Published online Apr 28, 2018. doi: 10.3748/wjg.v24.i16.1795
Peer-review started: December 5, 2017
First decision: January 18, 2018
Revised: March 6, 2018
Accepted: March 31, 2018
Article in press: March 30, 2018
Published online: April 28, 2018
Processing time: 142 Days and 12.1 Hours
To investigate the role of tacrolimus intra-patient variability (IPV) in adult liver-transplant recipients.
We retrospectively assessed tacrolimus variability in a cohort of liver-transplant recipients and analyzed its effect on the occurrence of graft rejection and de novo donor-specific antibodies (dnDSAs), as well as graft survival during the first 2 years posttransplantation. Between 02/08 and 06/2015, 116 patients that received tacrolimus plus mycophenolate mofetil (with or without steroids) were included.
Twenty-two patients (18.5%) experienced at least one acute-rejection episode (BPAR). Predictive factors for a BPAR were a tacrolimus IPV of > 35% [OR = 3.07 95%CI (1.14-8.24), P = 0.03] or > 40% [OR = 4.16 (1.38-12.50), P = 0.01), and a tacrolimus trough level of < 5 ng/mL [OR=3.68 (1.3-10.4), P =0.014]. Thirteen patients (11.2%) developed at least one dnDSA during the follow-up. Tacrolimus IPV [coded as a continuous variable: OR = 1.1, 95%CI (1.0-1.12), P = 0.006] of > 35% [OR = 4.83, 95%CI (1.39-16.72), P = 0.01] and > 40% [OR = 9.73, 95%CI (2.65-35.76), P = 0.001] were identified as predictors to detect dnDSAs. IPV did not impact on patient- or graft-survival rates during the follow-up.
Tacrolimus-IPV could be a useful tool to identify patients with a greater risk of graft rejection and of developing a de novo DSA after liver transplantation
Core tip: Tacrolimus intra-patient variability (Tac IPV) was associated with kidney-graft rejection and worse long-term outcomes, but until now, was not well studied after liver transplantation in adult recipients. We found that the coefficient of variability-IPV of tacrolimus was a predictive factor for acute rejection and the occurrence of de novo donor-specific antibodies (DSA) after liver transplantation in a retrospective cohort of 116 recipients treated with tacrolimus and mycophenolate mofetil. This could be a useful tool to identify patients with a greater risk of graft rejection and of developing a de novo DSA after liver transplantation.
- Citation: Del Bello A, Congy-Jolivet N, Danjoux M, Muscari F, Lavayssière L, Esposito L, Hebral AL, Bellière J, Kamar N. High tacrolimus intra-patient variability is associated with graft rejection, and de novo donor-specific antibodies occurrence after liver transplantation. World J Gastroenterol 2018; 24(16): 1795-1802
- URL: https://www.wjgnet.com/1007-9327/full/v24/i16/1795.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i16.1795
Tacrolimus (Tac) is considered a cornerstone within immunosuppression protocols to prevent T-cell and antibody-mediated rejection after liver transplantation[1-3] However, this treatment presents a narrow therapeutic index: overexposure can lead to clinically serious events[4] thus necessitating regular therapeutic drug monitoring, whereas underexposure can lead to acute or chronic graft rejection[4-6] Inter-individual variability from Tac therapy may be explained by the polymorphism of cytochromes P450 3A4 and 5 (responsible for biotransformation of Tac)[7] and the drug transporter ABCB1[8], circadian rhythms[9] and also drug-drug interactions[10]. In addition to inter-individual variability, the pharmacokinetics of Tac can vary within individual patients. The concept of intra-patient variability (IPV) refers to the fluctuations in Tac blood concentrations (and consequently episodes of over- and under-immunosuppression) that some patients experience over time[11].
Several non-modifiable and modifiable factors contribute to Tac IPV (e.g., polymorphism in CYP3A genes, the circadian rhythm of Tac exposure, gastrointestinal events such as diarrhea, cholestasis, changes in protein levels, anemia, but also drug-drug interactions with macrolides or azole anti-fungal treatments, foods, or changes in formulation or generic substitution)[11], but non-adherence to Tac seems to be the main cause of IPV[12,13]. It was previously suggested that higher degree of Tac IPV was associated with kidney-graft rejection and worse long-term outcomes after kidney transplantation[14,15]. Similar limited data have been reported after liver transplantation[16,17], mainly in pediatric cohorts. Moreover, little is known concerning the relationship between Tac variability and the occurrence of donor-specific antibodies (DSAs). Herein, we retrospectively assessed the variability of Tac in a cohort of liver-transplant recipients and analyzed its impact on the number of acute rejections, the occurrence of de novo DSAs, and patient- and graft-survival rates.
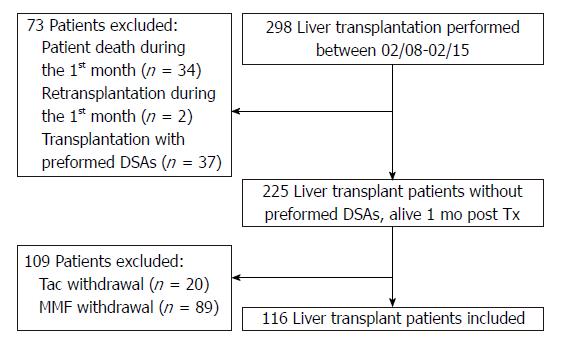
Between February 2008 (i.e., the date when the solid-phase Luminex assay was set up in our institution) and June 2015, a total of 298 adult patients received a liver transplant from deceased donors (DDLT) in our center. Patients excluded from the study were those that died within the first month posttransplantation (n = 34), those that needed a re-transplant during the first month (n = 2), and those that received a transplant with a preformed DSA (mean fluorescence intensity cut-off > 1000) directed against human leukocyte antigen (HLA) A, B, Cw, DR, DQ, or DP (n = 37). In order to avoid confounding factors associated with others immunosuppressive treatments, only patients that received and were maintained under Tac and mycophenolate mofetil (MMF) (with or without steroids) were included in this study (Figure 1). All patients but five received Tac given twice daily (Prograf®). The other five received Tac once daily (Advagraf®). We excluded patients that had Tac or MMF withdrawn. Moreover, to calculate intra-patient variability, at least three trough levels of Tac had to be available. Hence, 116 patients with a functioning liver allograft at 1 mo posttransplantation were included in this study after having given their informed consent and after we had obtained Toulouse University IRB approval.
The target concentration of Tac trough level was 7-10 ng/mL during the first 3 mo, and 5-10 ng/mL thereafter during the follow-up. Each participant was followed for 2 years or until re-transplantation (n = 3) or death (n = 6). The median follow-up was 24 mo (range: 6-24). All rejection episodes were biopsy proven. Biopsies were only performed for cause during the study period and were analyzed according to the Banff criteria[18-20]. Graft failure was defined as the need for re-transplantation or as death from liver failure.
Detection of cytomegalovirus was performed using real-time PCR, as previously described[21], at month 3, 6, 12, and 24, and at any other time if clinically indicated.
Tac trough levels were routinely assessed using high-performance liquid chromatography-linked tandem mass spectrometry (HPLC-MS) at discharge, then monthly between months 1-6, and thereafter at months 9, 12, 15, 18, and 24. To calculate the IPV of Tac, at least three Tac trough levels from each patient had to be available. The median number of available Tac measurements was 10 (range: 4-12).
Tac IPV was estimated using the coefficient of variability (CV). The CV-IPV was calculated as follows: CV-IPV (%) = (standard deviation/mean Tac trough-level concentration) × 100. Because all patients received the same drug dose between discharge and M24, the obtained levels were corrected for the corresponding daily dose of tacrolimus (CV C0/D-IPV). In addition, because some patients were converted from one formulation to another during the follow-up, we calculated CV and CV C0/D-IPV after excluding the Tac trough levels obtained during the adjustment dose period, i.e., the month following a switch.
To compare IPV with the two formulations of Tac, the Tac twice-daily CV-IPV was calculated using Tac trough levels obtained from patients that had received Tac twice daily since transplantation until last follow-up and those obtained in patients switched for Tac once daily before the switch. The Tac once-daily CV-IPV was calculated using Tac trough levels from patients that received Tac once daily since transplantation until the last follow-up, and those obtained from patients that were later switched from twice- to a once-daily formulation after the switch (this excluded Tac trough levels obtained in the month following the switch).
All patients were screened for anti-HLA DSAs at transplantation, and at month 3 and 12, and annually thereafter. Additional screening was performed in case of graft dysfunction. Luminex® assays were used to determine the specificity of class I HLAs in A/B/Cw and class II in DR/DQ/DP IgG antibodies in the recipients’ sera (centrifuged at 10000 g for 10 min) using Labscreen single Ag HLA class-I and class-II detection tests (One Lambda, Canoga Park, CA, United States), according to the manufacturer’s instructions. The presence and specificity of antibodies were then detected using a Labscan 100®, and the mean fluorescence (baseline) value for each sample in each bead was evaluated. The baseline value was calculated as follows: [raw sample mean fluorescence intensity (MFI)-raw negative serum control MFI-negative-bead raw MFI sample-negative-bead raw MFI negative serum control]. A baseline value of > 1000 was considered positive.
Categorical variables are expressed as percentages and comparisons between groups were made using the chi-squared test or, if appropriate, Fisher’s exact test. Continuous variables were expressed as medians and ranges, and compared using the Mann-Whitney test. Logistic regression analysis was used to determine the predictors for acute-rejection episodes and the occurrence of de novo anti-HLA DSAs. Variables with a P < 0.1 in the univariate analyses were included in the stepwise multivariable analyses. P < 0.05 was considered statistically significant.
The patients’ characteristics at transplantation are presented in Table 1. All liver transplantations performed in this study were performed from DDLT. The mean DDLT age was 51 ± 17 years. To note, one DDLT was < 18 years, and 4 DDLT were > 80 years.
| Variable | n = 116 |
| Donors’ age at transplantation, yr (range) | 53 (9-85) |
| Recipients’ age at transplantation, yr (range) | 57 (18-72) |
| Recipients’ gender: male, n (%) | 96 (83) |
| Initial liver disease, n (%) | |
| Alcohol | 49 (43) |
| Viral (HCV, HBV) | 36 (31) |
| Autoimmune disease (AIH, PSC, PBC) | 13 (11) |
| Other1 | 18 (17) |
| Median MELD score at transplantation (range) (%) | 22 (6-40) |
| Positive HCV RNA at transplantation, n (%) | 21 (18) |
| Re-transplantation, yes (%) | 3 (3) |
| Induction therapy, yes: n (%) | 87 (75) |
| Polyclonal antibodies, n (%) | 9 (8) |
| Interleukin-2 receptor blocker, n (%) | 78 (67) |
| Conversion during the follow-up from twice-daily to once daily tacrolimus, n (%) | 42 (36) |
| Number of patients receiving tacrolimus once daily, n (%) | 5 (4) |
| At discharge | |
| Month 1 | 8 (7) |
| Month 3 | 9 (8) |
| Month 6 | 12 (10) |
| Month 9 | 18 (16) |
| Month 12 | 26 (31) |
| Month 18 | 39 (34) |
| Month 24 | 47 (41) |
| Tacrolimus trough level (ng/mL) | 7.6 ± 3 |
| At discharge | |
| Month 1 | 8 ± 3 |
| Month 3 | 8.4 ± 3 |
| Month 6 | 8.4 ± 3 |
| Month 9 | 7.4 ± 3 |
| Month 12 | 7.8 ± 3 |
| Month 18 | 7.5 ± 2 |
| Month 24 | 6.9 ± 3 |
| Mycophenolate mofetil dose (mg/d) | 1700 ± 600 |
| At discharge | |
| Month 3 | 1250 ± 550 |
| Month 6 | 1100 ± 450 |
| Month 12 | 1000 ± 300 |
| Month 24 | 1000 ± 300 |
| Steroids (mg/d) | |
| At discharge: Yes (%) | 116 (100) |
| Dose (mg/d) | 20 ± 12 |
| Month 3: Yes (%) | 114 (98) |
| Dose (mg/d) | 8 ± 4 |
| Month 6: Yes (%) | 110 (95) |
| Dose (mg/d) | 7 ± 5 |
| Month 12: Yes (%) | 104 (90) |
| Dose (mg/d) | 6 ± 6 |
| Month 24: Yes (%) | 97 (84) |
| Dose (mg/d) | 5 ± 2 |
During the follow-up, 44 (38%) patients were switched from Tac immediate-release given twice a day (Prograf®), to Tac once a day to improve quality of life. The switch was performed at a mean of 15 (range: 1-18) mo posttransplantation.
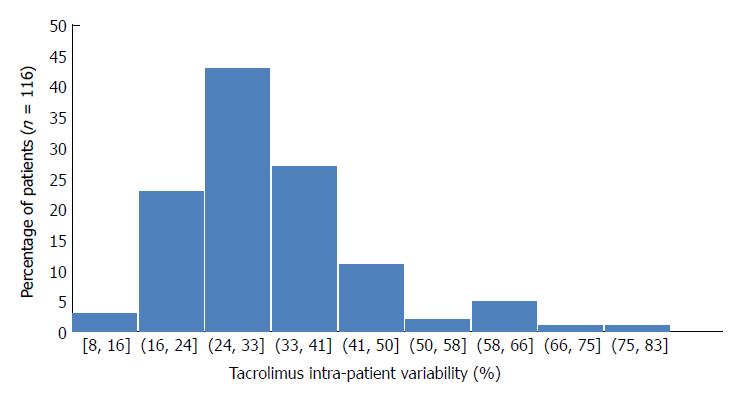
Mean tacrolimus trough level was 8 ± 3 ng/mL during the follow-up (Table 1). The mean dose of Tac was 6.8, 6.7, 6.4, 5.9, 5.4, 5.1, 4.8, and 4.6 mg/d, respectively, at discharge and at months 1, 3, 6, 9, 12, 18, and 24. Forty-five (38.8%) patients presented with a Tac trough level of < 5 ng/mL at least once during the follow-up. The overall mean Tac CV- IPV was 32 ± 12% [median CV-IPV 30.5% (7.6-80.6)]. Tac CV-IPV distribution is presented in Figure 2. The 1th, 2th, 3th, and 4th quartiles were, respectively, 25%, 30.5%, 36.5%, and 80.6%. The mean Tac CV-IPV was 30% ± 11% in patients given Tac once daily and was 32% ± 12% in patients that received Tac twice daily (P = 0.10). The mean Tac CV- IPV in the five patients that had received Tac once-daily since transplantation was 30% ± 7%. In the 44 patients that were converted from Tac twice-daily to once daily, the mean values of Tac CV-IPV were 32.3% ± 12% and 30% ± 12% before and after the switch, respectively (P = 0.21).
Overall mean CV C0/d- IPV was 73% ± 43%. It was 69% ± 29% with Tac twice-daily compared to 79% ± 50% for Tac given once daily (P = 0.9).
During the follow-up, 22 patients (19%) presented with at least one episode of acute rejection. The time between transplantation and a diagnosis of acute rejection (i.e., the date of the biopsy) was 3.5 mo (range: 0.5-12). Fourteen patients (12%) experienced a T-cell steroid-sensitive acute rejection, and six patients (5%) presented with a T-cell steroid-resistant acute rejection, which was treated with polyclonal antibodies. One patient presented with an acute antibody-mediated rejection at 4 mo posttransplantation. The Tac CV-IPV in this patient was high: CV-IPV of 63.2% and CV C0/d- IPV = 68.2%. The risk factors for acute rejection after liver transplantation are presented in Table 2. The predictive factors for a biopsy-proven acute rejection were a Tac trough level of < 5 ng/mL [OR = 3.68; 95%CI (1.30-10.41), P = 0.014], the Tac CV-IPV (coded as a continuous variable) [OR = 1.1; 95%CI (1.01-1.11), P = 0.008], a CV-IPV of > 35% [OR = 3.07; 95%CI (1.14-8.24), P = 0.03], and a CV-IPV of > 40% [OR = 4.16; 95%CI (1.38-12.50), P = 0.01]. Twenty-one of the 22 patients that presented with an acute-rejection episode were receiving Tac twice daily when the rejection was diagnosed.
| Variable | Univariate analyses | Multivariate analyses | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| MELD score > 30 (n = 31) | 0.55 | 0.12-1.90 | 0.42 | - | ||
| Initial liver disease | ||||||
| (1) Alcohol cirrhosis (n = 49) vs (2, 3, 4) | 0.58 | 0.18-1.68 | 0.34 | - | ||
| (2) Viral disease (n = 36) vs (1, 3, 4) | 1.34 | 0.44-3.90 | 0.61 | - | ||
| (3) Auto-immune ILD (n = 13) vs (1, 2,4) | 3.12 | 0.71-12.47 | 0.07 | 1.00 | 0.51-1.15 | 0.210 |
| (4) Other (n = 18) vs (1, 2, 3) | 0.49 | 0.05-2.37 | 0.52 | - | ||
| Induction therapy, yes (n = 87) | 0.66 | 0.22-2.15 | 0.42 | - | ||
| Polyclonal antibodies (vs other) | 3.89 | 0.70-20.13 | 0.06 | 2.87 | 0.61-13.47 | 0.180 |
| IL2R blockers (vs other) | 0.40 | 0.14-1.70 | 0.08 | 0.52 | 0.185-1.50 | 0.230 |
| Donors’ age > 50 yr (n = 69) | 0.98 | 0.35-2.88 | 1.00 | - | ||
| Recipients’ age > 50 yr (n = 92) | 0.61 | 0.20-2.01 | 0.41 | - | ||
| HCV-RNA + At transplantation (n = 21) | 1.96 | 0.54-6.45 | 0.22 | - | ||
| Steroid withdrawal during the FU (n = 19) | 2.30 | 0.63-7.82 | 0.20 | - | ||
| De novo DSAs during the FU (n = 13) | 2.80 | 0.64-11.19 | 0.13 | - | ||
| Tacrolimus trough level < 5 ng/mL (n = 34) | 3.00 | 1.05-8.96 | 0.02 | 3.68 | 1.30-10.41 | 0.014 |
| CV-IPV tacrolimus (continuous variable) | 2.70 | 1.88-13.45 | 0.01 | 1.10 | 1.01-1.11 | 0.008 |
| CV-IPV > 35% | 3.05 | 1.05-8.96 | 0.03 | 3.07 | 1.14-8.24 | 0.030 |
| CV-IPV > 0% | 2.97 | 0.91-9.30 | 0.04 | 4.16 | 1.38-12.50 | 0.010 |
| CV-C0/d-IPV | 1.89 | 0.67-5.74 | 0.24 | - | ||
Thirteen patients (11.2%) presented with at least one de novo DSA during the posttransplantation follow-up (nine anti-HLA class II, three anti-HLA class I, one anti-HLA classI and II). Only one of these patients developed an antibody-mediated rejection. The median time between transplantation and detection of a de novo DSA was 3.5 mo (range: 1-12). The risk factors for a de novo DSA are presented in Table 3. The Tac CV-IPV [coded as a continuous variable: OR = 1.1, 95%CI (1.0-1.12), P = 0.006), and a CV-IPV of > 35% [OR = 4.83, 95%CI (1.39-16.72), P = 0.01] or of > 40% [OR = 9.73, 95%CI (2.65-35.76), P = 0.001] were identified as predictors for the occurrence of de novo DSAs detection.
| Variable | Univariate analyses | Multivariate analyses | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| MELD score > 30 (n = 31) | 1.84 | 0.43-7.10 | 0.33 | - | ||
| Initial liver disease | ||||||
| (1) Alcohol cirrhosis (n = 49) vs (2, 3, 4) | 0.58 | 0.12-2.22 | 0.55 | - | ||
| (2) Viral disease (n = 36) vs (1, 3, 4) | 0.98 | 0.21-3.86 | 1.0 | - | ||
| (3) Autoimmune ILD (n = 13) vs (1, 2, 4) | 1.51 | 0.14-8.46 | 0.64 | - | ||
| (4) Other (n = 18) vs (1, 2, 3) | 2.79 | 0.55-11.83 | 0.64 | - | ||
| Induction therapy, yes (n = 87) | 1.61 | 0.41-7.61 | 0.55 | - | ||
| Polyclonal antibodies (vs other) | 0.59 | 0.70-18.00 | 0.60 | - | ||
| IL2R blockers (vs other) | 1.1 | 0.28-5.28 | 1.0 | - | ||
| Donors’ age > 50 yr (n = 69) | 0.78 | 0.20-3.00 | 0.77 | - | ||
| Recipients’ age > 50 yr (n = 92) | 0.36 | 0.09-1.58 | 0.10 | 0.2 | 0.07-0.85 | 0.3 |
| HCV RNA + at transplantation (n = 21) | 1.41 | 0.23-6.23 | 0.70 | - | ||
| Steroid withdrawal during the FU (n = 19) | 0.39 | 0.01-3.01 | 0.69 | - | ||
| Tacrolimus trough level < 5 ng/mL (n = 34) | 1.59 | 0.38-6.05 | 0.52 | - | ||
| CV-IPV tacrolimus (continuous variable) | 1.92 | -1.28-21.39 | 0.08 | 1.1 | 1.0-1.12 | 0.006 |
| CV-IPV > 35% | 4.66 | 1.22-19.82 | 0.02 | 4.83 | 1.39-16.72 | 0.01 |
| CV-IPV > 40% | 9.10 | 2.28-40.63 | < 0.001 | 9.73 | 2.65-35.76 | 0.001 |
| CV-C0/d-IPV | 3.15 | 5.47-27.31 | 0.005 | 1.0 | 0.97-1.02 | 0.09 |
During the follow-up, six patients died [at a mean of 13 mo (range: 6-23) posttransplantation]. The causes of death were infections (n = 3), cardiovascular (n = 2), and neoplastic (n = 1) complications. No difference in Tac CV- IPV was observed between patients that died during the follow-up (CV-IPV 33% ± 6%) and those that did not (CV-IPV 32% ± 12%; P = 0.70). Three patients required re-transplantation at month 5, 10, and 14, respectively, for ischemic cholangitis that occurred posttransplantation. During the follow-up, 24 patients presented with posttransplant replication of cytomegalovirus. No difference in Tac CV-IPV was observed between patients with replication of cytomegalovirus (CV-IPV 32% ± 9%) and those without replication (32% ± 12%, P = 0.90).
High IPV has been previously associated with a greater risk of graft rejection, an accelerated progression of chronic histological lesions, and worse long-term survival after kidney transplantation[11,14,22,23]. In pediatric liver-transplants, Tac variability was associated with late acute rejection[16]. In the present study, we investigated the impact of Tac variability in 116 adult liver-transplant recipients. In order to avoid confounding factors, we focused on patients that received a graft without preformed DSAs and that had received Tac associated with MMF. Although the mean Tac trough level was 8 ± 3 ng/mL during the study period, nearly 40% of patients had a Tac trough level of < 5 ng/mL at least once during the follow-up. Tac CV-IPV varied from 7.6%-80.6% (median 30.5%), and median Tac CV C0/d-IPV was 62% (18-147). Almost one-third of patients presented with a Tac CV-IPV of > 35%. This high value is similar to those reported in previous studies, mainly after kidney transplantation[24,25]. In kidney-transplant[13,25] and pediatric liver-transplant patients[16], high CV-IPV was associated with an increased risk of acute rejection. In the present study, we found that a Tac trough level of < 5 ng/mL, the Tac CV-IPV (coded as a continuous variable), a CV-IPV of > 35%, and a CV-IPV > 40% were independent predictive factors for a biopsy-proven graft rejection.
Posttransplant positive DSAs were associated with decreased graft survival and increased acute or chronic graft rejections[2,3,26]. It has been previously suggested that iterative transplantation, low levels of calcineurin inhibitors, the use of cyclosporine (compared to Tac), and non-adherence can promote the development of a de novo DSA after liver transplantation[2]. Herein, we found that the Tac CV-IPV (coded as a continuous variable), a CV-IPV of > 35%, and CV-IPV > 40% were independent predictive factors for the occurrence of a de novo DSA. Similar data, reported after kidney transplantation[24], from a cohort of 310 adult kidney-transplant patients given Tac twice-daily during the first year posttransplant, showed that a history of acute rejection, re-transplantation and a Tac CV greater than 30% were associated with the occurrence of a de novo DSA. In our study, one patient presented with an acute antibody-mediated rejection associated with an anti-class II de novo DSA at 3 mo after liver transplantation. Interestingly, this patient had high tacrolimus variability (CV-IPV 63.2%, CV C0/d-IPV 68.2%). None of the other 12 patients that developed a DSA experienced an acute antibody-mediated rejection. However, it was suggested that patients with positive DSAs would present lower graft survival, consecutive to chronic antibody mediated rejection[27] rather than to acute antibody-mediated rejection episodes.
In several studies, but not all, the use of once-daily tacrolimus compared to a twice daily formulation has been found to improve adherence and to reduce IPV[11,28-31]. In the present study, no difference between Tac formulations was observed.
This study has several limitations. Because of its retrospective design, we could not evaluate the cause of Tac variability. It has been suggested previously that non-adherence is the main cause of Tac variability[11]. However, in our study, adherence was not evaluated using objective methods, such as those previously reported using electronic devices[28]. Moreover, we did not evaluate MMF variability in our study because we do not perform this analysis routinely in our center. Of note, conflicting results have been reported concerning the use of MMF variability after solid-organ transplantation[14,25]. It was also previously suggested that pre-transplant determination of CYP3A5 and MDR1 polymorphisms[32] allows more rapid achievement of therapeutic Tac trough level. However, no association between the pharmacogenomics parameters and Tac intra-patient variability is expected and was reported.
In conclusion, we found that the CV-IPV of Tac was a predictive factor for acute rejection and the occurrence of a de novo DSA after liver transplantation. This could be a useful tool to identify patients with a greater risk of graft rejection and of developing a de novo DSA after liver transplantation. Future studies should investigate the role of Tac IPV on long-term outcomes, on chronic graft rejection, and over-immunosuppression-related diseases (cancer, and related immunocompromised infections).
Tacrolimus (Tac) is considered a cornerstone within immunosuppression protocols to prevent T-cell and antibody-mediated rejection after liver transplantation. However, this treatment presents a narrow therapeutic index: overexposure can lead to clinically serious events, thus necessitating regular therapeutic drug monitoring, whereas underexposure can lead to acute or chronic graft rejection. The concept of intra-patient variability (IPV) refers to the fluctuations in Tac blood concentrations (and consequently episodes of over- and under-immunosuppression) that some patients experience over time.
Tac-IPV is an inexpensive assay to explore fluctuations in Tac blood concentrations. We investigated the potential usefulness of Tac-IPV to predict the incidence of donor specific antibodies and graft rejection episodes.
Our aim was to investigate the role of tacrolimus IPV in adult liver-transplant recipients.
We retrospectively assessed tacrolimus variability and analyzed its effect on the occurrence of graft rejection and de novo donor-specific antibodies.
Twenty-two patients experienced at least one acute-rejection episode (BPAR). Predictive factors for a BPAR were a tacrolimus IPV of > 35% or > 40%, and a tacrolimus trough level of < 5 ng/mL. Thirteen patients developed at least one dnDSA during the follow-up. Tacrolimus IPV and tacrolimus IPV of > 35%, and > 40% were identified as predictors to detect dnDSAs. IPV did not impact on patient- or graft-survival rates during the follow-up.
In our study higher Tac-IPV was associated with graft rejection and occurrence of DSAs.
Tacrolimus-IPV could be a useful tool to identify patients with a greater risk of graft rejection and of developing a de novo DSA after liver transplantation.
| 1. | Price DC. Radioisotopic evaluation of the thyroid and the parathyroids. Radiol Clin North Am. 1993;31:991-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Kaneku H, O’Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, Terasaki PI. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant. 2013;13:1541-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol. 2016;64:433-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 744] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 4. | de Mare-Bredemeijer EL, Metselaar HJ. Optimization of the use of Calcineurin inhibitors in liver transplantation. Best Pract Res Clin Gastroenterol. 2012;26:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Rodríguez-Perálvarez M, Germani G, Papastergiou V, Tsochatzis E, Thalassinos E, Luong TV, Rolando N, Dhillon AP, Patch D, O’Beirne J. Early tacrolimus exposure after liver transplantation: relationship with moderate/severe acute rejection and long-term outcome. J Hepatol. 2013;58:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Del Bello A, Congy-Jolivet N, Muscari F, Lavayssière L, Esposito L, Cardeau-Desangles I, Guitard J, Dörr G, Suc B, Duffas JP. Prevalence, incidence and risk factors for donor-specific anti-HLA antibodies in maintenance liver transplant patients. Am J Transplant. 2014;14:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Hesselink DA, Bouamar R, Elens L, van Schaik RH, van Gelder T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2014;53:123-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Tada H, Satoh S, Iinuma M, Shimoda N, Murakami M, Hayase Y, Kato T, Suzuki T. Chronopharmacokinetics of tacrolimus in kidney transplant recipients: occurrence of acute rejection. J Clin Pharmacol. 2003;43:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | van Gelder T. Drug interactions with tacrolimus. Drug Saf. 2002;25:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Shuker N, van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev (Orlando). 2015;29:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Shemesh E, Shneider BL, Savitzky JK, Arnott L, Gondolesi GE, Krieger NR, Kerkar N, Magid MS, Stuber ML, Schmeidler J. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics. 2004;113:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, Solomon M, McCrindle BW, Grant D. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010;14:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Borra LC, Roodnat JI, Kal JA, Mathot RA, Weimar W, van Gelder T. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant. 2010;25:2757-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Ro H, Min SI, Yang J, Moon KC, Kim YS, Kim SJ, Ahn C, Ha J. Impact of tacrolimus intraindividual variability and CYP3A5 genetic polymorphism on acute rejection in kidney transplantation. Ther Drug Monit. 2012;34:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Shemesh E, Bucuvalas JC, Anand R, Mazariegos GV, Alonso EM, Venick RS, Reyes-Mugica M, Annunziato RA, Shneider BL. The Medication Level Variability Index (MLVI) Predicts Poor Liver Transplant Outcomes: A Prospective Multi-Site Study. Am J Transplant. 2017;17:2668-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Christina S, Annunziato RA, Schiano TD, Anand R, Vaidya S, Chuang K, Zack Y, Florman S, Shneider BL, Shemesh E. Medication level variability index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transpl. 2014;20:1168-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1014] [Article Influence: 35.0] [Reference Citation Analysis (1)] |
| 19. | Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, Fung J, Gouw A, Gustafsson B, Haga H. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. Hepatology. 2000;31:792-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 362] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 20. | Demetris AJ, Bellamy C, Hübscher SG, O’Leary J, Randhawa PS, Feng S, Neil D, Colvin RB, McCaughan G, Fung JJ. 2016 Comprehensive Update of the Banff Working Group on Liver Allograft Pathology: Introduction of Antibody-Mediated Rejection. Am J Transplant. 2016;16:2816-2835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 460] [Article Influence: 46.0] [Reference Citation Analysis (5)] |
| 21. | Mengelle C, Sandres-Sauné K, Pasquier C, Rostaing L, Mansuy JM, Marty M, Da Silva I, Attal M, Massip P, Izopet J. Automated extraction and quantification of human cytomegalovirus DNA in whole blood by real-time PCR assay. J Clin Microbiol. 2003;41:3840-3845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Taber DJ, Su Z, Fleming JN, McGillicuddy JW, Posadas-Salas MA, Treiber FA, Dubay D, Srinivas TR, Mauldin PD, Moran WP. Tacrolimus Trough Concentration Variability and Disparities in African American Kidney Transplantation. Transplantation. 2017;101:2931-2938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Vanhove T, Vermeulen T, Annaert P, Lerut E, Kuypers DRJ. High Intrapatient Variability of Tacrolimus Concentrations Predicts Accelerated Progression of Chronic Histologic Lesions in Renal Recipients. Am J Transplant. 2016;16:2954-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | Rodrigo E, Segundo DS, Fernández-Fresnedo G, López-Hoyos M, Benito A, Ruiz JC, de Cos MA, Arias M. Within-Patient Variability in Tacrolimus Blood Levels Predicts Kidney Graft Loss and Donor-Specific Antibody Development. Transplantation. 2016;100:2479-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 25. | Hsiau M, Fernandez HE, Gjertson D, Ettenger RB, Tsai EW. Monitoring nonadherence and acute rejection with variation in blood immunosuppressant levels in pediatric renal transplantation. Transplantation. 2011;92:918-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | O'Leary JG, Kaneku H, Jennings LW, Bañuelos N, Susskind BM, Terasaki PI, Klintmalm GB. Preformed class II donor-specific antibodies are associated with an increased risk of early rejection after liver transplantation. Liver Transpl. 2013;19:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | O'Leary JG, Cai J, Freeman R, Banuelos N, Hart B, Johnson M, Jennings LW, Kaneku H, Terasaki PI, Klintmalm GB. Proposed Diagnostic Criteria for Chronic Antibody-Mediated Rejection in Liver Allografts. Am J Transplant. 2016;16:603-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Kuypers DR, Peeters PC, Sennesael JJ, Kianda MN, Vrijens B, Kristanto P, Dobbels F, Vanrenterghem Y, Kanaan N; ADMIRAD Study Team. Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 29. | van Hooff J, Van der Walt I, Kallmeyer J, Miller D, Dawood S, Moosa MR, Christiaans M, Karpf C, Undre N. Pharmacokinetics in stable kidney transplant recipients after conversion from twice-daily to once-daily tacrolimus formulations. Ther Drug Monit. 2012;34:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Wehland M, Bauer S, Brakemeier S, Burgwinkel P, Glander P, Kreutz R, Lorkowski C, Slowinski T, Neumayer HH, Budde K. Differential impact of the CYP3A5*1 and CYP3A5*3 alleles on pre-dose concentrations of two tacrolimus formulations. Pharmacogenet Genomics. 2011;21:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Shuker N, Cadogan M, van Gelder T, Roodnat JI, Kho MM, Weimar W, Hesselink DA. Conversion from twice-daily to once-daily tacrolimus does not reduce intrapatient variability in tacrolimus exposure. Ther Drug Monit. 2015;37:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Tang JT, Andrews LM, van Gelder T, Shi YY, van Schaik RH, Wang LL, Hesselink DA. Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: recent developments and ethnic considerations. Expert Opin Drug Metab Toxicol. 2016;12:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chiu KW, Sergi CM, Sugawara Y S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y