reference Citation Analysis (9)] |
| 15. | Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009;158:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 492] [Article Influence: 28.9] [Reference Citation Analysis (1)] |
| 16. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19796] [Article Influence: 761.4] [Reference Citation Analysis (0)] |
| 17. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48804] [Article Influence: 3253.6] [Reference Citation Analysis (12)] |
| 18. | Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 19. | Koma Y, Onishi A, Matsuoka H, Oda N, Yokota N, Matsumoto Y, Koyama M, Okada N, Nakashima N, Masuya D. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS One. 2013;8:e80240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 195] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Albayrak S, Zengin K, Tanik S, Bakirtas H, Imamoglu A, Gurdal M. Red cell distribution width as a predictor of prostate cancer progression. Asian Pac J Cancer Prev. 2014;15:7781-7784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Chen GP, Huang Y, Yang X, Feng JF. A Nomogram to Predict Prognostic Value of Red Cell Distribution Width in Patients with Esophageal Cancer. Mediators Inflamm. 2015;2015:854670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Wan GX, Chen P, Cai XJ, Li LJ, Yu XJ, Pan DF, Wang XH, Wang XB, Cao FJ. Elevated red cell distribution width contributes to a poor prognosis in patients with esophageal carcinoma. Clin Chim Acta. 2016;452:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Hirahara N, Matsubara T, Kawahara D, Mizota Y, Ishibashi S, Tajima Y. Prognostic value of hematological parameters in patients undergoing esophagectomy for esophageal squamous cell carcinoma. Int J Clin Oncol. 2016;21:909-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Sun P, Zhang F, Chen C, Bi X, Yang H, An X, Wang F, Jiang W. The ratio of hemoglobin to red cell distribution width as a novel prognostic parameter in esophageal squamous cell carcinoma: a retrospective study from southern China. Oncotarget. 2016;7:42650-42660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Zhang F, Chen Z, Wang P, Hu X, Gao Y, He J. Combination of platelet count and mean platelet volume (COP-MPV) predicts postoperative prognosis in both resectable early and advanced stage esophageal squamous cell cancer patients. Tumour Biol. 2016;37:9323-9331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Hu D, Lin X, Chen Y, Chang Q, Chen G, Li C, Zhang H, Cui Z, Liang B, Jiang W. Preoperative blood-routine markers and prognosis of esophageal squamous cell carcinoma: The Fujian prospective investigation of cancer (FIESTA) study. Oncotarget. 2017;8:23841-23850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Perry R, Taylor M, Lewis L, Yellowlees A, Fleetwood K, Barata T. Estimating Survival Data from Published Kaplan-Meier Curves: a Comparison of Methods. Value Health. 2014;17:A326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 13637] [Article Influence: 852.3] [Reference Citation Analysis (1)] |
| 29. | Ellingsen TS, Lappegård J, Skjelbakken T, Braekkan SK, Hansen JB. Impact of red cell distribution width on future risk of cancer and all-cause mortality among cancer patients - the Tromsø Study. Haematologica. 2015;100:e387-e389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Balta S, Arslan Z, Unlu M, Demirkol S. The association between red cell distribution width and non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45:954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Seretis C, Seretis F, Lagoudianakis E, Gemenetzis G, Salemis NS. Is red cell distribution width a novel biomarker of breast cancer activity? Data from a pilot study. J Clin Med Res. 2013;5:121-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Warwick R, Mediratta N, Shackcloth M, Shaw M, McShane J, Poullis M. Preoperative red cell distribution width in patients undergoing pulmonary resections for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | Agarwal S. Red cell distribution width, inflammatory markers and cardiorespiratory fitness: results from the National Health and Nutrition Examination Survey. Indian Heart J. 2012;64:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Yeşil A, Senateş E, Bayoğlu IV, Erdem ED, Demirtunç R, Kurdaş Övünç AO. Red cell distribution width: a novel marker of activity in inflammatory bowel disease. Gut Liver. 2011;5:460-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Xu WS, Qiu XM, Ou QS, Liu C, Lin JP, Chen HJ, Lin S, Wang WH, Lin SR, Chen J. Red blood cell distribution width levels correlate with liver fibrosis and inflammation: a noninvasive serum marker panel to predict the severity of fibrosis and inflammation in patients with hepatitis B. Medicine (Baltimore). 2015;94:e612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Cetinkaya E, Senol K, Saylam B, Tez M. Red cell distribution width to platelet ratio: new and promising prognostic marker in acute pancreatitis. World J Gastroenterol. 2014;20:14450-14454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Wang FM, Xu G, Zhang Y, Ma LL. Red cell distribution width is associated with presence, stage, and grade in patients with renal cell carcinoma. Dis Markers. 2014;2014:860419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Lee H, Kong SY, Sohn JY, Shim H, Youn HS, Lee S, Kim HJ, Eom HS. Elevated red blood cell distribution width as a simple prognostic factor in patients with symptomatic multiple myeloma. Biomed Res Int. 2014;2014:145619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep. 2012;32:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 40. | Douglas SW, Adamson JW. The anemia of chronic disorders: studies of marrow regulation and iron metabolism. Blood. 1975;45:55-65. [PubMed] |
| 41. | Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2133] [Cited by in RCA: 2214] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 42. | Chai EZ, Siveen KS, Shanmugam MK, Arfuso F, Sethi G. Analysis of the intricate relationship between chronic inflammation and cancer. Biochem J. 2015;468:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 43. | McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 767] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 44. | Hébuterne X, Lemarié E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr. 2014;38:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 566] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 45. | Engström G, Smith JG, Persson M, Nilsson PM, Melander O, Hedblad B. Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. J Intern Med. 2014;276:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 46. | Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49 Suppl 2:24S-42S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 468] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 47. | Beyazit Y, Kekilli M, Ibis M, Kurt M, Sayilir A, Onal IK, Purnak T, Oztas E, Tas A, Yesil Y. Can red cell distribution width help to discriminate benign from malignant biliary obstruction? A retrospective single center analysis. Hepatogastroenterology. 2012;59:1469-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Ciezki JP, Jones G, Taylor ME S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Yin SY