Published online Mar 21, 2018. doi: 10.3748/wjg.v24.i11.1269
Peer-review started: December 5, 2017
First decision: December 14, 2017
Revised: January 27, 2018
Accepted: March 3, 2018
Article in press: March 3, 2018
Published online: March 21, 2018
Processing time: 102 Days and 13.3 Hours
To determine steatosis and fibrosis prevalence in hepatitis C patients after a sustained virological response achieved with direct-acting antivirals.
Transient elastography with controlled attenuation parameter (CAP) was used to assess hepatic steatosis post-sustained virological response (SVR); the CAP technology was not available in the United States at study initiation. Liver stiffness/fibrosis was measured before and 47 wk after treatment completion. Patients with genotype 3 and patients with cirrhosis were excluded.
One hundred and one patients were included in the study. Post-SVR there were decreases from baseline in alanine aminotransferase (ALT) (63.1 to 17.8 U/L), aspartate aminotransferase (51.8 to 21.5 U/L) and fibrosis score (7.4 to 6.1 kPa) (P < 0.05). Post-SVR, 48 patients (47.5%) had steatosis on CAP; of these, 6.25% had advanced fibrosis. Patients with steatosis had higher body mass index (29.0 vs 26.1 kg/m2), glucose (107.8 vs 96.6 mg/dL), ALT (20.4 vs 15.3 mg/dL), CAP score (296.3 vs 212.4 dB/m) and fibrosis score (7.0 vs 5.3 kPa); P < 0.05. Interestingly, compared to baseline, both patients with and without steatosis had change in fibrosis score post-SVR (7.7 kPa vs 7.0 kPa and 7.0 kPa vs 5.3 kPa); alternatively, (P < 0.05) and therefore patients with steatosis continued to have clinically significant stiffness (≥ 7 kPa).
Fatty liver is very common in hepatitis C virus (HCV) patients post-SVR. These patients continue to have elevated mean fibrosis score (≥ 7 kPa) compared to those without fatty liver; some have advanced fibrosis. Long term follow up is needed to assess steatosis and fibrosis in HCV patients post-SVR.
Core tip: This is the first prospective study to assess the prevalence of fatty liver in hepatitis C patients who have achieved a sustained virological response with direct-acting antivirals. The study’s findings that fatty liver is present in 47.5% of these patients and that some steatotic patients have clinically significant fibrosis despite normal liver enzymes should raise awareness of the post-sustained virological response (SVR) prevalence of fatty liver and the importance of post-SVR assessment of steatosis and fibrosis and long-term follow up with these patients.
- Citation: Noureddin M, Wong MM, Todo T, Lu SC, Sanyal AJ, Mena EA. Fatty liver in hepatitis C patients post-sustained virological response with direct-acting antivirals. World J Gastroenterol 2018; 24(11): 1269-1277
- URL: https://www.wjgnet.com/1007-9327/full/v24/i11/1269.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i11.1269
With the growing epidemic of obesity and type 2 diabetes mellitus, nonalcoholic fatty liver disease (NAFLD) currently has a worldwide prevalence of 25.24% (approximately 1.8 billion people)[1], making it the most common cause of chronic liver disease (CLD), followed by chronic hepatitis B (CHB, 257 million people), and chronic hepatitis C (CHC, 71 million people)[2]. In the United States, NAFLD and CHC are the two most common CLD causes[3], and nonalcoholic steatohepatitis (NASH)-associated cirrhosis is the second leading indication for liver transplant (LT) after hepatitis C virus (HCV)-associated end-stage liver disease[4]. With the recent study that showed that between 2004 and 2013 the number of adult patients with NASH awaiting LTs almost tripled[4], combined with the rapidly expanding population of CHC patients achieving sustained virological responses (SVRs) with direct-acting antivirals (DAAs), it is thought that NASH may soon become the leading indication for LT. NAFLD prevalence is now estimated to be approximately 30% in the United States[5].
NAFLD is usually diagnosed by detecting steatosis after excluding other causes of liver disease. However, hepatic steatosis may occur in patients with other liver diseases, often in those with obesity and other metabolic factors typical of NAFLD, potentially creating an additive or synergistic combination of steatosis, oxidative damage, cellular impairment and other factors that may worsen liver injury[6]. Steatosis is known to escalate liver necroinflammatory activity and accelerate fibrosis in CHC patients[7]. The hepatic steatosis prevalence in CHC patients has been reported to be approximately 50% (range 30%-70%)[8]. The mechanisms leading to steatosis in CHC have not been fully elucidated but may include host factors leading to insulin resistance and interactions between lipid metabolism pathways and the HCV core protein[9,10]. It has been proposed that HCV’s effects on hepatic lipid metabolism may inhibit the export proteins needed for the assembly and secretion of very low density lipoproteins (VLDL), resulting in triglyceride accumulation in the liver[8]. Therefore, hepatic steatosis in HCV patients may result from some combination of viral and metabolic factors, other than in genotype 3 (GNT3) patients in which the steatosis may be due to direct effects of genotype 3 viral proteins[11].
Historically, an SVR with interferon was not associated with steatosis resolution except in GNT3 patients which has a different steatosis etiology[10]. In patients with an SVR achieved with DAAs steatosis prevalence is unknown. In this prospective, cross-sectional study, we assessed steatosis prevalence and degree of fibrosis in CHC patients who achieved an SVR through treatment with DAAs.
This is a prospective, cross-sectional study of patients with CHC who achieved an SVR after treatment with DAAs. The patients in this cohort had been treated with a variety of direct-acting antiviral regimens: ledipasvir/sofosbuvir (Harvoni), 75 patients; elbasvir/grazoprevir (Zepatier), 1 patient; dasabuvir/ombitasvir/paritaprevir/ritonavir (Viekira), 7 patients; dasabuvir/ombitasvir/paritaprevir/ritonavir with ribavirin, 2 patients; sofosbuvir (Sovaldi) with ribavirin, 9 patients; sofosbuvir with daclatasvir (Daklinza), 1 patient; sofosbuvir with simeprevir (Olysio), 2 patients; sofosbuvir/velpatasvir (Epclusa), 4 patients. Between January 2016 and March 2017, 101 adult patients were enrolled, excluding patients with other liver diseases, secondary causes of steatosis (e.g., medications, excessive alcohol), and GNT3 which has a different steatosis etiology. After achieving an SVR, patients were invited to undergo standardized history and anthropometric examination, laboratory testing, and transient elastography (TE) at the California Liver Research Institute in Los Angeles. This study received approval and was done under IRB protocol CLRI-01. Ethical guidelines for human research were followed. All patients signed informed consent.
TE was performed using the FibroScan 502 Touch model (M Probe, XL Probe; Echosens, Paris, France) by an experienced TE-certified technician blinded to clinical data. Patients were asked to fast for at least 4 h prior to the examination. The procedure was performed in the supine position with the right arm adducted while holding the breath for 10 s. All patients were first scanned with the M probe (3.5 MHz) over the right liver lobe. If indicated by the machine, patients were re-evaluated using the XL probe (2.5 MHz). Ten measurements were made and the interquartile range was less than 30%. We defined test failure when no stiffness measurement was obtained or there were unreliable measurements (success rate < 60% or interquartile range/median > 30%)[12-14].
Liver stiffness/fibrosis scores were measured before and within one year after completion of HCV treatment with DAAs; the median time interval between treatment completion and post-SVR TE was 47 wk, with no significant difference between patients with and without steatosis. Simultaneous liver steatosis measurements were obtained using controlled attenuation parameter (CAP) values in dB/m only after SVR achievement as the technology was not available in the United States at the study’s initiation. Based on the recent large patient data meta-analysis of studies containing histology-verified CAP data for grading of steatosis that determined optimal cut-offs for CAP[15], steatosis was defined as ≥ 248 dB/M. Clinically significant stiffness was defined as ≥ 7 kilopascal (kPa)[16,17].
We included patients if they were 18 years or older, were treated for CHC using DAAs and were able to provide informed consent. We excluded patients if they (1) had a history of significant alcohol intake within 2 years of recruitment (14 drinks/wk for men or 7 drinks/wk for women) as assessed by the hepatologist as well as the Alcohol Use Disorders Identification Test-Consumption (AUDIT-C) questionnaire; (2) had secondary causes of fatty liver such as medications (for example, methotrexate) or other infectious causes (for example, human immunodeficiency virus); (3) had evidence of liver diseases other than hepatitis C; (4) were HCV GNT3 as it is thought to have a different underlying etiology of steatosis related to the virus (viral steatosis) and we sought to investigate this genotype separately; or (5) had cirrhosis based on imaging or FibroScan. All the following information was collected: medical history, age, sex, height, weight, body mass index (BMI), ethnic background, and vital signs.
The biochemical tests that were measured included aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, total bilirubin, direct bilirubin, albumin, fasting glucose, hemoglobin A1c, triglycerides, total cholesterol, high-density lipoprotein, and low-density lipoprotein. Other measurements included platelets, prothrombin time, and international normalized ratio.
The chi-square test was used to compare between categorical variables, and a paired t test to compare mean differences between continuous variables. Primary and secondary comparisons within groups were calculated with paired t tests, two-tailed, independent-sample t tests, or nonparametric tests including Wilcoxon signed-rank test as applicable. A two-tailed P < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 21.
Between January 2016 and March 2017, 101 adult CHC patients who achieved SVR were enrolled. At baseline the average age for the entire cohort was 60.3 ± 10.7 years and BMI was 27.6 ± 6.9 kg/m2; 37% were Caucasian and 26% were Hispanic. The average fibrosis score was 7.4 ± 1.9 kPa. HCV genotypes were: GNT1 (85%), GNT2 (14%), and GNT4 (1%) (Table 1).
| Prior to DAA treatment (baseline) | Post-SVR 12 | P1 value | |
| Demographics | |||
| Male | 49 (48) | 49 (48) | NS |
| Age (yr, mean ± SD) | 60.3 ± 10.7 | 60.3 ± 10.7 | NS |
| White | 37 (37) | 37 (37) | NS |
| Hispanic | 26 (26) | 26 (26) | NS |
| African American | 13 (13) | 13 (13) | NS |
| Asian | 7 (7) | 7 (7) | NS |
| Other | 2 (2) | 2 (2) | NS |
| Declined | 16 (15) | 16 (15) | NS |
| Clinical | |||
| Hypertension | 45 (43) | 45 (43) | NS |
| Type 2 diabetes | 13 (12.3) | 13 (12.3) | NS |
| Dyslipidemia | 8 (7.5) | 8 (7.5) | NS |
| Anthropometric (mean ± SD) | |||
| Body mass index (kg/m2) | 27.6 ± 6.9 | 27.5 ± 6.9 | NS |
| Weight (Lbs.) | 174.9 ± 46.9 | 172.7 ± 44.5 | NS |
| Laboratory panel (mean ± SD) | |||
| HCV vial load log10 IU/mL | 6.2 ± 0.9 | 0.0 ± 0.0 | < 0.0001 |
| HCV genotype | |||
| Genotype 1 | 86 (85) | ||
| Genotype 2 | 15 (14) | ||
| Genotype 4 | 1 (1) | ||
| AST (U/L) | 51.8 ± 41.1 | 21.5 ± 8.0 | < 0.0001 |
| ALT (U/L) | 63.1 ± 62.6 | 17.8 ± 12.3 | < 0.0001 |
| Alkaline phosphatase (U/L) | 77.5 ± 34.0 | 71.0 ± 24.3 | 0.004 |
| Albumin (g/dL) | 4.3 ± 0.4 | 4.4 ± 0.4 | NS |
| Bilirubin, total (mg/dL) | 0.6 ± 0.2 | 0.6 ± 0.3 | NS |
| Fasting glucose (mg/dL) | 99.1 ± 30.1 | 102.1 ± 23.5 | NS |
| FibroScan (mean ± SD) | |||
| Fibrosis Score (kPa) | 7.4 ± 1.9 | 6.1 ± 3.6 | 0.013 |
| IQR (%) | 12.6 ± 4.9 | 12.3 ± 5.5 | NS |
Changes in the Entire Cohort: As expected, post-SVR HCV viral load was undetectable compared to prior baseline (prior to starting treatment) (0.0 ± 0.0 IU/m vs 6.2 ± 0.9 IU/m, P < 0.0001). ALT and AST decreased to normal levels post-SVR compared to baseline (17.8 ± 12.3 U/L vs 63.1 ± 62.6 U/L for ALT, P < 0.0001 and 21.5 ± 8.0 U/L vs 51.8 ± 41.1 U/L for AST, P < 0.0001). There was no change in BMI post-SVR compared to baseline (27.5 ± 6.9 kg/m2vs 27.6 ± 6.9 kg/m2). In the overall cohort, post-SVR there was a significant decrease in fibrosis score on TE (7.4 ± 1.9 kPa to 6.1 ± 3.6 kPa; P = 0.013), a decline that is considered clinically significant.
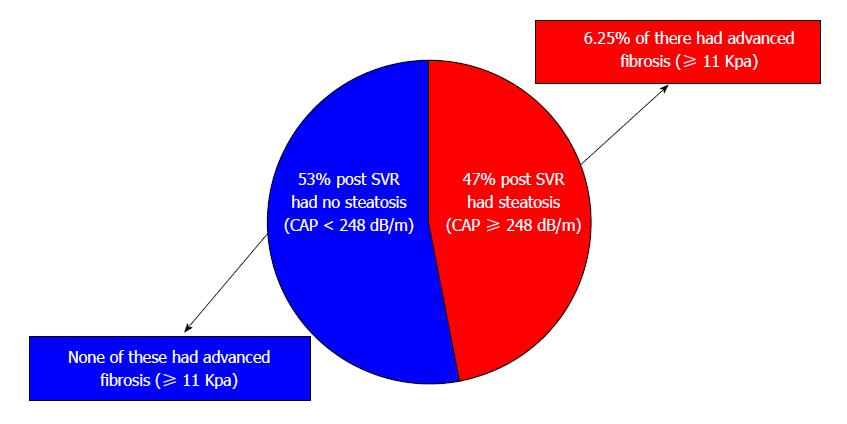
Changes in patients with and without steatosis post-SVR: Post-SVR, 48 patients (47.5%) had steatosis with mean CAP score 296.3 ± 37.4 compared to a mean CAP score 212.4 ± 29.4 dB/m in patients without steatosis (P < 0.0001) (Figure 1). Patients with steatosis were more likely than patients without steatosis to have type 2 diabetes (18.7% vs 7.5%, P = 0.04), dyslipidemia (10.4% vs 5.7%, P = 0.048), higher body mass index (28.9 ± 6.6 kg/m2vs 26.1 ± 6.9 kg/m2, P = 0.049), ALT (20.4 ± 16.5 U/L vs 15.3 ± 5.5 U/L, P = 0.048), fasting glucose (107.8 ± 30.5 mg/dL vs 96.5 ± 11.1 mg/dL, P = 0.023) and triglycerides (138.8 ± 77.9 mg/dL vs 109.7 ± 63.9 mg/dL, P = 0.05) (Table 2). None of the patients without steatosis had abnormal liver enzymes; only 6.25% of patients with steatosis had abnormal liver enzymes.
| Patients without steatosis(CAP < 248 dB/m) (n = 53) | Patients with steatosis(CAP ≥ 248 dB/m) (n = 48) | P1 value | |
| Demographics | |||
| Male | 25 (47) | 27 (56) | NS |
| Age (yr, mean ± SD) | 59.4 ± 11.6 | 60.9 ± 9.4 | NS |
| White | 18 (34) | 18 (38) | NS |
| Hispanic | 14 (26) | 12 (25) | NS |
| Clinical | |||
| Hypertension | 25 (47.2) | 20 (41.7) | NS |
| Dyslipidemia | 3 (5.7) | 5 (10.4) | 0.048 |
| Type 2 diabetes | 4 (7.5) | 9 (18.7) | 0.04 |
| Anthropometric (mean ± SD) | |||
| Body mass index (kg/m2) | 26.1 ± 6.9 | 28.9 ± 6.6 | 0.049 |
| Weight (Lbs.) | 161.0 ± 33.4 | 172.7 ± 44.5 | 0.005 |
| Hepatology and viral hepatitis panel (mean ± SD) | |||
| AST (U/L) | 20.2 ± 5.4 | 22.9 ± 9.8 | NS |
| ALT (U/L) | 15.3 ± 5.5 | 20.4 ± 16.5 | 0.048 |
| Alkaline phosphatase (U/L) | 70.7 ± 28.2 | 71.3 ± 19.4 | NS |
| Albumin (g/dL) | 4.3 ± 0.2 | 4.5 ± 0.6 | NS |
| Bilirubin, total (mg/dL) | 0.6 ± 0.3 | 0.6 ± 0.2 | NS |
| Other laboratory studies (mean ± SD) | |||
| Total cholesterol (mg/dL) | 184.8 ± 35.1 | 179 ± 37.2 | NS |
| HDL cholesterol (mg/dL) | 57.6 ± 18.6 | 50.8 ± 17.0 | NS |
| LDL cholesterol (mg/dL) | 102.6 ± 33.2 | 100.7 ± 31.5 | NS |
| Triglycerides (mg/dL) | 109.7 ± 63.9 | 138.9 ± 77.9 | 0.05 |
| HbA1c (%) | 5.7 ± 0.6 | 6.0 ± 0.9 | NS |
| Fasting serum glucose (mg/dL) | 96.5 ± 11.1 | 107.8 ± 30.5 | 0.023 |
| FibroScan (mean ±SD) | |||
| Fibrosis Score (kPa) | 5.3 ± 1.6 | 7.0 ± 4.8 | 0.0013 |
| CAP (dB/m) | 212.4 ± 29.0 | 296.3 ± 37.4 | < 0.0001 |
| % of patient with fibrosis score of (≥ 7 kPa) | 0% | 6.25% | 0.066 |
Changes in patients with and without steatosis between baseline and post-SVR: Interestingly, patients with steatosis continued to have clinically significant liver stiffness (mean baseline 7.7 ± 1.7 kPa; post-SVR 7.0 ± 4.8 kPa; P = 0.037) while patients without steatosis did not (mean baseline 7.1 ± 2.1; post-SVR 5.3 ± 1.5 kPa; P < 0.0001) (Table 3). Among patients with post-SVR steatosis, 6.25% had advanced fibrosis defined as ≥ 11 kPa. No patients without steatosis had advanced fibrosis (Table 3).
| Patients without steatosis n = 53 | Patients with steatosis n = 48 | |||||
| Pretreatment | Post SVR | P value | Pretreatment | Post SVR | P value | |
| Body mass index (kg/m2) | 25.5 ± 4.0 | 26.1 ± 6.9 | NS | 30.0 ± 8.5 | 29.0 ± 6.6 | NS |
| Weight (Lbs.) | 161.9 ± 32.6 | 161.0 ± 33.4 | NS | 187.3 ± 55.8 | 186.1 ± 51.3 | NS |
| Laboratory panel (mean ± SD) | ||||||
| HCV vial load log10 IU/mL | 6.1 ± 1.0 | 0.0 ± 0.0 | < 0.0001 | 6.3 ± 0.8 | 0.0 ± 0.0 | < 0.0001 |
| AST (U/L) | 43.3 ± 35.6 | 20.2 ± 5.4 | < 0.0001 | 61.3 ± 44.7 | 22.9 ± 9.8 | < 0.0001 |
| ALT (U/L) | 55.6 ± 60.9 | 15.3 ± 5.5 | < 0.0001 | 68.78 ± 52.8 | 20.4 ± 16.5 | < 0.0001 |
| Alkaline phosphatase (U/L) | 78.5 ± 43.1 | 70.8 ± 28.8 | 0.01 | 75.5 ± 21.8 | 71.3 ± 19.4 | 0.04 |
| Albumin (g/dL) | 4.2 ± 0.5 | 4.4 ± 0.3 | 0.006 | 4.3 ± 0.2 | 4.5 ± 0.6 | 0.006 |
| Bilirubin total (mg/dL) | 0.6 ± 0.2 | 0.6 ± 0.3 | NS | 0.6 ± 0.3 | 0.6 ± 0.2 | NS |
| Fasting glucose (mg/dL) | 95.6 ± 31.9 | 96.6 ± 11.1 | NS | 103.0 ± 27.5 | 107.8 ± 30.5 | NS |
| FibroScan (mean ± SD) | ||||||
| Fibrosis score (kPa) | 7.1 ± 2.1 | 5.3 ± 1.5 | < 0.0001 | 7.7 ± 1.7 | 7.0 ± 4.8 | 0.0037 |
Post-SVR, neither weight nor BMI changed while levels of transaminases and other liver enzymes dropped in patients both with and without steatosis, including ALT (55.6 ± 60.9 U/L to 15.3 ± 5.5 U/L in patients with steatosis, P < 0.0001, and 68.78 ± 52.8 U/L to 20.4 ± 16.5 U/L in patients without steatosis; P < 0.0001, respectively); AST (43.3 ± 35.6 U/L to 20.2 ± 5.4 U/L; P < 0.0001 and 61.3 ± 44.7 U/L to 22.9 ± 9.8 U/L; P < 0.0001, respectively); and alkaline phosphatase (78.5 ± 43.1 U/L to 70.8 ± 28.8 U/L; P = 0.01 and 75.5 ± 21.8 U/L to 71.3 ± 19.4 U/L; P = 0.04 (Table 3).
Since hepatic steatosis prevalence in CHC patients has previously been reported to be approximately 50%[8] our findings of a 47.5% prevalence post-SVR achieved with DAAs should perhaps not be surprising. However, this very high prevalence with continuing clinically significant fibrosis in the steatotic patients despite normal liver enzymes should be of concern to clinicians. The current European guidelines recommend assessing ALT and HCV RNA 48 wk post-treatment in non-cirrhotic patients with SVR, with no further follow up with normal ALT/undetectable HCV RNA[18]. The current United States guidelines for patients post-SVR recommend follow up only for those with advanced fibrosis; assessing other liver disease causes is only recommended in cases of persistently abnormal transaminases[19]. Importantly, we show that fatty liver may be present despite normal liver enzymes, confirming previous studies that have shown this[20]. Therefore, we recommend post-SVR assessment of steatosis and fibrosis in those with abnormal BMI or other risk factors typical of NAFLD. In patients found to have hepatic steatosis long-term follow up is warranted.
To our knowledge this is the first prospective study to assess the prevalence of fatty liver in HCV patients who achieved an SVR with DAAs. We hope that our study will raise awareness of the post-SVR prevalence of fatty liver and the need for screening and long-term follow up. Our study’s strengths include the community-based hepatology setting, which likely accurately represents real life experience. In addition, we used TE, which is highly sensitive and specific, and is widely used and easy to perform. Although liver biopsy is still the gold standard to assess fatty liver and staging with MRI proton density fat fraction may be more accurate[21], biopsy is invasive and costly and many patients are reluctant to undergo the procedure because of concerns about pain and, although limited, possible complications. With biopsy there is also the possibility of inter-and intra-observer variability and sampling error[22]. MRI techniques are quite expensive. Neither of these is likely to be performed in post-SVR patients with normal liver enzymes. Thus, the use of TE with CAP is realistic in a real-world setting.
There is substantial data showing good sensitivity and specificity for the use of TE in determining either presence of advanced fibrosis or no fibrosis. In eight studies that compared the usefulness of TE and liver biopsy for assessment of liver fibrosis in NAFLD patients it was shown that TE is very good for diagnosis of F ≥ 3, with 84%-100% sensitivity and 83%-97% specificity[23-30]. Similar findings were reported in a recent large systematic review and meta-analysis that confirmed that TE was excellent for diagnosis of F ≥ 3 in NAFLD patients[31]. Although there is reduced accuracy using TE for distinguishing early fibrosis stages (F1-F2), in our study we were mainly comparing results in patients with and without advanced fibrosis. There is also substantial data showing good sensitivity and specificity of TE with CAP for assessing hepatic steatosis[32]. Although cutoff values for defining steatosis with CAP have not been fully formalized, we chose the value that defined steatosis (≥ 248 dB/M) based on a very recent large (2735 patients) meta-analysis of studies containing histology-verified CAP data for grading of steatosis that determined optimal cut-offs for CAP[15].
Although until relatively recently, obesity (BMI > 30 kg/m2) was associated with a reduced ability of TE to accurately determine fibrosis and steatosis, this problem has been largely addressed with the development of the obese-specific XL probe which we used in our study, confirmed in multiple studies to obtain reliable liver stiffness measurement in obese patients[33-35]. Another strength of our study is our inclusion of a detailed metabolic profile and alcohol questionnaire, with other causes carefully ruled out. It has been suggested that post-SVR some patients might feel free to indulge in alcohol consumption, with a resulting increase in liver stiffness measurements. Importantly, we ruled out increased alcohol intake through both medical records and use of the AUDIT-C at the time of the TE CAP assessment post-SVR.
Although our exclusion of HCV GNT3 patients means that our findings cannot be applied to the approximately 30.1% of HCV patients with this genotype[36], the exclusion is a strength of the study in other ways. Steatosis has been shown to correlate with intrahepatic viral replication in GNT3, with resolution of steatosis seen after effective antiviral treatment, suggesting a direct steatogenic effect of GNT3 virus[8]. In a study of patients treated with interferon, steatosis improvement post-SVR was seen in 91% of GNT3 patients vs 43% of patients with other genotypes (P < 0.04)[37]. In a study that compared the effects of interferon treatment in GNT1 and GNT3 patients, hepatic steatosis did not change in GNT1 patients, regardless of the treatment response, while steatosis was significantly reduced in GNT3 patients who achieved an SVR (P < 0.001) but not in patients who did not[38], again suggesting a direct steatogenic effect of GNT3 HCV. Thus, GNT3 patients represent a unique population in terms of steatosis that should be studied separately. Inclusion of these patients in our study could have substantially altered our findings regarding post-SVR steatosis, likely substantially reducing the prevalence due to steatosis reduction in GNT3 patients, resulting in an overall steatosis prevalence which would not be representative of the almost 70% of HCV patients with other genotypes[36].
A limitation of our study is that, because the CAP technology was not available in the United States at the time of study initiation, we were unable to estimate steatosis prevalence with CAP prior to the initiation of DAAs in order to determine treatment effect. However, regardless of baseline steatosis prevalence, there is real clinical value in assessing post-SVR prevalence so that appropriate long-term follow up can be recommended. Another limitation is the length of follow-up as the median time interval in our study is 47 wk between treatment completion and the post-SVR TE. Lengthier studies are definitely needed to assess NAFLD progression and steatosis and fibrosis changes over time in this population. However, by assessing patients at almost a year post-SVR we have at least provided a foundation upon which lengthier studies could expand. The sample size could be considered as a limitation; however, this is a proof of concept study that this is first of its kind and warrants larger studies. Finally, we excluded patients with cirrhosis. However, these patients are usually followed up closely post-SVR and steatosis has been found to be low when patients have advanced fibrosis[39].
In conclusion, our findings that 47.5% of HCV patients had steatosis post-SVR and that some steatotic patients had clinically significant fibrosis, despite normal liver enzymes, highlight the importance of post-SVR assessment of steatosis and fibrosis in these patients. We believe these patients should be followed longitudinally, both to provide appropriate patient care and to advance our understanding of the long-term consequences of hepatic steatosis in post-SVR patients. In addition, we note that despite SVR these steatotic CHC patients are excluded from most NAFLD clinical trials, predominantly because of the current guidelines’ definition of NAFLD as a diagnosis of exclusion[40,41]. We propose revisiting this and implementing new definitions of those with concomitant liver diseases, including those with HCV SVRs, that might allow patients’ participation in trials, an unmet need in the rising epidemic of NAFLD.
It is known that the hepatic steatosis prevalence in hepatitis C patients who have achieved a sustained virological response with interferon is approximately 50%. However, the prevalence of fatty liver in hepatitis C patients who have achieved a sustained virological response with direct-acting antivirals has not previously been studied. Knowledge of this is important in order to direct appropriate long-term follow up for patients.
Post-sustained virological response (SVR), hepatitis C patients, many of whom have normal liver enzymes, are too often being discharged from their hepatologists’ care with no further plans for follow up. The current European and United States guidelines only recommend long-term follow up in patients with elevated enzymes. In addition, many hepatitis C patients who have achieved an SVR are excluded from nonalcoholic fatty liver disease (NAFLD) clinical trials. We think it is important to determine the prevalence of NAFLD post-SVR and assess the severity of liver disease in these patients. Determining these things can provide a basis for future research aimed at determining the long-term natural history of the disease in these patients, and may prompt changes in both liver society guidelines for follow up and in clinical trial exclusion criteria.
The main objective, to determine the prevalence of fatty liver in hepatitis C patients who have achieved a sustained virological response with direct-acting antivirals, was achieved. This knowledge provides a basis for future research aimed at determining the long-term natural history of the disease in these patients.
In this study we used transient elastography with controlled attenuation parameter to measure steatosis and fibrosis in hepatitis C patients post-SVR. This was the first study to measure both fibrosis and steatosis in hepatitis C patients using the FibroScan technology.
Our findings have added knowledge previously unknown in this field that may help to guide the need for long-term monitoring of hepatitis C patients post-SVR, with a particular focus on the possible occurrence of NAFLD in these patients, whether or not there are elevated liver enzymes. The most important future research will be to carry out long-term follow up on hepatitis C patients post-SVR to determine the prevalence of fatty liver over time.
This is the first prospective study to assess the prevalence of fatty liver in hepatitis C patients who have achieved a sustained virological response with direct-acting antivirals. The study’s findings that fatty liver is present in 47.5% of these patients and that some steatotic patients have clinically significant fibrosis despite normal liver enzymes should raise awareness of the high post-SVR prevalence of fatty liver and the importance of post-SVR assessment of steatosis and fibrosis and long-term follow up with these patients. The study’s findings raise concern that the recommendations found in the current U.S. and European guidelines for follow up of patients post-SVR could result in a lack of adequate long-term monitoring of these patients. In particular, the very high prevalence of fatty liver (47.5%) with continuing clinically significant fibrosis in the steatotic patients despite normal liver enzymes should be of concern to clinicians. Therefore, we recommend post-SVR assessment of steatosis and fibrosis in those with abnormal BMI or other risk factors typical of NAFLD. In patients found to have hepatic steatosis long-term follow up is clearly warranted.
Our study’s assessment of steatosis and fibrosis in hepatitis C patients at almost a year post-SVR has shown that long-term monitoring of these patients to assess the possibility of fatty liver and fibrosis is important. With this study, we have provided a foundation upon which lengthier and larger studies should expand, using regularly scheduled transient elastography with controlled attenuation parameter assessments in order to determine whether this high level of steatosis is still present multiple years post-SVR and the clinical ramifications for patients.
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7938] [Article Influence: 793.8] [Reference Citation Analysis (8)] |
| 2. | World Health Organization. Global hepatitis report 2017. Geneva, Switzerland: World Health Organization 2017; 83. |
| 3. | Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The multiethnic cohort. Hepatology. 2016;64:1969-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 232] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 4. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1418] [Article Influence: 128.9] [Reference Citation Analysis (1)] |
| 5. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2325] [Article Influence: 155.0] [Reference Citation Analysis (1)] |
| 6. | Powell EE, Jonsson JR, Clouston AD. Steatosis: co-factor in other liver diseases. Hepatology. 2005;42:5-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 261] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 774] [Article Influence: 31.0] [Reference Citation Analysis (3)] |
| 8. | Lonardo A, Adinolfi LE, Loria P, Carulli N, Ruggiero G, Day CP. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586-597. [PubMed] |
| 9. | Castera L. Steatosis, insulin resistance and fibrosis progression in chronic hepatitis C. Minerva Gastroenterol Dietol. 2006;52:125-134. [PubMed] |
| 10. | Bondini S, Younossi ZM. Non-alcoholic fatty liver disease and hepatitis C infection. Minerva Gastroenterol Dietol. 2006;52:135-143. [PubMed] |
| 11. | Abenavoli L, Masarone M, Peta V, Milic N, Kobyliak N, Rouabhia S, Persico M. Insulin resistance and liver steatosis in chronic hepatitis C infection genotype 3. World J Gastroenterol. 2014;20:15233-15240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1100] [Article Influence: 61.1] [Reference Citation Analysis (1)] |
| 13. | Han MA, Saouaf R, Ayoub W, Todo T, Mena E, Noureddin M. Magnetic resonance imaging and transient elastography in the management of Nonalcoholic Fatty Liver Disease (NAFLD). Expert Rev Clin Pharmacol. 2017;10:379-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 292] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 15. | Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, Kumar M, Lupsor-Platon M, Han KH, Cardoso AC. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 932] [Article Influence: 103.6] [Reference Citation Analysis (2)] |
| 16. | Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, Hooker J, Sy E, Savides MT, Alquiraish MH. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:598-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 569] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 17. | Alkhouri N, Feldstein AE. Noninvasive diagnosis of nonalcoholic fatty liver disease: Are we there yet? Metabolism. 2016;65:1087-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2016. J Hepatol. 2017;66:153-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 809] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 19. | American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Summary of the recommendations for monitoring patients who are starting HCV treatment, are on treatment, or have completed therapy. Available from: https://www.hcvguidelines.org/evaluate/monitoring. |
| 20. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 2721] [Article Influence: 123.7] [Reference Citation Analysis (3)] |
| 21. | Noureddin M, Khoyilar C, Palmer SL. MRI, CT scan, and ultrasound in the diagnosis of nonalcoholic fatty liver disease. J Clin Gastroenterol. 2015;49:351-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Zeng DW, Dong J, Liu YR, Jiang JJ, Zhu YY. Noninvasive models for assessment of liver fibrosis in patients with chronic hepatitis B virus infection. World J Gastroenterol. 2016;22:6663-6672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Cassinotto C, Boursier J, de Lédinghen V, Lebigot J, Lapuyade B, Cales P, Hiriart JB, Michalak S, Bail BL, Cartier V. Liver stiffness in nonalcoholic fatty liver disease: A comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 401] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 24. | Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, Fujita K, Yoneda M, Taguri M, Hyogo H. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 627] [Article Influence: 62.7] [Reference Citation Analysis (0)] |
| 25. | Kumar R, Rastogi A, Sharma MK, Bhatia V, Tyagi P, Sharma P, Garg H, Chandan Kumar KN, Bihari C, Sarin SK. Liver stiffness measurements in patients with different stages of nonalcoholic fatty liver disease: diagnostic performance and clinicopathological correlation. Dig Dis Sci. 2013;58:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Lupsor M, Badea R, Stefanescu H, Grigorescu M, Serban A, Radu C, Crişan D, Sparchez Z, Iancu S, Maniu A. Performance of unidimensional transient elastography in staging non-alcoholic steatohepatitis. J Gastrointestin Liver Dis. 2010;19:53-60. [PubMed] |
| 27. | Pathik P, Ravindra S, Ajay C, Prasad B, Jatin P, Prabha S. Fibroscan versus simple noninvasive screening tools in predicting fibrosis in high-risk nonalcoholic fatty liver disease patients from Western India. Ann Gastroenterol. 2015;28:281-286. [PubMed] |
| 28. | Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, Choi PC, Kowo M, Chan AW, Merrouche W. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 985] [Article Influence: 61.6] [Reference Citation Analysis (1)] |
| 29. | Yoneda M, Yoneda M, Fujita K, Inamori M, Tamano M, Hiriishi H, Nakajima A. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD). Gut. 2007;56:1330-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 199] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, Nozaki Y, Yonemitsu K, Higurashi T, Takahashi H. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis. 2008;40:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 301] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 31. | Kwok R, Tse YK, Wong GL, Ha Y, Lee AU, Ngu MC, Chan HL, Wong VW. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease--the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 32. | Noureddin M, Mato JM, Lu SC. Nonalcoholic fatty liver disease: update on pathogenesis, diagnosis, treatment and the role of S-adenosylmethionine. Exp Biol Med (Maywood). 2015;240:809-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease - Where do we stand? World J Gastroenterol. 2016;22:7236-7251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 205] [Cited by in RCA: 213] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 34. | Myers RP, Pomier-Layrargues G, Kirsch R, Pollett A, Duarte-Rojo A, Wong D, Beaton M, Levstik M, Crotty P, Elkashab M. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 383] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 35. | Sporea I, Sirli R, Mare R, Popescu A, Ivascu SC. Feasibility of Transient Elastography with M and XL probes in real life. Med Ultrason. 2016;18:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1077] [Cited by in RCA: 1160] [Article Influence: 105.5] [Reference Citation Analysis (0)] |
| 37. | Castéra L, Hézode C, Roudot-Thoraval F, Lonjon I, Zafrani ES, Pawlotsky JM, Dhumeaux D. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004;53:420-424. [PubMed] |
| 38. | Kumar D, Farrell GC, Fung C, George J. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology. 2002;36:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 257] [Article Influence: 10.7] [Reference Citation Analysis (9)] |
| 39. | Permutt Z, Le TA, Peterson MR, Seki E, Brenner DA, Sirlin C, Loomba R. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 40. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterologyh. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1384] [Article Influence: 98.9] [Reference Citation Analysis (5)] |
| 41. | European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3284] [Article Influence: 328.4] [Reference Citation Analysis (7)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Abenavoli L, Ho SB, Rodriguez-Frias F S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y