Published online May 7, 2018. doi: 10.3748/wjg.v24.i17.1911
Peer-review started: March 7, 2018
First decision: March 21, 2018
Revised: April 2, 2018
Accepted: April 9, 2018
Article in press: April 9, 2018
Published online: May 7, 2018
Processing time: 60 Days and 6.5 Hours
To explore the value of three-dimensional (3D) visualization technology in the minimally invasive treatment for infected necrotizing pancreatitis (INP).
Clinical data of 18 patients with INP, who were admitted to the PLA General Hospital in 2017, were retrospectively analyzed. Two-dimensional images of computed tomography were converted into 3D images based on 3D visualization technology. The size, number, shape and position of lesions and their relationship with major abdominal vasculature were well displayed. Also, percutaneous catheter drainage (PCD) number and puncture paths were designed through virtual surgery (percutaneous nephroscopic necrosectomy) based on the principle of maximum removal of infected necrosis conveniently.
Abdominal 3D visualization images of all the patients were well reconstructed, and the optimal PCD puncture paths were well designed. Infected necrosis was conveniently removed in abundance using a nephroscope during the following surgery, and the median operation time was 102 (102 ± 20.7) min. Only 1 patient underwent endoscopic necrosectomy because of residual necrosis.
The 3D visualization technology could optimize the PCD puncture paths, improving the drainage effect in patients with INP. Moreover, it significantly increased the efficiency of necrosectomy through the rigid nephroscope. As a result, it decreased operation times and improved the prognosis.
Core tip: As a lethal disease, infected necrotizing pancreatitis is gradually treated by minimally invasive surgery. Percutaneous catheter drainage (PCD) is the prerequisite of various minimally invasive treatment, which has been of great significance for prognosis of the disease. In this study, three-dimensional (3D) visualization technology was used preoperatively to optimize the puncture position and direction of PCD path. As a result, it improved the drainage effect and increased the efficiency of subsequent necrosectomy. So, the 3D visualization technology was great help for the prognosis of infected necrotizing pancreatitis.
- Citation: Wang PF, Liu ZW, Cai SW, Su JJ, He L, Feng J, Xin XL, Lu SC. Usefulness of three-dimensional visualization technology in minimally invasive treatment for infected necrotizing pancreatitis. World J Gastroenterol 2018; 24(17): 1911-1918
- URL: https://www.wjgnet.com/1007-9327/full/v24/i17/1911.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i17.1911
Infected necrotizing pancreatitis (INP), which often leads to sepsis and multiple organ failure, is one of the most severe complications of acute pancreatitis[1,2]. In recent years, various kinds of minimally invasive treatments have achieved good results in treating INP and improved prognosis of the patients[3,4]. However, irrespective of the kind of minimally invasive surgeries applied, the prerequisite has been to establish a convenient surgical approach through preoperative percutaneous catheter drainage (PCD)[5,6]. Whether the puncture paths of PCD were ultimately appropriate was related to not only the effect of drainage but also the efficiency of subsequent minimally invasive removal of infected necrotic tissues.
In recent years, three-dimensional (3D) visualization technology has been widely applied in hepatobiliary and pancreatic surgeries, helping surgeons to intuitively identify the relationship between the shape of lesions and the important anatomical structures around[7,8]. Therefore, this technique was applied in our center on patients with INP to improve the quality of PCD since January 2017. This study retrospectively analyzed the clinical data of enrolled patients to investigate the value of the 3D visualization technique in guiding PCD of INP.
Eighteen patients [12 males and 6 females, with an average age of 46 (51 ± 12.9) years] were enrolled in this study. The inclusion criteria were as follows: (1) acute pancreatitis with pancreatic or peripancreatic necrosis accompanied by infection but without any invasive treatment; and (2) patients or their families accepting the evaluation of 3D visual reconstruction. The exclusion criteria were as follows: patients with acute pancreatitis without local complications or liquefied necrotic lesions without infection in a stable condition. According to the 2012 Atlanta classification of acute pancreatitis[9], 9 patients had moderately severe acute pancreatitis and the other 9 patients had severe acute pancreatitis. This study was approved by the ethics committee of the PLA General Hospital.
Data acquisition and 3D reconstruction: The 128-slice spiral computed tomography (CT) scanner (GE Corporation, Stamford, CT, United States) was used for obtaining abdominal enhanced CT scans of arterial and portal venous phases. The layer thickness was 1.5 mm, and the layer distance was 1.5 mm. Lipiodol was injected as the contrast medium (Beilu Pharmaceutical Co., Beijing, China); the concentration was 350 mg/mL. A high-pressure syringe was used for elbow vein injection. The dose was 60 mL, and the injection rate was 4 mL/s. The collected image data were stored in the form of Digital Imaging and Communications in Medicine and introduced into 3D visualization system (Mimics 17.0; Materialise Co., Leuven, Belgium) for reconstruction.
Anatomical evaluation and determination of position and paths of optimal puncture points: The reconstructed model could be viewed from any direction (magnified, contracted, rotated, or transparent) through the 3D visualization system. Therefore, the size, shape, position and number of the lesions and surrounding structure were intuitively demonstrated. Moreover, virtual operations could be done on the 3D image to observe different debridement ranges through various puncture points. At last, the optimal puncture number and paths were determined based on the following principles. First, retroperitoneal access was preferred to transabdominal access, which meant less intraperitoneal contamination. Second, the paths were established along the longitudinal axis of the necrotic cavity, and the puncture point should be as close to the necrotic cavity as possible, facilitating the maximum removal of necrosis. Third, multiple drains should be placed during the same procedure, if necessary, to avoid the visual blind area or operational blind area, which were also estimated using the 3D virtual system.
PCD and percutaneous nephroscopic necrosectomy for removing necrotic tissues: PCD was performed under the guidance of CT in strict accordance with the position and direction of puncture points designed by the 3D visualization system. Subsequently, an antiinflammatory drug was administered according to the drainage culture and the results of drug sensitivity test. The changes in the disease were assessed with a reexamination of abdominal CT scan weekly. If the condition did not improve obviously or continued to aggravate, the percutaneous nephroscopic necrosectomy was performed under general anesthesia. Briefly, a 1.2-cm skin incision was made that was centered on the PCD. Amplatz renal dilators (Cook Urological Incorporated, Bloomington, IN, United States) were used to serially dilate to create a 30F tract, following which a 12-mm trocar was inserted.
An operating nephroscope (Hopkins Telescopes; Karl Storz-Endoskope, Tuttingen, Germany) with an 8-mm working channel was then passed through the trocar into the necrotic cavity. Subsequently, the piecemeal removal of solid necrosis was performed repeatedly using a fenestrated grasper through the working channel. Finally, a 10F catheter sutured to a tube drain of 28F was placed into the distal end of the necrotic cavity to allow continuous lavage after surgery. All patients underwent CT scanning reexamination weekly to evaluate the results of necrosectomy and drain placement. The operation might be performed again, if necessary according to the changes in the condition. The position and range of the lesion, the time and number of surgery, postoperative complications, and the time of hospitalization of each patient were recorded.
The SPSS 17.0 software (SPSS Inc., Chicago, IL, United States) was used for statistical analysis. Measurement data were expressed as χ ± s.
The 3D reconstruction was successfully completed for all of the 18 patients. The results clearly showed the shape, size and number of necrotic lesions, as well as the anatomical relationship with surrounding blood vessels and organs. Thus, the stereoscopic visual observation of the lesions from any angle and virtual surgery using the aforementioned software system were performed. Further, the removal range and residual blind area of different puncture points were defined. Also, the individualized optimal position, direction and number of puncture paths suitable for patients were determined.
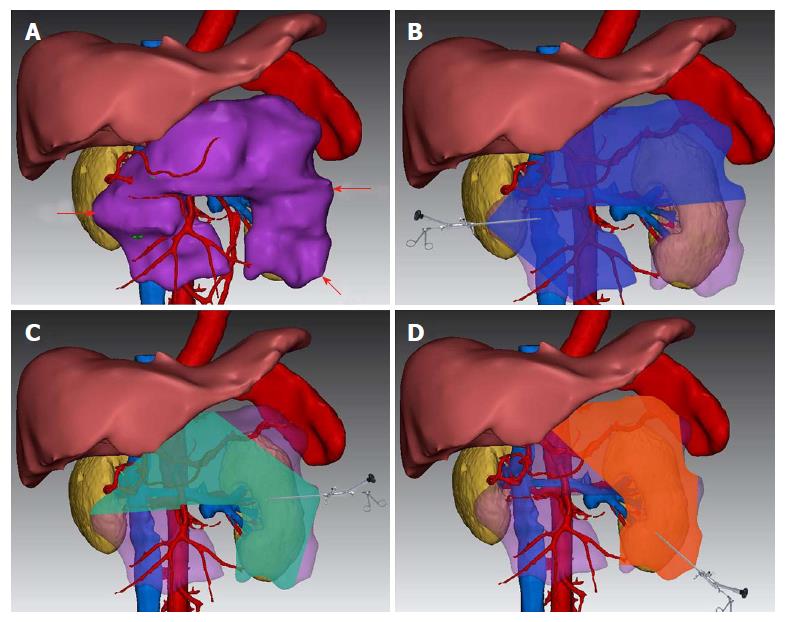
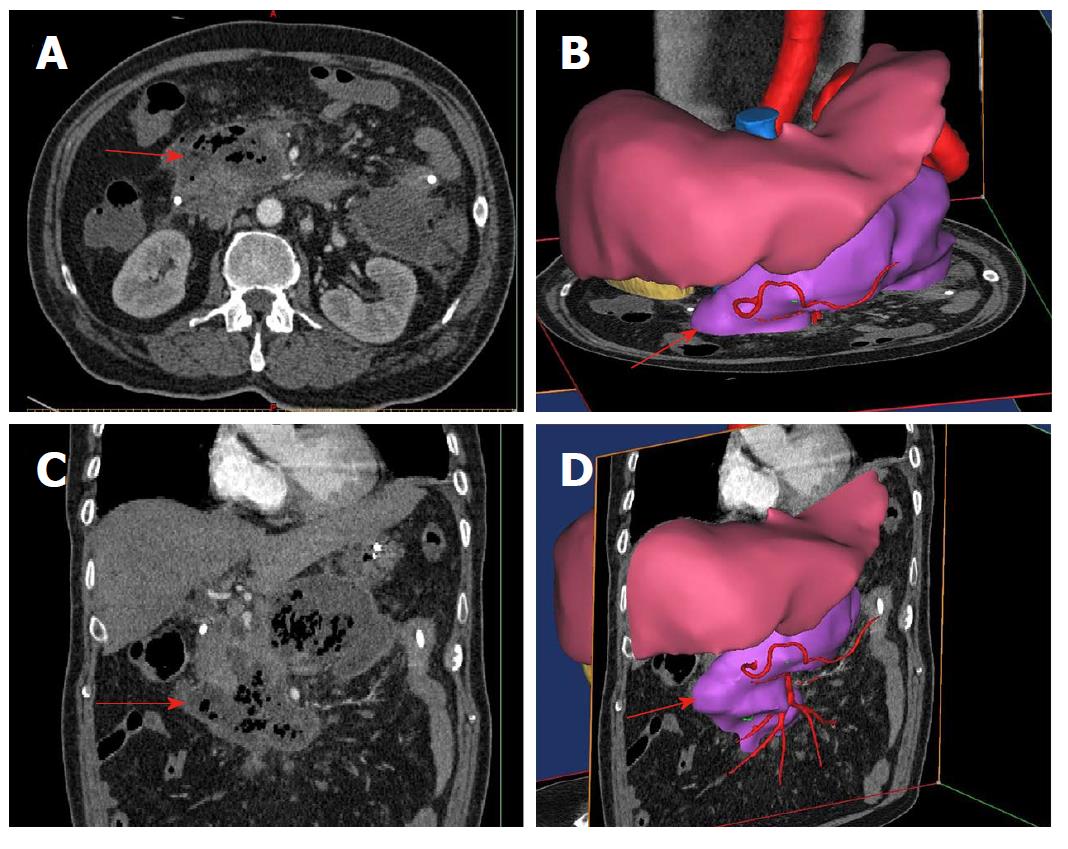
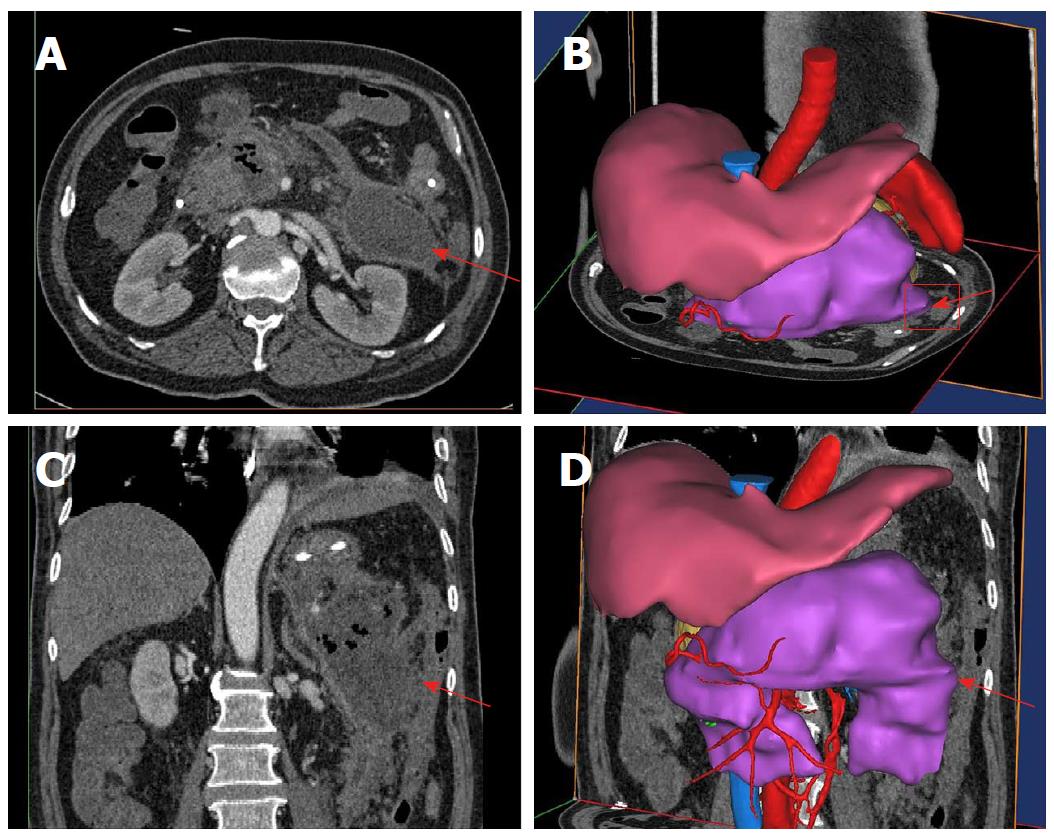
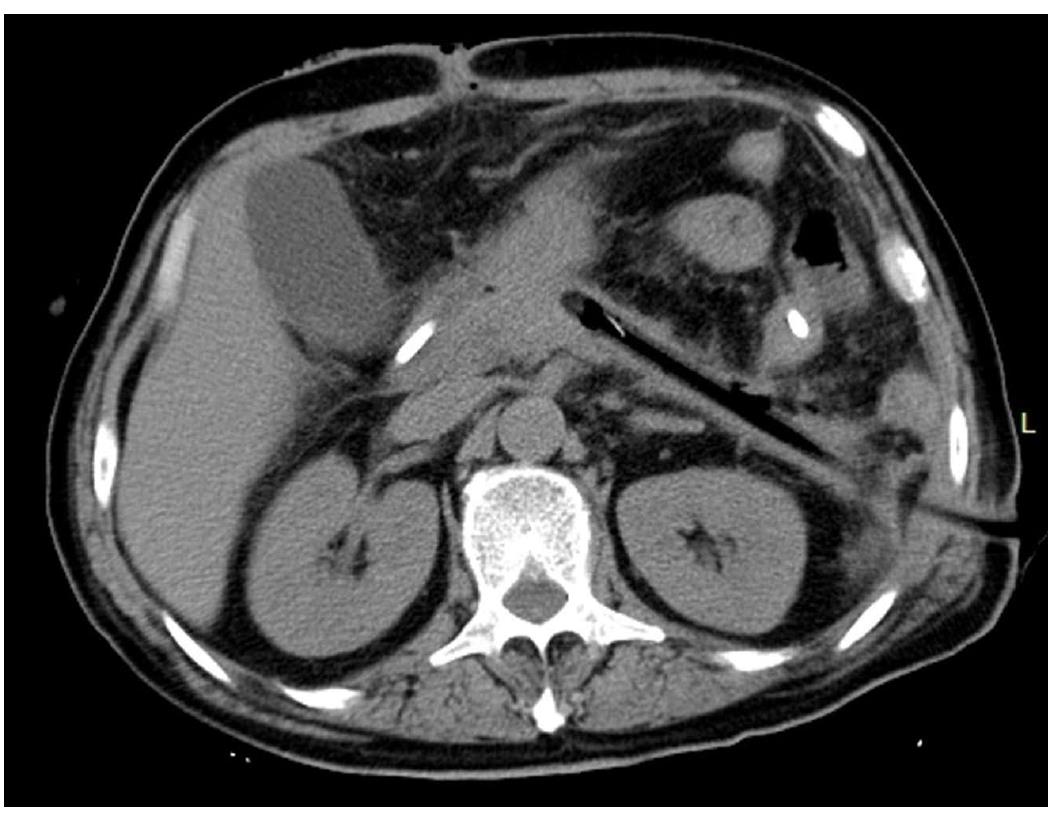
A typical case is given here to illustrate the use of 3D visualization technology: Patient No. 4, a 43-year-old male with alcoholic pancreatitis. The patient suffered from a high fever and abdominal pain for more than 2 wk till he was transferred to the hospital. The CT examination showed extensive peripancreatic necrosis in the retroperitoneal space. The results of 3D reconstruction clearly showed horseshoe-shaped necrosis, as the purple lesion shown in Figure 1A, involving the head of pancreas, uncinate process, root of mesentery, body and tail of pancreas, splenic hilus and left paracolic sulcus, which was closely related to surrounding organs and blood vessels. Virtual percutaneous nephroscopic necrosectomy was performed through the 3D virtual system to determine the three optimal puncture paths. As shown in Figure 1B, the necrosis in the blue area could be debrided from the right puncture path. As shown in Figure 1C, the necrosis in the green area could be debrided from the upper left puncture path. As shown in Figure 1D, the necrosis in the orange area could be debrided from the lower left puncture path. Residual necrosis was found without any single puncture path. Therefore, the three puncture paths were used simultaneously to avoid the operational blind area or residual necrosis. Figures 23 and 4 are the cross-section, coronal-section and 3D reconstruction images of puncture points on the right side, upper left side, and lower left side, respectively. These were critical to ensure the accuracy of PCD under the guidance of CT.
The CT-guided PCD of the 18 patients was successfully performed strictly according to 3D visualization design, and subsequent percutaneous nephroscopic necrosectomy was carried out according to the changes in the condition, debriding maximum necrosis. Twelve patients were cured by conducting one-time surgery. Five patients were cured after two-time surgery and only one patient underwent the surgery three times. Patient No. 14 underwent additionally endoscopic necrosectomy for removing residual small lesions due to the winding sinus. The median operation time was 102 (102 ± 20.7) min, and the postoperative hospitalization time was 35 (35 ± 8.1) d. No major surgical complications occurred. All the patients were cured and discharged from the hospital eventually (Table 1).
| No. | Sex | Age | Severity | Infected necrosis position | No. of PCD | Operation time in minute | No. of operation times | Surgical complications | Postoperative hospitalization time in day |
| 1 | M | 58 | Moderate to severe | Body and tail of pancreas | 1 | 85 | 1 | None | 34 |
| 2 | F | 38 | Severe | Around the pancreas | 2 | 145 | 2 | None | 48 |
| 3 | M | 39 | Moderate to severe | Lesser peritoneal sac, and body and tail of pancreas | 1 | 90 | 1 | None | 23 |
| 4 | M | 43 | Severe | Head of pancreas, uncinate process, root of mesentery, neck of pancreas, body and tail of pancreas, porta lienis, and left paracolic sulcus | 3 | 150 | 1 | None | 29 |
| 5 | M | 58 | Moderate to severe | Body and tail of pancreas | 1 | 90 | 1 | None | 28 |
| 6 | M | 44 | Moderate to severe | Lesser peritoneal sac, and body and tail of pancreas | 2 | 105 | 1 | None | 36 |
| 7 | F | 62 | Severe | Body and tail of pancreas, paracolic sulcus, and head of pancreas | 3 | 100 | 2 | None | 32 |
| 8 | F | 36 | Severe | Head of pancreas, uncinate process, body and tail of pancreas, porta lienis, and left paracolic sulcus | 3 | 120 | 3 | None | 48 |
| 9 | M | 32 | Severe | Lesser peritoneal sac, and body and tail of pancreas | 2 | 105 | 1 | None | 39 |
| 10 | M | 67 | Severe | Head of pancreas, uncinate process, body and tail of pancreas, porta lienis, and left paracolic sulcus | 3 | 120 | 1 | None | 34 |
| 11 | M | 51 | Moderate to severe | Body and tail of pancreas, paracolic sulcus, and head of pancreas | 2 | 95 | 1 | None | 30 |
| 12 | F | 27 | Moderate to severe | Lesser peritoneal sac, and body and tail of pancreas | 1 | 80 | 1 | None | 28 |
| 13 | M | 33 | Severe | Body and tail of pancreas, paracolic sulcus, and head of pancreas | 3 | 95 | 2 | None | 29 |
| 14 | F | 62 | Severe | Body and tail of pancreas, paracolic sulcus, and head of pancreas | 2 | 95 | 1 | Residual uncinate process lesions; endoscopic necrosectomy was used for removal | 41 |
| 15 | M | 44 | Moderate to severe | Lesser peritoneal sac, and body and tail of pancreas | 1 | 90 | 1 | None | 45 |
| 16 | F | 39 | Severe | Body and tail of pancreas, paracolic sulcus, and head of pancreas | 3 | 105 | 2 | None | 48 |
| 17 | M | 67 | Moderate to severe | Lesser peritoneal sac, and body and tail of pancreas | 1 | 70 | 2 | None | 25 |
| 18 | M | 35 | Moderate to severe | Lesser peritoneal sac, and body and tail of pancreas | 2 | 80 | 1 | None | 31 |
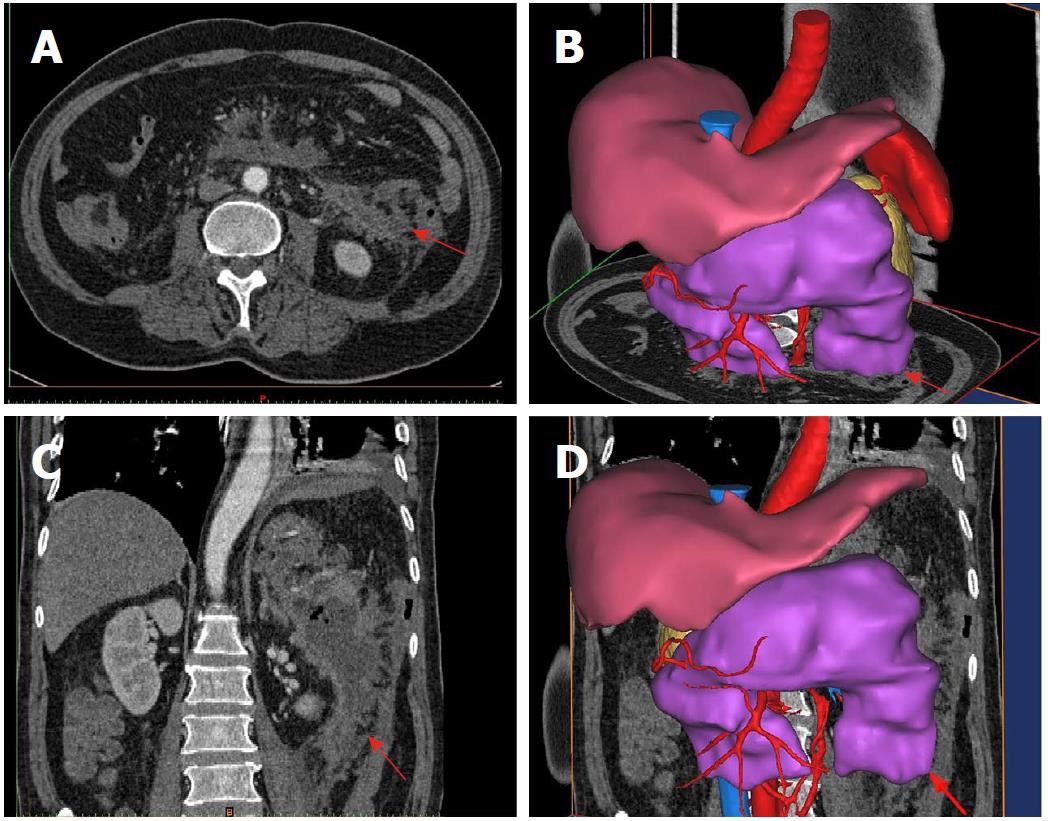
The patient in the aforementioned typical case underwent percutaneous nephroscopic necrosectomy for removing necrotic tissues 14 d after PCD. A large number of infected necrotic tissues were removed from the three puncture points. The clinical condition of the patient improved obviously after surgery. Figure 5 shows the results of abdominal CT re-examination on the 15th day after surgery. Finally, he was discharged from the hospital on the 29th day postoperatively.
INP is a serious disease with a mortality of approximately 30% and up to 80% of cases having multiple organ failure[10,11]. Open necrosectomy has been considered the gold standard treatment for decades. However, the morbidity and mortality rates of the surgery were high[12,13]. In 2000, Carter et al[14] reported a new treatment method called minimal access retroperitoneal pancreatic necrosectomy, which yielded good results. Since then, various kinds of minimally invasive approaches have been increasingly used worldwide[14-17].
The first randomized controlled trial compared a minimally invasive necrosectomy with traditional laparotomy necrosectomy and showed a significant decrease in the incidence of complications[18]. Recently, another clinical study that summed up 394 cases in the last 17 years found that the mortality and incidence of complications in the minimally invasive nephroscope group were 15.3% and 63.5%, respectively, which were significantly lower than those in the laparotomy group (23.3% and 81.7%, respectively)[19]. All these studies were based on the belief that the necessary precondition of the minimally invasive surgery was correct PCD.
As the initial step of minimally invasive treatment, the purpose of PCD was to attenuate sepsis and establish an access track for further necrosectomy. Some patients with infected necrotic pancreatitis could even be cured only using PCD[20,21]. More importantly, PCD established a guide channel for subsequent minimally invasive removal of necrotic tissues. The blind area in percutaneous nephroscopic necrosectomy could be avoided through a reasonable and correct PCD path, which was critical for prognosis. In our hospital, the minimally invasive method has been used since 2008[22] to treat more than 200 patients with INP until now. A number of them were referrals from other hospitals, and some had undertaken PCD by interventional doctors without considering the convenience for subsequent surgical operation. A few of the patients even undertook a wrong PCD due to the doctors’ lack of experience; the catheter passed through the colon, stomach or other hollow organs, which was a disaster for patients with severe pancreatitis. Therefore, it was believed that preoperatively reliable and visualized imaging guidance was crucial for correct PCD.
With the development of digital medicine, 3D visua–lization software has been gradually applied to clinical practice and proved useful in many diseases. Compared with the traditional two-dimensional ultrasound, CT or magnetic resonance imaging, the 3D visualization image has shown huge advantages in the objective, direct and visual image display of lesions and surrounding anatomical structures. Therefore, it could reduce doctor “mistakes” due to lack of experience.
In this study, the technique was first adopted to treat patients with INP. The site, shape and number of infected necrotic lesions, as well as their relationship with peripheral vessels and important organs, could be identified through image reconstruction based on CT before surgery, which instructed doctors on how to select the optimal puncture position and direction to establish an ideal PCD path. The infected necrotic tissue was fully drained using multiple ideal PCD paths, which was critically important to attenuate sepsis. Moreover, it helped avoid the blind area and maximally debride the necrosis conveniently in the subsequent surgery. This was the most important reason why two-thirds of patients were cured through a single operation, no patient was switched to laparotomy, and all were cured finally. Consequently, the operation-related complications and time of hospitalization decreased significantly.
The disadvantage of this study was the limited number of patients. Therefore, analysis of more cases is needed to support the findings. However, the initial good results of the study indicated that the 3D visualization technology was meaningful for this terrible disease.
In conclusion, 3D visualization technique could help clinicians in selecting the optimal puncture path of catheterization, which could not only maximize the degree of drainage of infected lesions in pancreatitis patients but also significantly improve the efficiency of subsequent percutaneous nephroscopic necrosectomy and therefore improve the prognosis.
Infected necrotizing pancreatitis (INP) is a severe disease with high mortality, which generally requires percutaneous catheter drainage (PCD) and following surgical debridement if necessary. Whether the puncture paths of PCD are appropriate or not is related to not only the effect of drainage but also the efficiency of subsequent minimally invasive removal of infected necrosis. However, a number of patients’ PCDs were insufficient or even the catheter passed through the hollow organs due to the doctors’ lack of experience, which was a disaster for INP patients.
Three-dimensional (3D) visualization technology has been proved to be of great help for precise intervention or surgery, which also might be useful to optimize the puncture paths of multiple PCDs for INP patients.
To explore the value of 3D visualization technology for PCDs in INP patients.
Preoperative computed tomography images were converted into 3D modellings through a software and the lesions were well displayed. PCD number and puncture paths were designed through virtual surgery (percutaneous nephroscopic necrosectomy) based on the principle of maximum removal of infected necrosis conveniently. We retrospectively analyzed 18 INP patients’ clinical data and present a typical case in detail.
All the patients’ 3D modellings was well reconstructed, through which the optimal PCD paths were designed. As a result, infected necrosis was conveniently removed in abundance using a nephroscope during the following surgery and two-thirds of the patients were cured after only one-time operation. Postoperative hospitalization time was 35 d on average, no major surgical complications occurred, and no one died.
3D visualization technology was useful for INP patients to maximize the PCD effect. Moreover, it significantly improved the efficiency of subsequent percutaneous nephroscopic necrosectomy, which was critically important for improving the prognosis.
Although the case number of this study was limited, the initial result indicated the value of 3D visualization technology for this terrible disease. Of course, analysis of more cases from multiple centers is needed to support the findings.
| 1. | Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006;354:2142-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 525] [Article Influence: 26.3] [Reference Citation Analysis (2)] |
| 2. | Werner J, Feuerbach S, Uhl W, Büchler MW. Management of acute pancreatitis: from surgery to interventional intensive care. Gut. 2005;54:426-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Raraty MG, Halloran CM, Dodd S, Ghaneh P, Connor S, Evans J, Sutton R, Neoptolemos JP. Minimal access retroperitoneal pancreatic necrosectomy: improvement in morbidity and mortality with a less invasive approach. Ann Surg. 2010;251:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 511] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 5. | van Baal MC, van Santvoort HC, Bollen TL, Bakker OJ, Besselink MG, Gooszen HG; Dutch Pancreatitis Study Group. Systematic review of percutaneous catheter drainage as primary treatment for necrotizing pancreatitis. Br J Surg. 2011;98:18-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 237] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1095] [Article Influence: 84.2] [Reference Citation Analysis (10)] |
| 7. | Sasaki R, Kondo T, Oda T, Murata S, Wakabayashi G, Ohkohchi N. Impact of three-dimensional analysis of multidetector row computed tomography cholangioportography in operative planning for hilar cholangiocarcinoma. Am J Surg. 2011;202:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Andolfi C, Plana A, Kania P, Banerjee PP, Small S. Usefulness of Three-Dimensional Modeling in Surgical Planning, Resident Training, and Patient Education. J Laparoendosc Adv Surg Tech A. 2017;27:512-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4711] [Article Influence: 362.4] [Reference Citation Analysis (48)] |
| 10. | Banks PA, Freeman ML; Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines in acute pancreatitis. Am J Gastroenterol. 2006;101:2379-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1175] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 11. | Working Party of the British Society of Gastroenterology; Association of Surgeons of Great Britain and Ireland; Pancreatic Society of Great Britain and Ireland; Association of Upper GI Surgeons of Great Britain and Ireland. UK guidelines for the management of acute pancreatitis. Gut. 2005;54 Suppl 3:iii1-iii9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 12. | Neoptolemos JP, Raraty M, Finch M, Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut. 1998;42:886-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 194] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 13. | Götzinger P, Sautner T, Kriwanek S, Beckerhinn P, Barlan M, Armbruster C, Wamser P, Függer R. Surgical treatment for severe acute pancreatitis: extent and surgical control of necrosis determine outcome. World J Surg. 2002;26:474-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Carter CR, McKay CJ, Imrie CW. Percutaneous necrosectomy and sinus tract endoscopy in the management of infected pancreatic necrosis: an initial experience. Ann Surg. 2000;232:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 269] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Connor S, Ghaneh P, Raraty M, Sutton R, Rosso E, Garvey CJ, Hughes ML, Evans JC, Rowlands P, Neoptolemos JP. Minimally invasive retroperitoneal pancreatic necrosectomy. Dig Surg. 2003;20:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 123] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 483] [Article Influence: 32.2] [Reference Citation Analysis (2)] |
| 17. | Babu RY, Gupta R, Kang M, Bhasin DK, Rana SS, Singh R. Predictors of surgery in patients with severe acute pancreatitis managed by the step-up approach. Ann Surg. 2013;257:737-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | van Santvoort HC, Besselink MG, Bakker OJ, Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF, van Eijck CH, Bollen TL. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1075] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 19. | Gomatos IP, Halloran CM, Ghaneh P, Raraty MG, Polydoros F, Evans JC, Smart HL, Yagati-Satchidanand R, Garry JM, Whelan PA. Outcomes From Minimal Access Retroperitoneal and Open Pancreatic Necrosectomy in 394 Patients With Necrotizing Pancreatitis. Ann Surg. 2016;263:992-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Segal D, Mortele KJ, Banks PA, Silverman SG. Acute necrotizing pancreatitis: role of CT-guided percutaneous catheter drainage. Abdom Imaging. 2007;32:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Rocha FG, Benoit E, Zinner MJ, Whang EE, Banks PA, Ashley SW, Mortele KJ. Impact of radiologic intervention on mortality in necrotizing pancreatitis: the role of organ failure. Arch Surg. 2009;144:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Cai SW, Wang PF, Liu ZW, He L, Liu H, Kang HJ, Xiao YY, Song Q, Gu WQ, Dong JH. Improvement and effect of retroperitoneoscopic necrosectomy for infected necrotizing pancreatitis. Zhonghua Gandan Waike Zazhi. 2012;18:439-441. [DOI] [Full Text] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Ominami M, Park SJ, Queiroz DM S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y