Published online Apr 21, 2018. doi: 10.3748/wjg.v24.i15.1583
Peer-review started: February 2, 2018
First decision: February 24, 2018
Revised: March 20, 2018
Accepted: March 30, 2018
Article in press: March 30, 2018
Published online: April 21, 2018
Processing time: 77 Days and 3.7 Hours
Ultrasound findings in autoimmune hepatitis (AIH) have not been reported systematically so far. The use of reliable and accurate noninvasive methods for determining fibrosis stage is important in evaluation of treatment efficacy and fibrosis regression in AIH. Imaging plays an important role in detection of complications and ruling out other possible causes of chronic liver diseases. Ultrasound elastography cut-off values in AIH patients are not the same as those in patients with chronic viral hepatitis or non-alcoholic fatty liver disease. AIH is characterized by wide fluctuations in inflammatory activity. Here we report on current knowledge of ultrasound findings in AIH.
Core tip: Accurate noninvasive imaging to determine fibrosis stages is of importance in the evaluation of treatment efficacy and fibrosis regression in autoimmune hepatitis (AIH). The cut-off values in AIH patients are not the same as those in patients with chronic viral hepatitis or non-alcoholic fatty liver disease.
- Citation: Dong Y, Potthoff A, Klinger C, Barreiros AP, Pietrawski D, Dietrich CF. Ultrasound findings in autoimmune hepatitis. World J Gastroenterol 2018; 24(15): 1583-1590
- URL: https://www.wjgnet.com/1007-9327/full/v24/i15/1583.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i15.1583
Autoimmune hepatitis (AIH) is a chronic immune mediated liver disease of unknown etiology[1,2]. About one-third of the patients already have developed advanced fibrosis and liver cirrhosis at the time of diagnosis. AIH mainly affects women and is usually characterized by chronic inflammation of the liver, hypergammaglobulinemia with increased immunoglobulin G (IgG) levels and circulating autoantibodies associated with human leukocyte antigens DR3 or DR4, typical liver histology with interface hepatitis[3], and a favorable response to immunosuppressive treatment[1,2,4]. Once other liver diseases such as viral hepatitis have been excluded, the diagnosis of AIH can be made by serological and histological findings. AIH can range from a mild or severe course to fulminant hepatic failure. Despite corticosteroid therapy, hepatic fibrosis develops in 25% of patients with AIH[5]. To find reliable and accurate noninvasive imaging methods for determining fibrosis stages is of importance in the evaluation of treatment efficacy and fibrosis regression in AIH[6]. Here we report on ultrasound findings in AIH.
AIH has a global distribution. It is considered as a rare disease affecting all ages and ethnic groups with a female predominance (F:M ratio 3.6:1). The incidence of AIH is around 1 per 100000 persons per year[7]. In 1992, the International AIH Group (IAIHG) reported diagnostic criteria[8], which were remarkably simplified in 2008[9]. AIH is classified into two major types: AIH type 1 (AIH-1) and AIH type 2 (AIH-2). Antinuclear antibodies (ANA) and/or smooth muscle autoantibodies (SMA) could be detected in AIH-1. Also perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA) could be detected in 60%-90% of AIH-1 patients[4,5,9,10]. AIH-2 is characterized by the detection of anti-liver/kidney microsomal antibody type 1 or anti-LKM type 3[4,10] and/or antibodies against liver cytosol type 1 antigen[1,4]. AIH-1 accounts for about 75%-80% of all patients, however, AIH-2 is more frequently seen in children and young patients, which might present with acute onset and severe histological changes at time of diagnosis. Poor treatment prognosis, recurrence after treatment and need for lifelong treatment are more common in AIH-2[4,11]. AIH-1 patients might also show antibodies against soluble liver/liver-pancreas-antigen SLA/LP-[12,13].
According to the dominant pathogenetic hypothesis, AIH develops in genetically susceptible individuals by several triggers. The liver is attacked through mechanisms of “molecular mimicry”, and is promoted by down regulation of regulatory T-cells[3,4].
AIH may develop after the use of some drugs and biological agents or after viral infections and other events, including de novo after orthotopic liver transplantation[14-16]. AIH may first develop during pregnancy and after delivery.
Clinically AIH is characterized by fluctuation of disease activity. Its clinical symptoms range from no obvious manifestations to severe and acute hepatitis [4,17]. Clinical manifestations range from merely elevated transaminases to liver cirrhosis and/or fulminant liver failure requiring liver transplantation[18]. Acute AIH presents in approximately 25% patients with similar symptoms as patients suffering from acute toxic or viral hepatitis[19]. At time of diagnosis, about one third of patients have established cirrhosis[3]. A specific and common clinical characteristic of AIH is its association with other autoimmune diseases including first degree relatives[20]. Concurrent extrahepatic autoimmune conditions mostly affect the thyroid gland (10%-23%)[13]. Clinical presentation of AIH might be similar to primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC). These diseases may coexist leading to overlap or variant syndromes[21,22].
Due to the absence of specific diagnostic features and diversity of clinical manifestations, serological and histological features, AIH diagnosis may be a challenge[3]. According to the International Autoimmune Hepatitis Group (IAIHG), the clinical diagnosis of AIH is based on biochemical, immunological, and histological features. Viral hepatitis should be excluded[9]. The simplified diagnostic criteria of IAIHG for AIH is based mainly on four parameters, including autoantibodies detection, serum IgG levels, absence of viral hepatitis markers and liver histology[9]. Histological changes including interface hepatitis, and hepatic rosette formation and emperipolesis[9]. Autoantibodies detectionis regarded as the hallmark for a timely diagnosis although not pathognomonic[3].
Liver biochemistry is not characteristic in most of AIH patients, with elevated bilirubin and transaminases. In most patients, polyclonal hypergammaglobulinemia with particular elevated level of serum IgG is observed. However, it should be mentioned that 15%-25% of patients (especially children, elderly and acute cases) have normal IgG levels. Therefore, AIH diagnosis should not be excluded depending on a normal IgG testing[3]. The standard laboratory assessments include elevated LFTs, hypergammaglobulinemia, and the detection of autoantibodies (ANA, anti-SMA, and anti-LKM).
Liver biopsy is strongly recommended to confirm AIH[13], first to make the diagnosis and second to determine the stage of disease. The diagnostic histological features of AIH include moderate to severe interface hepatitis without biliary lesions or well-defined granulomas. However, it must be noted that pathognomonic histologically characteristics for AIH are missing. Regular assessment of hepatic fibrosis is important in patients with AIH because progressive fibrosis ultimately leads to cirrhosis and liver failure[23]. It has been recommended that clinical decisions about duration of treatment or immunosuppressive therapy should be based on clinical remission and histological features[2].
Laboratory methods can differentiate liver cirrhosis from non-cirrhosis, but their accuracy in distinguishing changes of AIH in histological stages is uncertain. Biochemical markers can reflect the therapeutic response during treatment, but they cannot reflect the severity of liver fibrosis[5]. Many non-invasive markers for assessing liver fibrosis and cirrhosis have been applied in clinical practice[24,25]. However, their ability to detect early stages of liver fibrosis and cirrhosis in AIH patients is still uncertain[26]. All calculated non-invasive markers are not specific. However, it has been consider feasible to predict the degree of liver fibrosis in patients with AIH using laboratory parameters. Platelet count as well as AAR could be used to predict the presence of advanced fibrosis[27,28].
Differential diagnosis of AIH includes chronic viral hepatitis (B and C), primary sclerosing cholangitis, alpha-1 antitrypsin deficiency, primary biliary cirrhosis, hemochromatosis, Wilson’s disease and drug induced hepatitis (e.g., minocycline, nitrofurantoin, isoniazid, methyldopa). However, to differentiate AIH from drug-induced liver injury (DILI) might be a challenge in cholestatic and severe clinical presentations, in particular when circulating liver autoantibodies are detectable in serum[2] Elevated IgG serum-levels and the histological presence of plasma cells can be found as well in a significant proportion of DILI patients[29].
In order to prevent progressive liver fibrosis/cirrhosis, treatment aims on complete biochemical (defined by normalization of aminotransferases and IgG level) and histological remission[13,30]. Most patients respond well to immunosuppressive therapy, which usually results in an excellent prognosis[1,31,32]. Steroids are used as initial therapy leading to a treatment response in 80% of patients with AIH[33-35]. In adults, Azathioprine is effective as maintenance therapy[5,10,30]. Treatment should be continued until normalization of laboratory tests and liver histology[36]. Incomplete response and treatment failure occur in 14%[37] and 7% of patients[38], respectively[36,39,40]. Treatment failure is characterized by a missing decrease of aminotransferase levels and, in some patients, rapid progression to cirrhosis. Consequently, alternative therapeutic regimens have to be considered[5,41,42]. In cases of treatment failure, overlap with other etiologies should be considered. In those patients with liver failure, liver transplantation might be indicated and carries a 10-year survival rate exceeding 70%[1]. Future anti-fibrotic therapies and monitoring fibrosis progression are essential in patients with in AIH.
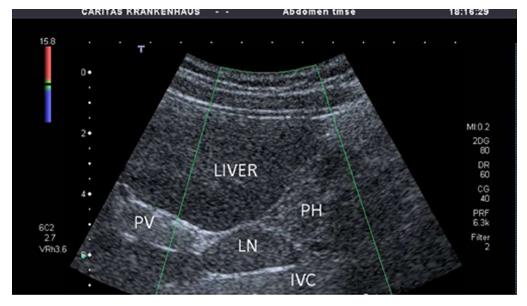
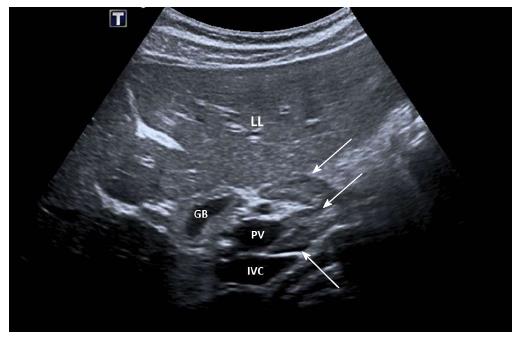
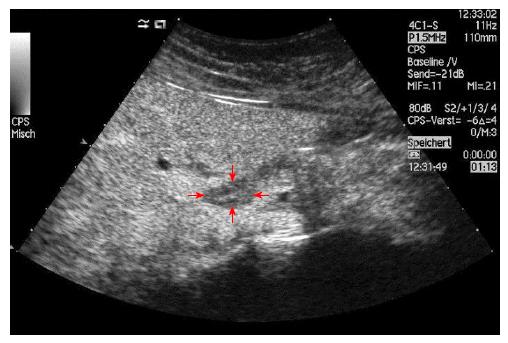
No characteristic conventional ultrasound imaging features of AIH have been described. For initial diagnosis of AIH, ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) are valuable methods to detect liver cirrhosis and its complications. Imaging of AIH play a role in the detection of complications[21]. Enlarged perihepatic lymph nodes are a typical ultrasound feature, similar to virus hepatitis C[43-46] (Figure 1), PBC[47,48], PSC[49], sarcoidosis[50,51] and other inflammatory liver diseases[50] in adults and children[46,50] (Figures 1 and 2). These enlarged inflammatory perihepatic lymph nodes show typical contrast behavior and elastographic architecture[52-59] (Figure 3).
About 1%-9% of AIH patients with liver cirrhosis develop HCC. Therefore, ultrasound follow-up examinations are recommended every six month[60]. Characteristically, involvement of the biliary tract is absent or minimal in AIH. Magnetic resonance cholangiography (MRC) is recommended in all children and adult patients with elevated markers of cholestasis in order to detect concurrent overlap syndromes, particularly PSC.
Non-invasive liver ultrasound elastography methods are useful for detection and staging of liver fibrosis initially as well as during clinical follow-up. Transient elastography (TE) has been introduced first to assess liver stiffness in patients with chronic liver diseases[61,62]. Other newer ultrasound-elastography methods include point shear-wave elastography (pSWE) and two-dimensional shear-wave elastography (2D-SWE)[63]. These tools are integrated in standard ultrasound devices.
Ultrasound elastography methods are proved to be accurate and reliable in the diagnosis of advanced fibrosis and cirrhosis, however, the diagnostic performances may be compromised by inflammation, congestion, biliary obstruction and obesity[63]. Magnetic resonance elastography (MRE) has excellent performance parameters for all histological stages in diverse liver diseases[64], which represents to be a reliable alternative to SWE. MRE is less influenced by body habitus and inflammatory activity in the evaluation of fibrosis in AIH[65-67] but its availability is limited and the investigation is more expensive.
The cut-off values in AIH patients are not the same as those in patients with chronic viral hepatitis. A recent study which enrolled 108 AIH patients who underwent liver biopsies, AUROC value of liver stiffness measurement (LSM) was 0.885 for stage F2 (n = 24), 0.897 for stage F3 (n = 30), and 0.878 for stage F4 (n = 24). The optimal LSM cut-off value was 6.27 kPa for stage F2, 8.18 kPa for F3, and 12.67 kPa for F4[68]. LSM was superior to other non-invasive markers in differentiating the stages of fibrosis in AIH patients[68]. Liver stiffness measured by TE correlated significantly with the stage of liver fibrosis in a study which compared accuracy of TE and liver biopsy in AIH patients. TE correlated better than non-invasive laboratory markers[69,70]. This study demonstrated similar cut-off values, with LSM cut-off values of 6.45 kPa for F2, 8.75 kPa for F3, and 12.5 kPa for F4[70].
A previous study[70] evaluated the accuracy of LSM, APRI, and FIB-4 in 100 AIH patients. TE outperformed the other non-invasive markers. LSM was proven to be closely associated with fibrosis stages (r = 0.752, P < 0.01). Patients with more advanced fibrosis stages are associated with higher LSM values. Of importance, serum ALT levels had minor effect on LSM values and hepatic inflammatory activity had no significant effect on LSM determination.
TE proved also to be more accurate than APRI score in study published by Halasz et al[71] including 22 cases of AIH.
Wang et al[69] conducted a retrospective study with 36 histologically confirmed AIH patients (19 treated and 17 untreated). They reported that TE was accurate for distinguishing hepatic fibrosis in AIH between stages F0-F2 and F3-F4.
While comparing to other etiologies, the higher LSM values for different Ishak stages in AIH patients are in line with the results in the literature[69,72-74]. In a pediatric study of Behairy et al[6], a total of 90 children (HCV n = 50, AIH n = 20, Wilson´s disease n = 20) were included and underwent LSM using TE. AIH patients had both higher values of LSM and (necro) inflammation scores compared to patients with HCV and Wilson´s disease. Inflammatory activity accompanying with increased serum aminotransferase levels, can increase liver stiffness may be misinterpreted as fibrosis[75,76]. Therefore, the higher grade of (necro-) inflammatory activity in AIH patients compared to other etiologies could be a possible explanation[2,3].
Long-term treatment with mono corticosteroids or in combination with azathioprine is proposed when the AIH diagnosis is established. The effect of treatment on the diagnostic performance of LSM has been studied as well. Hartl et al[77] reported that performance of TE in the detection of cirrhosis is better for AIH patients who received longer treatment compared to treatment-naïve patients and patients with shorter duration of treatment. Using the cut-off of 16 kPa, the diagnostic accuracy for cirrhosis was excellent in patients (n = 36) under immunosuppressive treatment for 6 months or longer[77]. A non-invasive inflammatory score has been proposed to discriminate patients with and without significant hepatic inflammation[78]. Those scores are easy to calculate, however, they would be only suitable to patients without co-morbidities and not for patients with low inflammatory activity[79]. Weight gain is a common consequence of corticosteroid treatment[80,81].
Acoustic radiation force impulse imaging (ARFI) can help to distinguish liver fibrosis patients with autoimmune liver diseases from healthy subjects[82,83]. In AIH patients after at least 2 years of biochemical remission, ARFI allowed to differentiate significant (F ≥ 2) from non-significant liver fibrosis (F < 2) (2.28 ± 0.68 m/s vs 1.20 ± 0.24 m/s, P = 0.002)[23]. Although large studies on ARFI elastography in AIH patients are still lacking preliminary data indicate that ARFI is a promising non-invasive method for detection and staging of fibrosis also in AIH patients.
SuperSonic shear wave imaging (SuperSonic Imagine, Aix-en-Provence, France) had higher values in liver fibrosis with AIH of stages S2-S4[84] similar to the results with ARFI[82]. The shear moduli were 9.41 ± 2.5 kPa in S0 stage; 10.42 ± 5.1kPa in S1 stage; 13.25 ± 5.6 kPa in S2 stage; 19.03 ± 7.8 kPa in S3 stage and 24.99 ± 9.5 kPa in S4 stage[84].
In an animal based study, Hao et al[85] investigated the inflammation effect on fibrosis staging by measuring quantitative elasticity parameters in AIH rats (HiVision Preirus, Hitachi Medical Systems Co, Ltd, Tokyo, Japan). The grade of inflammation will influence the accuracy of Real-time elastography (RTE) measurements. The liver fibrosis index had the highest correlation with inflammation grading (r = 0.766; P< 0.05).
Currently, ultrasound findings in AIH have been limited use so far. No characteristic ultrasound imaging features of AIH have been described in the literature. There is a need for studies to determine the better use of ultrasound in AIH patients.
In conclusion, AIH is characterized by wide fluctuations in inflammatory activity, Thus, stage of fibrosis can be overestimated by transient elastography[86] due to concomitant hepatic necroinflammatory activity. It can be also concluded that LSM using TE reflects the stages of liver fibrosis and correlates better than non-invasive laboratory markers in patients with treated AIH[77,87,88]. Other non-invasive ultrasound based techniques as ARFI, 2D-SWE or Real Time Elastography are not well investigated in the population of AIH patients yet. Further studies are needed. However, current non-invasive markers/methods for the evaluation of liver fibrosis in AIH could not replace liver biopsy, especially in differentiating mild from severe stages of fibrosis[87].
| 1. | Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis--Update 2015. J Hepatol. 2015;62:S100-S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 260] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 2. | Liwinski T, Schramm C. Autoimmune hepatitis - update on clinical management in 2017. Clin Res Hepatol Gastroenterol. 2017;41:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Gatselis NK, Zachou K, Koukoulis GK, Dalekos GN. Autoimmune hepatitis, one disease with many faces: etiopathogenetic, clinico-laboratory and histological characteristics. World J Gastroenterol. 2015;21:60-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (1)] |
| 4. | Zachou K, Muratori P, Koukoulis GK, Granito A, Gatselis N, Fabbri A, Dalekos GN, Muratori L. Review article: autoimmune hepatitis -- current management and challenges. Aliment Pharmacol Ther. 2013;38:887-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1027] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 6. | Behairy Bel-S, Sira MM, Zalata KR, Salama el-SE, Abd-Allah MA. Transient elastography compared to liver biopsy and morphometry for predicting fibrosis in pediatric chronic liver disease: Does etiology matter? World J Gastroenterol. 2016;22:4238-4249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Jepsen P, Grønbæk L, Vilstrup H. Worldwide Incidence of Autoimmune Liver Disease. Dig Dis. 2015;33 Suppl 2:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Krawitt EL. Can you recognize autoimmune hepatitis? Postgrad Med. 1998;104:145-149, 152. [PubMed] |
| 9. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1308] [Article Influence: 72.7] [Reference Citation Analysis (1)] |
| 10. | Gleeson D, Heneghan MA; British Society of Gastroenterology. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Liberal R, Grant CR, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: a comprehensive review. J Autoimmun. 2013;41:126-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Manns M, Gerken G, Kyriatsoulis A, Staritz M, Meyer zum Büschenfelde KH. Characterisation of a new subgroup of autoimmune chronic active hepatitis by autoantibodies against a soluble liver antigen. Lancet. 1987;1:292-294. [PubMed] |
| 13. | Deutsche Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) (federführend). Deutsche Gesellschaft für Innere Medizin (DGIM); Deutsche M. Crohn/Colitis ulcerosa Vereinigung (DCCV); Deutsche Leberhilfe e.V; Deutsche Gesellschaft für Ultraschall in der Medizin (DEGUM); Deutsche Gesellschaft für Endoskopie und Bildgebende Verfahren (DGE-BV); Deutsche Gesellschaft für Kinder- und Jugendmedizin (DGKJ); Gesellschaft für Pädiatrische Gastroenterologie (GPGE); Deutsche Gesellschaft für Rheumatologie (DGRh); Deutsche Röntgengesellschaft (DRG); Deutsche Transplantationsgesellschaft (DTG); Deutsche Gesellschaft für Pathologie (DGP) und Bundesverband Deutscher Pathologen (BDP); Österreichische Gesellschaft für Gastroenterologie (ÖGG); Schweizer Gastroenterologische Gesellschaft (SGG); Authors; Collaborators:; Externe Begutachtung durch:. [Practice guideline autoimmune liver diseases - AWMF-Reg. No. 021-27]. Z Gastroenterol. 2017;55:1135-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Westbrook RH, Yeoman AD, Kriese S, Heneghan MA. Outcomes of pregnancy in women with autoimmune hepatitis. J Autoimmun. 2012;38:J239-J244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Castiella A, Zapata E, Lucena MI, Andrade RJ. Drug-induced autoimmune liver disease: A diagnostic dilemma of an increasingly reported disease. World J Hepatol. 2014;6:160-168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Björnsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, Neuhauser M, Lindor K. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 350] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 17. | Stravitz RT, Lefkowitch JH, Fontana RJ, Gershwin ME, Leung PS, Sterling RK, Manns MP, Norman GL, Lee WM; Acute Liver Failure Study Group. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. 2011;53:517-526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 219] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | Acharya GK, Liao HI, Frunza-Stefan S, Patel R, Khaing M. Autoimmune Hepatitis: Diagnostic Dilemma When It Is Disguised as Iron Overload Syndrome. J Clin Exp Hepatol. 2017;7:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Takahashi H, Zeniya M. Acute presentation of autoimmune hepatitis: Does it exist? A published work review. Hepatol Res. 2011;41:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 20. | Teufel A, Weinmann A, Kahaly GJ, Centner C, Piendl A, Wörns M, Lohse AW, Galle PR, Kanzler S. Concurrent autoimmune diseases in patients with autoimmune hepatitis. J Clin Gastroenterol. 2010;44:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 21. | Malik N, Venkatesh SK. Imaging of autoimmune hepatitis and overlap syndromes. Abdom Radiol (NY). 2017;42:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Weiler-Normann C, Lohse AW. Variant Syndromes of Autoimmune Liver Diseases: Classification, Diagnosis and Management. Dig Dis. 2016;34:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Efe C, Gungoren MS, Ozaslan E, Akbiyik F, Kav T. Acoustic Radiation Force Impulse (ARFI) for Fibrosis Staging in Patients with Autoimmune Hepatitis. Hepatogastroenterology. 2015;62:670-672. [PubMed] |
| 24. | Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, Janssen HL, Lampertico P, Lau D, Bornstein JD. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 25. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1665] [Article Influence: 87.6] [Reference Citation Analysis (1)] |
| 26. | Abdollahi M, Pouri A, Ghojazadeh M, Estakhri R, Somi M. Non-invasive serum fibrosis markers: A study in chronic hepatitis. Bioimpacts. 2015;5:17-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Anastasiou J, Alisa A, Virtue S, Portmann B, Murray-Lyon I, Williams R. Noninvasive markers of fibrosis and inflammation in clinical practice: prospective comparison with liver biopsy. Eur J Gastroenterol Hepatol. 2010;22:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Lüth S, Herkel J, Kanzler S, Frenzel C, Galle PR, Dienes HP, Schramm C, Lohse AW. Serologic markers compared with liver biopsy for monitoring disease activity in autoimmune hepatitis. J Clin Gastroenterol. 2008;42:926-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (2)] |
| 29. | de Boer YS, Kosinski AS, Urban TJ, Zhao Z, Long N, Chalasani N, Kleiner DE, Hoofnagle JH; Drug-Induced Liver Injury Network. Features of Autoimmune Hepatitis in Patients With Drug-induced Liver Injury. Clin Gastroenterol Hepatol. 2017;15:103-112.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 30. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63:971-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 659] [Cited by in RCA: 888] [Article Influence: 80.7] [Reference Citation Analysis (1)] |
| 31. | Lamers MM, van Oijen MG, Pronk M, Drenth JP. Treatment options for autoimmune hepatitis: a systematic review of randomized controlled trials. J Hepatol. 2010;53:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 32. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J Gastroenterol. 2017;23:6030-6048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 33. | Jothimani D, Cramp ME, Mitchell JD, Cross TJ. Treatment of autoimmune hepatitis: a review of current and evolving therapies. J Gastroenterol Hepatol. 2011;26:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Chen J, Eslick GD, Weltman M. Systematic review with meta-analysis: clinical manifestations and management of autoimmune hepatitis in the elderly. Aliment Pharmacol Ther. 2014;39:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Roberts SK, Therneau TM, Czaja AJ. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996;110:848-857. [PubMed] |
| 36. | Czaja AJ. Review article: the management of autoimmune hepatitis beyond consensus guidelines. Aliment Pharmacol Ther. 2013;38:343-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Czaja AJ. Rapidity of treatment response and outcome in type 1 autoimmune hepatitis. J Hepatol. 2009;51:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 38. | Montano-Loza AJ, Carpenter HA, Czaja AJ. Features associated with treatment failure in type 1 autoimmune hepatitis and predictive value of the model of end-stage liver disease. Hepatology. 2007;46:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 40. | Muratori L, Muratori P, Lanzoni G, Ferri S, Lenzi M. Application of the 2010 American Association for the study of liver diseases criteria of remission to a cohort of Italian patients with autoimmune hepatitis. Hepatology. 2010;52:1857; author reply 1857-1857; author reply 1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Hübener S, Oo YH, Than NN, Hübener P, Weiler-Normann C, Lohse AW, Schramm C. Efficacy of 6-Mercaptopurine as Second-Line Treatment for Patients With Autoimmune Hepatitis and Azathioprine Intolerance. Clin Gastroenterol Hepatol. 2016;14:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (3)] |
| 42. | Hennes EM, Oo YH, Schramm C, Denzer U, Buggisch P, Wiegard C, Kanzler S, Schuchmann M, Boecher W, Galle PR. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol. 2008;103:3063-3070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Dietrich CF, Lee JH, Herrmann G, Teuber G, Roth WK, Caspary WF, Zeuzem S. Enlargement of perihepatic lymph nodes in relation to liver histology and viremia in patients with chronic hepatitis C. Hepatology. 1997;26:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Dietrich CF, Stryjek-Kaminska D, Teuber G, Lee JH, Caspary WF, Zeuzem S. Perihepatic lymph nodes as a marker of antiviral response in patients with chronic hepatitis C infection. AJR Am J Roentgenol. 2000;174:699-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Dietrich CF, Viel K, Braden B, Caspary WF, Zeuzem S. Mediastinal lymphadenopathy: an extrahepatic manifestation of chronic hepatitis C? Z Gastroenterol. 2000;38:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Schreiber-Dietrich D, Pohl M, Cui XW, Braden B, Dietrich CF, Chiorean L. Perihepatic lymphadenopathy in children with chronic viral hepatitis. J Ultrason. 2015;15:137-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Dietrich CF, Leuschner MS, Zeuzem S, Herrmann G, Sarrazin C, Caspary WF, Leuschner UF. Peri-hepatic lymphadenopathy in primary biliary cirrhosis reflects progression of the disease. Eur J Gastroenterol Hepatol. 1999;11:747-753. [PubMed] |
| 48. | Braden B, Faust D, Ignee A, Schreiber D, Hirche T, Dietrich CF. Clinical relevance of perihepatic lymphadenopathy in acute and chronic liver disease. J Clin Gastroenterol. 2008;42:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Hirche TO, Russler J, Braden B, Schuessler G, Zeuzem S, Wehrmann T, Seifert H, Dietrich CF. Sonographic detection of perihepatic lymphadenopathy is an indicator for primary sclerosing cholangitis in patients with inflammatory bowel disease. Int J Colorectal Dis. 2004;19:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Barreiros AP, Chiorean L, Braden B, Dietrich CF. Ultrasound in rare diffuse liver disease. Z Gastroenterol. 2014;52:1247-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Tana C, Silingardi M, Dietrich CF. New trends in ultrasound of hepatosplenic sarcoidosis. Z Gastroenterol. 2015;53:283-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Dietrich CF. Contrast-enhanced endobronchial ultrasound: Potential value of a new method. Endosc Ultrasound. 2017;6:43-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Hocke M, Ignee A, Dietrich C. Role of contrast-enhanced endoscopic ultrasound in lymph nodes. Endosc Ultrasound. 2017;6:4-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Chiorean L, Barr RG, Braden B, Jenssen C, Cui XW, Hocke M, Schuler A, Dietrich CF. Transcutaneous Ultrasound: Elastographic Lymph Node Evaluation. Current Clinical Applications and Literature Review. Ultrasound Med Biol. 2016;42:16-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Dietrich CF, Jenssen C, Arcidiacono PG, Cui XW, Giovannini M, Hocke M, Iglesias-Garcia J, Saftoiu A, Sun S, Chiorean L. Endoscopic ultrasound: Elastographic lymph node evaluation. Endosc Ultrasound. 2015;4:176-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Dietrich CF, Annema JT, Clementsen P, Cui XW, Borst MM, Jenssen C. Ultrasound techniques in the evaluation of the mediastinum, part I: endoscopic ultrasound (EUS), endobronchial ultrasound (EBUS) and transcutaneous mediastinal ultrasound (TMUS), introduction into ultrasound techniques. J Thorac Dis. 2015;7:E311-E325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 57. | Jenssen C, Annema JT, Clementsen P, Cui XW, Borst MM, Dietrich CF. Ultrasound techniques in the evaluation of the mediastinum, part 2: mediastinal lymph node anatomy and diagnostic reach of ultrasound techniques, clinical work up of neoplastic and inflammatory mediastinal lymphadenopathy using ultrasound techniques and how to learn mediastinal endosonography. J Thorac Dis. 2015;7:E439-E458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 58. | Cui XW, Hocke M, Jenssen C, Ignee A, Klein S, Schreiber-Dietrich D, Dietrich CF. Conventional ultrasound for lymph node evaluation, update 2013. Z Gastroenterol. 2014;52:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Dietrich CF, Jenssen C, Herth FJ. Endobronchial ultrasound elastography. Endosc Ultrasound. 2016;5:233-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 60. | Czaja AJ. Hepatocellular carcinoma and other malignancies in autoimmune hepatitis. Dig Dis Sci. 2013;58:1459-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 61. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] |
| 62. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 63. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e16-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 608] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 64. | Czaja AJ. Review article: The prevention and reversal of hepatic fibrosis in autoimmune hepatitis. Aliment Pharmacol Ther. 2014;39:385-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Cui J, Heba E, Hernandez C, Haufe W, Hooker J, Andre MP, Valasek MA, Aryafar H, Sirlin CB, Loomba R. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 66. | Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626-637.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 627] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 67. | Wang J, Malik N, Yin M, Smyrk TC, Czaja AJ, Ehman RL, Venkatesh SK. Magnetic resonance elastography is accurate in detecting advanced fibrosis in autoimmune hepatitis. World J Gastroenterol. 2017;23:859-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 68. | Guo L, Zheng L, Hu L, Zhou H, Yu L, Liang W. Transient Elastography (FibroScan) Performs Better Than Non-Invasive Markers in Assessing Liver Fibrosis and Cirrhosis in Autoimmune Hepatitis Patients. Med Sci Monit. 2017;23:5106-5112. [PubMed] |
| 69. | Wang QX, Shen L, Qiu DK, Bao H, Chen XY, Zeng MD, Mao YM, Ma X. [Validation of transient elastography (Fibroscan) in assessment of hepatic fibrosis in autoimmune hepatitis]. Zhonghua Gan Zang Bing Za Zhi. 2011;19:782-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 70. | Xu Q, Sheng L, Bao H, Chen X, Guo C, Li H, Ma X, Qiu D, Hua J. Evaluation of transient elastography in assessing liver fibrosis in patients with autoimmune hepatitis. J Gastroenterol Hepatol. 2017;32:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 71. | Halász T, Horváth G, Kiss A, Pár G, Szombati A, Gelley F, Nemes B, Kenessey I, Piurkó V, Schaff Z. Evaluation of Histological and non-Invasive Methods for the Detection of Liver Fibrosis: The Values of Histological and Digital Morphometric Analysis, Liver Stiffness Measurement and APRI Score. Pathol Oncol Res. 2016;22:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Fitzpatrick E, Quaglia A, Vimalesvaran S, Basso MS, Dhawan A. Transient elastography is a useful noninvasive tool for the evaluation of fibrosis in paediatric chronic liver disease. J Pediatr Gastroenterol Nutr. 2013;56:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 73. | Wilder J, Patel K. The clinical utility of FibroScan(®) as a noninvasive diagnostic test for liver disease. Med Devices (Auckl). 2014;7:107-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Abdalla AF, Zalata KR, Ismail AF, Shiha G, Attiya M, Abo-Alyazeed A. Regression of fibrosis in paediatric autoimmune hepatitis: morphometric assessment of fibrosis versus semiquantiatative methods. Fibrogenesis Tissue Repair. 2009;2:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 75. | Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 390] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 76. | Kim SU, Kim DY, Park JY, Lee JH, Ahn SH, Kim JK, Paik YH, Lee KS, Chon CY, Choi EH. How can we enhance the performance of liver stiffness measurement using FibroScan in diagnosing liver cirrhosis in patients with chronic hepatitis B? J Clin Gastroenterol. 2010;44:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 77. | Hartl J, Denzer U, Ehlken H, Zenouzi R, Peiseler M, Sebode M, Hübener S, Pannicke N, Weiler-Normann C, Quaas A. Transient elastography in autoimmune hepatitis: Timing determines the impact of inflammation and fibrosis. J Hepatol. 2016;65:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 78. | Gutkowski K, Hartleb M, Kacperek-Hartleb T, Kajor M, Mazur W, Zych W, Walewska-Zielecka B, Habior A, Sobolewski M. Laboratory-based scoring system for prediction of hepatic inflammatory activity in patients with autoimmune hepatitis. Liver Int. 2013;33:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Fabbri A, Lenzi M. Non-invasive markers of inflammation in autoimmune hepatitis. Liver Int. 2013;33:1295-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Czaja AJ. Safety issues in the management of autoimmune hepatitis. Expert Opin Drug Saf. 2008;7:319-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (2)] |
| 81. | Sporea I, Bota S, Jurchis A, Sirli R, Grădinaru-Tascău O, Popescu A, Ratiu I, Szilaski M. Acoustic radiation force impulse and supersonic shear imaging versus transient elastography for liver fibrosis assessment. Ultrasound Med Biol. 2013;39:1933-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Righi S, Fiorini E, De Molo C, Cipriano V, Cassani F, Muratori L, Lenzi M, Morselli Labate AM, Serra C. ARFI elastography in patients with chronic autoimmune liver diseases: A preliminary study. J Ultrasound. 2012;15:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Bota S, Sporea I, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Saito H, Ebinuma H, Lupsor M, Badea R. The influence of aminotransferase levels on liver stiffness assessed by Acoustic Radiation Force Impulse Elastography: a retrospective multicentre study. Dig Liver Dis. 2013;45:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 84. | Sun LL, Chang W, Jiao LQ, Cui X, Dong G. Hepatic fibrosis and supersonic shear imaging in patients with different etiological chronic hepatic diseases. J Biol Regul Homeost Agents. 2016;30:761-765. [PubMed] |
| 85. | Hao L, Tu JZ, Wang XH, Shi Y, Liu XL, Zhang HH. Effect of Inflammation on Fibrosis Staging Measured by Quantitative Elasticity Parameters in Rats With Immune Hepatitis. J Ultrasound Med. 2016;35:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Romanque P, Stickel F, Dufour JF. Disproportionally high results of transient elastography in patients with autoimmune hepatitis. Liver Int. 2008;28:1177-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | E Anastasiou O, Büchter M, A Baba H, Korth J, Canbay A, Gerken G, Kahraman A. Performance and Utility of Transient Elastography and Non-Invasive Markers of Liver Fiibrosis in Patients with Autoimmune Hepatitis: A Single Centre Experience. Hepat Mon. 2016;16:e40737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 88. | Hartl J, Ehlken H, Sebode M, Peiseler M, Krech T, Zenouzi R, von Felden J, Weiler-Normann C, Schramm C, Lohse AW. Usefulness of biochemical remission and transient elastography in monitoring disease course in autoimmune hepatitis. J Hepatol. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gatselis NK, Tai DI, Trifan A S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y