Published online Mar 21, 2018. doi: 10.3748/wjg.v24.i11.1181
Peer-review started: January 28, 2018
First decision: February 10, 2018
Revised: February 12, 2018
Accepted: February 25, 2018
Article in press: February 25, 2018
Published online: March 21, 2018
Processing time: 48 Days and 6.4 Hours
Macrophages (MΦ) differentiate from blood monocytes and participate in innate and adaptive immunity. Because of their abilities to recognize pathogens and activate bactericidal activities, MΦ are always discovered at the site of immune defense. MΦ in the intestine are unique, such that in the healthy intestine, they possess complex mechanisms to protect the gut from inflammation. In these complex mechanisms, they produce anti-inflammatory cytokines, such as interleukin-10 and transforming growth factor-β, and inhibit the inflammatory pathways mediated by Toll-like receptors. It has been demonstrated that resident MΦ play a crucial role in maintaining intestinal homeostasis, and they can be recognized by their unique markers. Nonetheless, in the inflamed intestine, the function of MΦ will change because of environmental variation, which may be one of the mechanisms of inflammatory bowel disease (IBD). We provide further explanation about these mechanisms in our review. In addition, we review recent discoveries that MΦ may be involved in the development of gastrointestinal tumors. We will highlight the possible therapeutic targets for the management of IBD and gastrointestinal tumors, and we also discuss why more details are needed to fully understand all other effects of intestinal MΦ.
Core tip: The manuscript involves three components. First, after briefly describing the origin of macrophages (MΦ), it summarizes their general biologic features and common functions. The second component reveals the differences between resident MΦ in the intestine and those in other tissues. Notably, we depicted how resident MΦ participate in maintaining intestinal homeostasis and why they can maintain intestinal health by comparison between each of these distinct features. The third part discusses how the deficiency of this anti-inflammatory system leads to autoimmune diseases. However, we also discuss the many details of why intestinal MΦ and the underlying mechanism of inflammatory bowel disease and gut tumors remain obscure.
- Citation: Liu YH, Ding Y, Gao CC, Li LS, Wang YX, Xu JD. Functional macrophages and gastrointestinal disorders. World J Gastroenterol 2018; 24(11): 1181-1195
- URL: https://www.wjgnet.com/1007-9327/full/v24/i11/1181.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i11.1181
The intestine is organized into distinct specialized and functional tissues, such as the epithelium and lamina propria (LP). As the major site of bacterial colonization (102 cfu/mL in the duodenum, 102 cfu/mL in the jejunum, 103 cfu/mL in the proximal ileum, 107-108 cfu/mL in the distal ileum, and 1011-1012 cfu/mL in the colon[1]), it is crucial to maintain intestinal homeostasis in which the intestinal immune system contributes to such maintenance under physiological conditions. Meanwhile, both commensal bacteria and their products play important roles[2].
The mammalian intestine is considered the largest immune organ in the body. It is estimated that 65%-80% of the immune cells, such as macrophages (MΦ), dendritic cells (DCs), T cells and B cells[3], exist in the intestine. There are many lymphocytes and natural killer (NK) cells in the region of the epithelial base[4,5]. Most of the intraepithelial lymphocytes are T cells, and they express CD3, CD8[6], TCRαβ[5] or TCRγδ[7] (mainly in mice). Goblet cells of the intestinal epithelium secrete net-like MUC2 mucins that compose the surface mucus layer, which can filter out microbes[8,9]. Both the intestinal epithelium and mucus layer constitute the double-protective barrier to maintain homeostasis at the entrance where pathogens invade. With the background described above, it seems that MΦ are insignificant in the intestinal immune system. In fact, they play a unique supporting role in maintaining the balance of intestinal immunity, and they are by no means as simple as we thought.
MΦ are one of the nonhematopoietic cells in all mammalian species that are distributed throughout the tissues of individuals. Their origin is relatively clear, and their biologic features have long been explored. In terms of immune defense, their name reveals their function: phagocytosis. They participate in innate immune responses and adaptive immune responses, especially in the intestine, which is the largest pool of MΦ and commensal bacteria. They can be considered as regulators instead of inflammation propellants (see below).
Emerging evidence suggests that intestinal resident MΦ contribute to maintaining intestinal homeostasis by several mechanisms (see below), and the production of immunosuppressive cytokines and their inhibitory biologic behavior suppress cascaded inflammatory responses. This is beneficial to the host because they protect the intestine from over-responding to commensal bacteria, resulting in severe tissue damage. Thus, they have attracted increasing attention in research on intestinal homeostasis and the correlative mechanisms of intestinal autoimmune diseases, represented by inflammatory bowel disease (IBD).
IBD includes two types of diseases: ulcerative colitis (UC) and Crohn’s disease (CD). IBD has long been considered a typical autoimmune disease. Several reports have confirmed that multiple factors, for example, epithelial defects, disturbance of commensal or pathogenic bacteria and destruction of the mucus layer, lead to the development of IBD. In addition, intestinal MΦ highlight the defects of their protective function in IBD.
In addition, we propose some promising targets for the studies and treatments of IBD and gastrointestinal tumors. These comprehensive descriptions and findings of MΦ above have been summarized in figures of our manuscript to make the unique function of intestinal MΦ more understandable.
In 1884, Ilya Ilyich Mechnikov, an immunologist and pathologist in Russia, identified MΦ. Hereafter, the exploration of this cell type has never waned. Regarding the origin of MΦ, the mononuclear phagocyte system arises from hematopoietic stem cells in the bone marrow and from progenitors in the embryonic yolk sac[10], as well as from fetal liver during early development. As early as 1980, it was verified by using the Chediak-Higashi marker that both interstitial and intraalveolar MΦ of the lung are derived from bone marrow precursor cells[11]. The family of mononuclear phagocytes consists of monocytes (Mo), MΦ, osteoclasts and DCs.
Granulocyte-macrophage colony stimulating factor (GM-CSF) is a major factor that can promote hematopoietic stem cell differentiation into granulocyte-monocyte cells, promonocytes and Mo[12,13]. Thereafter, Mo circulate in the blood stream in different types of tissues (the environment with different types of tissues controls the differentiation and maturation of resident MΦ by several molecular mechanisms[14-19]), a part of the blood MΦ undergo maturation, adapt to their local microenvironment and turn into various resident MΦ. Resident MΦ may remain as relatively long-life span cells, although they usually cease to proliferate[20]. The remaining blood Mo differentiate into free MΦ, migrating between diverse tissues like amoebae.
To be more rigorous, some researchers further showed that Mo in the bone marrow can be classified as Ly6Chi Mo and Ly6Clo Mo by their expression of Ly6C/Gr1, CCR2 and CX3CR1. Ly6Chi Mo express high levels of Ly6C/Gr-1, CCR2 and CD62L, but low levels of CX3CR1. CCR2 is a chemokine receptor, which is essential for Ly6C+Gr1+CX3CL1- Mo to enter the circulation. Ly6Clo Mo express low levels of Ly6C/Gr1, CCR2 and CD62L but high levels of CX3CR1[21]. Ly6Clo Mo are proposed to be the precursors of resident MΦ[4,22], but there are some conflicts about this hypothesis if the Mo entering the blood stream rely on expressing CCR2, and there is no abundant evidence to support this conclusion. Moreover, MΦ differentiate from blood Mo, a finding that has been challenged recently. Some researchers have suggested that blood Mo contribute little to MΦ in the steady state, and emerging evidence indicates that resident MΦ can undergo self-renewal[23]. However, other researchers demonstrated that blood Ly6Chi Mo are responsible for turning into resident MΦ because they convert into Ly6Clo Mo and can return to the bone marrow, differentiating into Ly6Clo Mo[21]. This explanation may be helpful to understand the origin of resident MΦ.
The volume of MΦ is 5-10 times that of Mo, and they have more organelles (especially lysosomes), folds and pseudopodia. Resident MΦ are widely distributed throughout the body with distinctive phenotypes - for example, dust cells in lung, Langerhans cells in skin, histiocytes in connective tissue, Kupffer cells in the liver, mesangial cells in the kidney and microglial cells in the central nervous system.
A considerable amount of MΦ exists in the intestine, and specific markers expressed by MΦ can be used to study the heterogeneity. For instance, the F4/80[24] antigen and macrosialin in mice are proven to be useful markers in most of the tissues to define the distribution of MΦ, while several antigens such as sialoadhesin, a lectin-like receptor for sialylated glycoconjugates, are particularly strongly present in populations of MΦ in lymphoid organs that do not express F4/80 or CD68. In humans, the CD68 antigen (the human homolog of macrosialin) is widely found in MΦ expressing EMR2 (the human homolog of F4/80)[20].
Presently, many promising markers are awaiting identification, and some detected materials have already generated new hypotheses. For example, matrix metalloproteinase-9, produced by MΦ in the early phase of mouse peritonitis, may be used as an inflammatory marker[25]. In addition, the protein dehydrogenase/reductase-9 was identified as a specific and stable marker of human regulatory MΦ (Mregs)[26], which contributed greatly to the existing body of knowledge on immunosuppressive therapy.
MΦ can be classified as M1 and M2, functionally within the Mregs. M1 MΦ produce high interleukin (IL)-12 and low IL-10, while M2 MΦ show the opposite trend. Additionally, M2 MΦ express IL-13α1, but M1 MΦ do not[27]. A recent study has shown that a novel marker, MS4A4A (a member of the membrane-spanning 4A gene family), is only expressed in M2 MΦ - that is, MS4A4A might be a surface marker of M2 MΦ[28]. M2 MΦ were largely mysterious in the past, while the importance of M1 MΦ in mucosal biology has been appreciated for decades; the immune regulatory function of M2 MΦ has only begun to be understood in the last few years. Additionally, their differentiation, as well as their differences from M1 MΦ in cell biology, will become clearer in the future. Thus, regarding Mregs, it is also important that they are activated by different pathways and play diverse roles in the immune system, which will be described below.
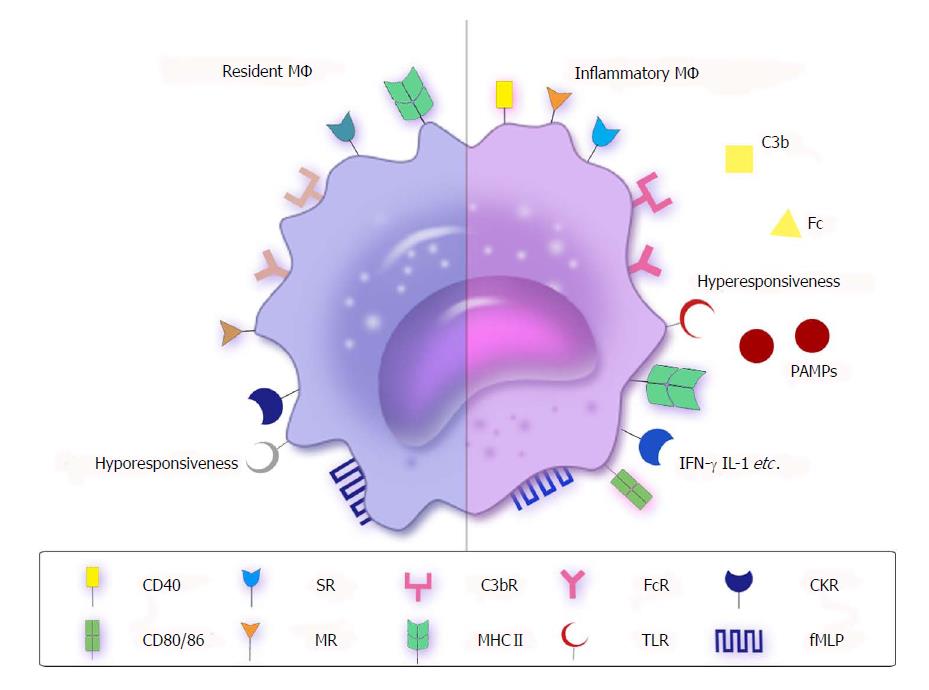
MΦ, “big eaters”, are named after their major function: phagocytosis, involving the uptake of particulate materials (> 5.0 μm) by opsonic (Fc receptors and C3b receptors) or non-opsonic receptors such as mannose receptors, scavenger receptors, formyl-methionine-leucyl-phenylalanine, and pattern recognition receptors (PRRs), especially the Toll-like receptors (TLRs). With the existence of these receptors, MΦ can participate in innate immunity and adaptive immunity (Figure 1).
MΦ dispose of approximately 2 × 1011 erythrocytes a day and clear damaged or dying cells[20]. Activated MΦ can recognize microorganisms that break into the epithelial or mucosal barriers with their special/nonspecial receptors and stretch the pseudopodia to swallow these microbes, followed by their digestion by oxygen-dependent/-independent pathways in phagolysosomes. Beyond that, MΦ can be activated by IL-8 and release chemotactic factors and mediators of inflammation (IL-1, IL-6, IL-12 and tumor necrosis factor (TNF)-α, which recruit neutrophils to the inflammatory site.
The neutrophils produce bactericidal compounds, causing the liquefaction of tissue and formation of pus to eliminate the invading as well as missing pathogens. To complement MΦ, neutrophils secrete several preformed proteins stored in the granules, such as lactoferrin, lipocalin, lysozyme, IL-37, defensins and myeloperoxidase (converts H2O2 to hypochlorous acid)[20]. However, MΦ are not so bellicose. To maintain homeostasis of innate immunity, several self-regulative mechanisms restrain inflammation. NK cells inhibit the activation of MΦ by releasing IFN-γ or reducing the number of overactive MΦ by cytotoxicity. IL-1β, IL-10 and transforming growth factor (TGF)-β, produced by MΦ, are responsible for down-regulating the innate immune response. Moreover, the dead neutrophils are phagocytosed by mononuclear phagocytes, and lipoxins, protectins and resolvins contribute to the restoration of normal function[20].
In adaptive immunity, MΦ are an antigen-presenting cell type, like DCs. In the marginal sinus of a lymphoid organ, after digestion, MΦ present fragments at the cell surface on MHCII molecules. Indeed, MΦ are less effective than DCs in antigen presentation to naïve T cells because they only express appropriate costimulatory molecules (e.g., CD40, CD80 and CD86) following infection or contact with microbial productions. However, DCs express high levels of MHCII molecules as well as costimulatory molecules. In fact, several microbial productions promote the expression of MHCII molecules and costimulatory molecules in MΦ, which probably enhance the autoimmune response[29].
Gut-associated lymphoid tissues, including dispersed and aggregative tissues, are the primary part of the intestinal immune system[30-32]. The latter type is represented by Peyer’s patches (PPs), settled in the LP of the appendix and small intestine, and the solitary lymphoid follicles, widely distributed in the intestinal LP[33,34]. The PPs look like an arch, and they are covered by follicle-associated epithelium, which involves special cells named microfold/membranous cells (M cells)[34,35]. T cells, B cells[36], DCs and MΦ exist in a pocket-like structure outside the base of M cells. M cells efficiently uptake antigens. However, instead of processing and presenting antigens, they are only responsible for transporting antigens and communicating with the resident B cells in the center of PPs.
Most PP cells are B cells, and only a few are T cells, which has been explored in mature mice. The B cells located in the germinal centers of PPs can produce IgA[37-40] (ingredient of sIgA) to participate in pathogen defense. In addition, M cells transport antigens to epithelial cells or antigen presenting cells (DCs and MΦ) to induce the adaptive immune response. It has been certified that the cell-bound antigen transportation can affect mucosal tolerance with the participation of regional lymph nodes[41].
M1 MΦ or classically activated MΦ develop in cell-mediated immune responses, which are mainly driven by interferon (IFN)-γ and TNF. IFN-γ can be produced in innate immunity and adaptive immunity. In the former, NK cells are important, but the production of IFN-γ in NK cells is too transient for the persistence of this population of MΦ. Consequently, it is necessary to depend on the adaptive immune response; T helper (Th)1 cells release sustainable IFN-γ and induce classical activated MΦ to kill the microbes indiscriminately[42].
Endogenously produced IFN-β is another factor that can replace IFN-γ to activate classically activated MΦ[43]. M1 MΦ are the major component of host defense. They produce pro-inflammatory cytokines (e.g., IL-1, IL-6 and IL-23) and associate with Th cells, but it has been reported that their connection with Th17 cells, which produce IL-17, results in serious tissue damage. Thus, their over-activation may be the cause of autoimmune diseases[42].
M2 MΦ or alternatively-activated MΦ are produced during the innate or adaptive immune response. Basophils and mast cells produce innate IL-4, one of the first innate signals released during tissue injury, and IL-4 turns the resident MΦ into this population of cells to promote wound healing. IL-4 can also be released in adaptive immune responses that can be thought as particularly important pathways to develop and persist the alternatively-activated MΦ[42]. In addition, the Th2-type immune responses have been documented to work at the intestinal mucosal surface to respond to the disturbances by cytokines, such as IL-4 and IL-13[44]. However, compared with M1 MΦ, there is no sufficient evidence to show that M2 MΦ directly participate in the bactericidal activities, but they do have indirect regulatory effects[45], which may explain why it is hotly debated in the field of neoplasms[46-56], fibrosis[57-60], metabolic syndrome (might relate to insulin resistance)[61-65] and intestinal autoimmune diseases.
Mregs are a type of immunosuppressive cells, which have been illustrated comprehensively by Mosser et al[42]. Those authors summarized the mechanisms of producing Mregs in innate and adaptive immune responses and the stimuli of these processes. In addition, they mentioned that Mregs produce IL-10 and decrease the production of IL-12 to dampen inflammation. However, their helpful antiinflammatory function might be exploited by parasites to safely survive in the host’s defense, which is an interesting point and powerful evidence to confirm the role of Mregs in the immune system.
To summarize, MΦ are extraordinarily complicated in their structure and functions. On the one hand, they are pioneers of pathogen defense in vivo, and one of the regulators that control the immune responses. On the other hand, they can be considered a bridge between innate immunity and adaptive immunity. It has been proven that they are very important in diseases such as asthma[66-70], atherosclerosis[71-76], retinopathy[77-80], neoplasm and autoimmune diseases.
The differentiation of intestinal MΦ rely on intestinal epithelial cells, which have been proven by an extracorporeal three-dimensional coculture model[81]. MΦ are found in the intestinal tract of all mammals, both in the mucosa and deeper layers[82]. They are found mostly frequently in the LP and produce PGF2 to replenish deficient epithelial cells[23]. Several studies have summarized a rule about the quantity of intestinal MΦ, as follows: in different parts of the intestine, the numbers of MΦ correlate with the quantity of bacteria. An experiment provided the supporting evidence by recording the weight of each mouse organ or tissue and calculating their F4/80 antigen levels. The total F4/80 antigen levels in the small bowel were 1.3 × 107, and 1.4 × 107 in the large bowel. In the intestine of germ-free mice, the numbers of MΦ are decreased[24], likely indicating that the pathogen defense should also be the basic function of intestinal MΦ.
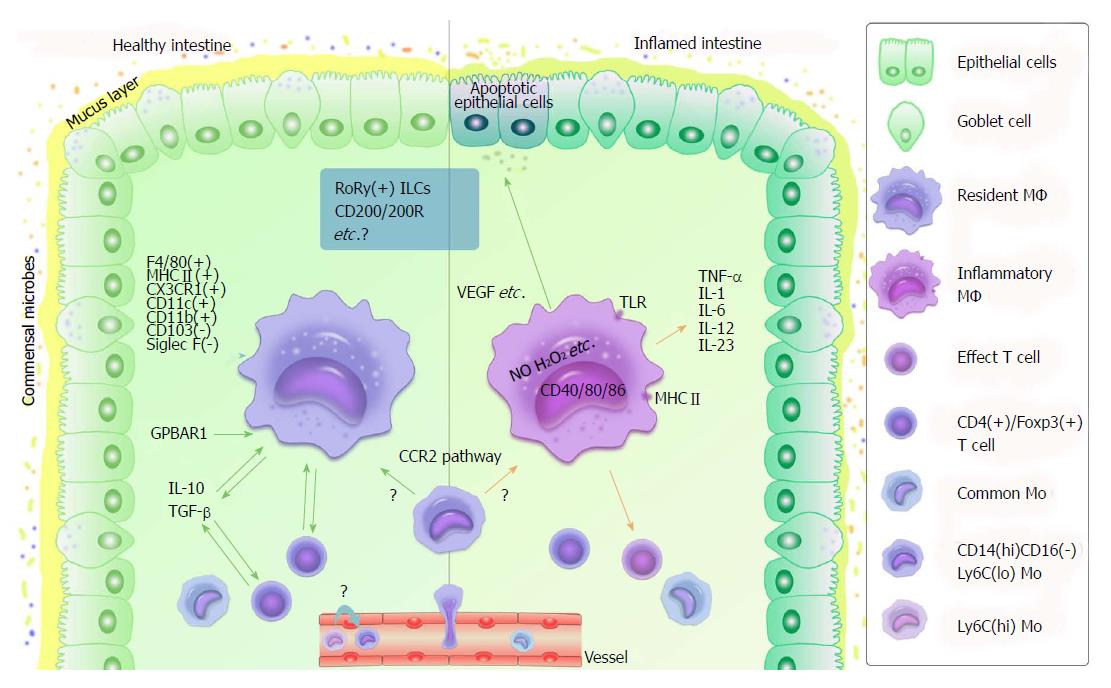
The general markers of MΦ have been mentioned above. Regarding intestinal MΦ, they can be recognized by their unique markers. Resident MΦ in the healthy mouse colon are F4/80hi, class II MHChi (also found in humans[83]), CX3CR1hi, CD11c+, CD103- and Siglec F-[82]. Unlike resident MΦ in other tissues, the highly expressed CX3CR1 is unique. Furthermore, the intestinal MΦ express CD13[84], CD14 and CD70, and they can be subdivided according to their size[85]. Previously, it was difficult to distinguish between intestinal DCs and MΦ; however, a small population of mucosal MΦ has recently been found to express CD11c, which is a specific marker of DCs. The F4/80+, CD11b+, and CD68+ cells are more likely to be MΦ rather than DCs. They do not present antigens to naïve T cells, and only the CD103+CX3CR1- cells are classical DCs[82,86-90]. These findings resolved a few puzzles concerning intestinal DCs and MΦ-like cells with the emergence of a possible hypothesis about the relationship between intestinal MΦ and DCs.
Differences between macrophages in the intestine and other tissues are illustrated in Figure 1. Unlike MΦ in other tissues, resident MΦ[91] in the healthy intestine do not express high levels of costimulatory molecules, such as CD40, CD80 and CD86[83], and they do not up-regulate costimulatory molecules or induce a respiratory burst to exterminate microbes[92-94]. Additionally, their responses to TLR ligands are unexpected[83,95]. TLRs are membrane glycoproteins located at the cell surface or within endosomes. They have an extracellular region to bind ligand and an ectoplasmic domain to trigger the intracellular signaling cascade. They can form hetero- or homodimers with each other, or complex with other receptors to recognize a wide range of microbes.
In general, with the TLRs, MΦ can be activated through many pathways mediated by MyD88, TRIF and NF-κB[20]. It is widely accepted that TLRs are the most characteristic PRRs. However, the intestinal resident MΦ do not respond to TLR ligands and produce proinflammatory cytokines or chemokines, such as IL-1, IL-6, IL-12, IL-23, TNF-α and CXCL10[82,91], which can be considered the inertia of mucosal MΦ. It has been conjectured that such is likely due to the absence of TLRs and other receptors (NOD-1/NOD-2) or malfunction of signaling pathways (via inhibitors or other mechanisms[96])[82,97,98]. However, this does not mean that the intestinal resident MΦ do not express TLRs or that TLRs are not necessary. In fact, they are essential to protect the intestinal epithelium under pathological circumstances[97,99,100].
These differences between intestinal mucosal MΦ and their homogeneity in other tissues reveal that they are more likely to control inflammation and maintain homeostasis in healthy individuals. However, what will occur if the balance has become broken?
It is less rigorous to use the word “change[101]” in the subtitle because there is little detail to describe that the intestinal resident MΦ change into inflammatory MΦ (classical MΦ) under pathological circumstances with the changes in the environment, or that these two types of MΦ coexist in healthy intestine, working respectively. Nonetheless, there is another possibility. A credible concept has been explained[21] involving CD14hiCD16- Mo, which can be considered to enter the intestinal LP only in a CCR2-dependent[102] manner and turn into the resident CD14lo MHCIIhiCD163hiCD64+ MΦ or inflammatory CD14hiMHCIIhiCD163loCD64+ MΦ in different circumstances. However, confusion concerning the relationship between CD14hiCD16- Mo and Ly6Chi/ Ly6Clo Mo has emerged and remains to be directly described.
It is clear that intestinal resident MΦ produce antiinflammatory cytokines, especially IL-10 and TGF-β[4,84,103-111], whereas inflammatory MΦ work at the inflammatory site and have strong bactericidal activity, as explained above. In healthy intestine, IL-10 is produced by mucosal MΦ themselves and is a component of T cells[112]. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide increase the production of IL-10 by mucosal MΦ in vitro and in vivo[113]. IL-10 prevents the NF-κB pathway, and inhibiting the autocrine/paracrine production of IL-10 reverses TLR unresponsiveness in MΦ[82]. Maintaining Foxp3 expression of regulatory T cells (Tregs) has been reported as one of the important functions of IL-10 produced by MΦ[114]. CD4+Foxp3+ Tregs greatly contribute to the immune regulatory networks with the complement of other T cells and B cells, maintaining intestinal homeostasis[115]. Recently, research[107] on Citrobacter rodentium-infected mice with cell type-specific deletion of Il-10 demonstrated that IL-10 prevents excessive inflammation in acute bacterial infection by controlling IL-23[116,117] production to limit innate immunity. Another study indicated that the deficiency of IL-10 results in stable chromatin alterations in intestinal MΦ[118]. These results showed that IL-10 indeed plays a critical role in limiting inflammation.
Another factor for antiinflammation is TGF-β. Intestinal resident MΦ express high levels of TGF-β receptors and show constitutively-active TGF-β signaling[82]. TGF-β also connects with Foxp3, expressed by Tregs, and CD4+Foxp3+ Tregs decrease the ability of mucosal MΦ to activate and translocate NF-κB[115]. Intestinal resident MΦ do not respond to TLR ligands with the existence of TGF-β[82]. In contrast to IL-10, their production in murine MΦ is inhibited by VIP[111]. Moreover, the expression of Smad7 (a member of the Smad family that mediates a pathway for TGF-β and BMP-2 signal transduction) interrupts TGF-β signaling and activates inflammatory MΦ, a finding that was demonstrated in an experiment of necrotizing enterocolitis MΦ[110].
Currently, the study of CD200 for antiinflammation has received less attention. CD200L is a member of the protective system, with the ability to restrain the activity of MΦ. Inhibitory signaling of CD200L is triggered by the interaction with CD200 in nonhematopoietic cells as well as MΦ[20]. This process protects tissues from severe damage. A study reported that knock-out of CD200 or CD200R1 produces MΦ hyperactivity and autoimmune diseases[119]. Enlightened by this, it is possible to assume CD200 maintains intestinal homeostasis. There are some relevant studies in the respiratory system[120], but the existing evidence in the intestine remains insufficient.
The enteric nervous system (ENS) plays a crucial role in controlling gastrointestinal physiology and interacting with microbes and immune cells, functions that have been explored for decades. Accumulating evidence indicates they closely contact MΦ. The development of CX3CR1hiMHCIIhi CD11b+CD11cloCD103- muscularis MΦ (MMs) requires CSF1, and enteric neurons selectively express bone morphogenetic protein (BMP; expressed by MMs) receptor 2, which produces CSF1. By contrast, the expression of BMP2 activates enteric neurons. The correlation of MMs and ENS contributes to gut motility[121]. Additionally, MMs have been found to express tissue-protective and wound-healing genes resembling M2 MΦ, reacting in intestinal infection[122].
More importantly, neurotransmitters are essential for neuronal immune control. VIP is known to exhibit antiinflammatory effects, depending on promoting the production of IL-10. Nitric oxide is well known for its antimicrobe ability in the respiratory burst. However, it suppresses excitability in neurons[121] and influences ENS during intestinal inflammation[91]. Interestingly, serotonin (5-HT), which was considered a trigger of inflammation, has been demonstrated to act, indirectly, on MMs by 5-HT4 receptors in neurons and to stimulate an antiinflammatory cascade in MΦ. It has been indicated that 5-HT2 and 5-HT7 are related to the development of M1 and M2 MΦ[91]. In addition, γ-amino butyric acid has been suggested to have an immunosuppressive effect on resident MΦ of the central nervous system[91]. However, in the intestine, it remains unclear. It is worth investigating the functions of ENS and how they act on MΦ to understand the gut immune system and associated disease treatments in the future.
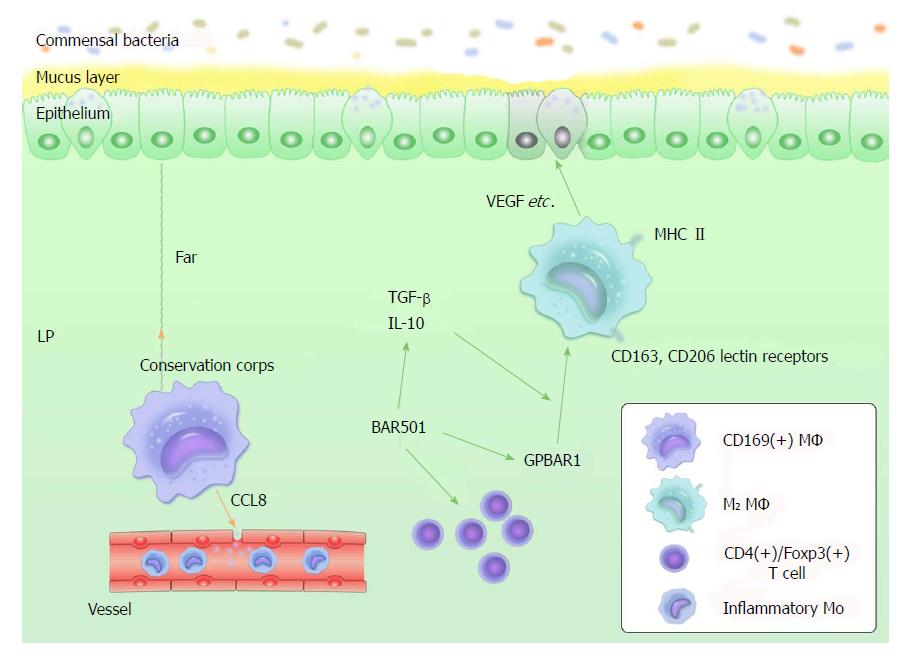
First, Kennichi et al[123] provided an exhaustive experimental result concerning LP-resident CD169+ MΦ that mainly persist in secondary lymphoid organs. They indicate that CD169+ MΦ reside at the bottom-end of the LP microenvironment, far away from the epithelium-LP border. Most importantly, the CD169+ MΦ recruit inflammatory monocytes by producing CCL8, selective depletion of CD169+ MΦ and anti-CCL8 antibody promotion of dextran sulfate sodium-induced colitis in mice. The comparison of CD109- and CD109+ MΦ led to an interesting hypothesis. Unlike CD109- MΦ, CD109+ MΦ are located in a region distant from the perimeter where they can be interrupted by commensal bacteria and dead epithelial cells, and they can directly release CCL8 into the systemic circulation in the vascular-rich environment. CD109+ MΦ probably respond to the collapse of the frontline defense - i.e. they can be considered as a “conservation corps” in the intestine (Figure 2).
Second, M2 MΦ struggle for attention. As another regulative population, M2 MΦ produce IL-10 and express CD163 and CD206 lectin receptors. They do not produce proinflammatory mediators with signals of stimulation. Certainly, they produce tissue-repairing factors, such as vascular endothelial growth factor (VEGF), actin and metalloproteinases, due to their function in wound healing. M2 MΦ are MHCII+, which may be helpful in exploring their potential in bactericidal activities[82,83,124,125]. Unlike M2 MΦ, Mregs express high levels of costimulatory molecules, such as CD40, CD80 and CD86, to submit antigens to T cells more effectively[42], highlighting the hypothesis that the regulation of M2 MΦ in the intestine might be different from that of Mregs. However, the antiinflammatory function of Mregs mentioned above has not been directly verified in the intestine. Therefore, we are unsure about the role of Mregs in intestinal homeostasis, and some questions remain concerning the meaning of the difference between M2 MΦ and Mregs (Figure 2).
Finally, a novel finding[126] concerning GPBAR1 (a G protein-coupled receptor for secondary bile acids) suggests that GPBAR1 is essential to maintain intestinal immune homeostasis by regulating M1/M2 MΦ. BAR501 (a small-molecule stimulus of GPBAR1) contributes to this regulatory process, depending on the production control of IL-10. Absence of the GPBAR1 gene causes the recruitment of M1 macrophages and severe inflammation in the colon. Exposure to BAR501 leads to the increased expression of IL-10 and TGF-β mRNA, and percentage of CD4+/Foxp3+ cells. Based on this study, GPBAR1 deserves attention for its potential to protect intestinal health (Figure 2).
According to the mechanisms of intestinal MΦ in maintaining homeostasis, any defect of the antiinflammation system may bring the reduction of immune tolerance, resulting in IBD. In 1998, it was found that intestinal MΦ displayed low expression of class II MHC molecules in mouse colitis[127]. A hypothesis arose from this study that there could be dysfunction of MΦ participating in adaptive immune responses when inflammation occurs.
From the origin of MΦ, emerging evidence suggests that GM-CSF plays a central role and has a protective effect in human CD and acute colitis by activating specific Mo[128,129]. Classical CD14hiCD16- Mo differentiate into large numbers of inflammatory MΦ in the inflamed mucosa of patients with CD[21]. CD14+ Mo in the mucosa from IBD patients increase the production of TNF-α[130,131], IL-1β and IL-6, and enhance respiratory burst activity[21]. Moreover, IL-10 knock-out mice develop spontaneous IBD[82]. An intrinsic resistance to TGF-β receptor signaling has been shown in the mucosa from patients with CD[132]. CD4+Foxp3+ T cells fail to protect the intestine from chronic inflammation without IL-10- and TGF-β-dependent mechanisms[115]. M2 MΦ have been certified to be activated by the Wnt signaling pathway, which is associated with UC[133]. These studies showed that intestinal MΦ are of great value for IBD. Following this result, promising treatments for IBD, such as CD109+ MΦ Tregs and GPBAR1, can be considered new therapeutic targets.
MΦ are clearly associated with IBD, but there remain a few puzzles regarding some details. The first study[134] observed that RoRy+ innate lymphoid cells (ILCs; the primary source of GM-CSF in the gut) promote MΦ to respond to the microbial signals and produce IL-1β, which enhances inflammation. By contrast, another study[135] discovered that with the regulation of RoRy+ ILCs, MΦ promote a negative feedback pathway through the activation of IL-22 production, which might be protective. Indeed, the quantity of RoRy+ ILCs could increase in human CD. This finding inspires the question of whether the possibility exists that a portion of MΦ still tries to restore intestinal homeostasis when the intestine is trapped in a vicious cycle for inflammatory macrophages. The second item concerns CD200/CD200R1 mentioned above. Knock-out of CD200 results in MΦ hyperactivity in vitro, but CD200R1 knock-out mice have normal intestinal MΦ populations, and they neither develop spontaneous IBD nor become more susceptible to colitis induced by the dextran sulfate sodium model[82]. This indicates that CD200R1 may not be as important as we had previously considered, but the reasons remain unclear.
Since the end of the last century, many studies have certified the connection between MΦ and tumors in various systems. There are considerable numbers of investigations concerning tumor-associated macrophages (TAMs). They promote immunosuppression, tumor immune evasion[136], tumorigenesis, tumor metastasis and angiogenesis as well as invasion by releasing various cytokines and inflammatory mediators, such as IL-6, IL-10, TFG-β, CCL2, CCL17, VEGF and cathepsins[137]. However, different populations of TAM have different functions. M1 MΦ have been confirmed to recognize and clear tumor cells, a function that is beneficial to health. By contrast, the development and movement of tumors benefit from M2 MΦ. TAMs are one of the promising targets of tumor therapy, especially M2 MΦ. Gut tumors are also included. We provide more details about TAMs and references in Box 4 to further illustrate the relationship between TAMs and tumors.
Similar to other MΦ, TAMs arise from hematopoietic stem cells in the bone marrow and from progenitors in the embryonic yolk sac. With different environmental signals, Mo differentiate into distinctive macrophages[137,138]. Tumor signals contribute to the development of TAMs. Mantovani et al[139] summarized the signals associated with TAMs. For example, lactic acid, CCL2, CSF1, VEGF and TGF-1 from tumor cells, IL-1β from tumor-associated fibroblasts, and IL-10 from Tregs, all can drive TAMs into tumor-promoting MΦ. Moreover, they also list the products of TAMs which have different functions. For instance, IL-6, MFG-E8 and osteopontin from TAMs can active tumor stem cells; TAMs produce epidermal growth factor to promote tumor growth, invasion and metastasis. Nitric oxide and reactive oxygen species can be released to destroy tumor cells. However, they might result in genetic instability, causing tumor formation. Nevertheless, further studies have indicated that not all the macrophages that have emerged into the tumor microenvironment are tumor promoting.
M1 MΦ (having antitumor function) can recognize tumors and kill tumor cells by the cytotoxic effect, representing a double-edged sword. They have been verified as an independent predictor of survival time in patients with non-small cell lung cancer[140]. M2 MΦ have a protumor function. They promote the metastasis of K7M2 wild-type osteosarcoma cells in mice. Additionally, all-trans retinoic acid dampens the profunction of M2 MΦ by suppressing the production of IL-13 or IL-14 (from M2 m MΦ) to inhibit the metastasis of osteosarcoma[141]. CHI3L1, a protein secreted by M2 MΦ, promotes the metastasis of gastric and breast cancer cells[55]. In addition, it was confirmed that patients with peritoneal dissemination in gastric cancer have more M2 MΦ and low expression of M1-related messengers[142]. MFG-E8, a powerful angiogenic factor, is induced by bone marrow-derived mesenchymal stromal cells in mice. Attenuated tumor growth and the decreasing function of M2 MΦ can be found in MFG-E8-deficient mice[143], which represent M2 MΦ that contribute to tumor angiogenesis; whether the correlation of M2 MΦ and MFG-E8 is parallel or antiparallel should be further clarified.
Above all, TAMs have advantages and disadvantages to both human physiology and tumors. They are members of our defensive line, but they are also tumor helpers. Compared with the favorable contributions of TAMs, such as M1 MΦ in tumor resistance, the promising therapeutic targets they provide might be more useful. In the 1990s, some scientists systematically revealed that TAMs were worth exploring for antitumor therapy[144], and more and more findings were uncovered during the last 50 years. On the one hand, TAMs are hopeful antitumor targets; on the other hand, as Mantovani and Allavena[139] illustrated, the mechanisms of TAMs in tumor development and antitumor processes are intricate, which limits researchers’ ability to find the antitumor target precisely. This phenomenon is the yin-yang of antitumor therapy and the challenge[145] of future antitumor studies.
Several studies have presented recent research progress in gastrointestinal tumors. First, tumor angiogenesis and survival in intestinal-type gastric cancer is closely associated with the infiltration of thymidine phosphorylase-positive MΦ[146]. Therefore, thymidine phosphorylase could be a useful marker for tumor angiogenesis, and the prognosis of intestinal-type gastric cancer. Second, there is a hotspot induced by M2 MΦ. A portion of M2 MΦ, cooperating with TNF, were shown to be recruited to tumors[56,147]. The macromolecular contrast agent PG-Gd-NIR813 shows a dual magneto-optical imaging probe of tumor-associated M2 MΦ[50], and a few new factors have been evaluated as mediators of the development of gastrointestinal tumors, such as M2 MΦ-secreted CHI3L1 protein[55] and monocyte chemoattractant protein-1[148]. All are likely to become novel approaches for antitumor therapy.
In summary (Figure 3), MΦ with their various receptors act as sentinels in innate immunity and adaptive immunity. In healthy intestinal mucosa, they are indispensable to suppress inflammation and play an essential role in maintaining homeostasis by producing many inhibitors, such as IL-10 and TGF-β. However, they show strong bactericidal activities. Intestinal resident MΦ create a harmonious environment for commensal bacteria and their host. Any defect in keeping this balance can reduce immune tolerance, causing acute tissue damage or chronic autoimmune diseases, explaining their close association with IBD. New findings concerning intestinal MΦ and IBD, as well as tumors, can be very helpful for studies and disease treatments. Meanwhile, there are many details awaiting clarification as well as many unresolved issues.
We wish to thank Prof. Zhang Min (School of Biological Engineering, Capital Medical University) for her contribution in polishing the language of this manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Caboclo JL, Contini S, Grizzi F S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y
| 1. | Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1339] [Cited by in RCA: 1394] [Article Influence: 77.4] [Reference Citation Analysis (1)] |
| 2. | Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1157] [Article Influence: 52.6] [Reference Citation Analysis (6)] |
| 3. | Tang L, Cheng CY, Sun X, Pedicone AJ, Mohamadzadeh M, Cheng SX. The Extracellular Calcium-Sensing Receptor in the Intestine: Evidence for Regulation of Colonic Absorption, Secretion, Motility, and Immunity. Front Physiol. 2016;7:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 477] [Article Influence: 43.4] [Reference Citation Analysis (14)] |
| 5. | Altmeyer S, Zentek J, Vahjen W, Scharek-Tedin L. The expression of NKG2D on porcine IEL and its possible relation to the adaptive intestinal immune system. Vet Immunol Immunopathol. 2017;187:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Goodyear AW, Kumar A, Dow S, Ryan EP. Optimization of murine small intestine leukocyte isolation for global immune phenotype analysis. J Immunol Methods. 2014;405:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Weitkamp JH, Rosen MJ, Zhao Z, Koyama T, Geem D, Denning TL, Rock MT, Moore DJ, Halpern MD, Matta P. Small intestinal intraepithelial TCRγδ+ T lymphocytes are present in the premature intestine but selectively reduced in surgical necrotizing enterocolitis. PLoS One. 2014;9:e99042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 1005] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 9. | Johansson ME, Hansson GC. Mucus and the goblet cell. Dig Dis. 2013;31:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Takata K, Kozaki T, Lee CZW, Thion MS, Otsuka M, Lim S, Utami KH, Fidan K, Park DS, Malleret B. Induced-Pluripotent-Stem-Cell-Derived Primitive Macrophages Provide a Platform for Modeling Tissue-Resident Macrophage Differentiation and Function. Immunity. 2017;47:183-198.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 268] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 11. | Johnson KJ, Ward PA, Striker G, Kunkel R. A study of the origin of pulmonary macrophages using the Chédiak-Higashi marker. Am J Pathol. 1980;101:365-374. [PubMed] |
| 12. | Fuad MB, Robert MN. Allergy and Immunology of the Upper Airway. 6th ed. Philadelphia, PA, US: Elsevier Saunders 2015; 593-625. |
| 13. | MacDonald KP, Palmer JS, Cronau S, Seppanen E, Olver S, Raffelt NC, Kuns R, Pettit AR, Clouston A, Wainwright B. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955-3963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 406] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 14. | Dubertret L, Breton-Gorius J, Fosse M, Touraine R. A cytochemical marker for epidermal differentiation, Langerhans cells, skin resident macrophages and mitochondria. Br J Dermatol. 1982;107 Suppl 23:96-100. [PubMed] |
| 15. | Hoefsmit EC, Schadee-Eestermans IL, Beelen RH. The development of the resident pattern of endogenous peroxidatic activity in mouse peritoneal macrophages coincides with the expression of the differentiation antigen F4/80. A combined method for immunoperoxidase labeling and endogenous peroxidase cytochemistry. J Histochem Cytochem. 1986;34:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Iwama A, Wang MH, Yamaguchi N, Ohno N, Okano K, Sudo T, Takeya M, Gervais F, Morissette C, Leonard EJ. Terminal differentiation of murine resident peritoneal macrophages is characterized by expression of the STK protein tyrosine kinase, a receptor for macrophage-stimulating protein. Blood. 1995;86:3394-3403. [PubMed] |
| 17. | Satoh T, Kidoya H, Naito H, Yamamoto M, Takemura N, Nakagawa K, Yoshioka Y, Morii E, Takakura N, Takeuchi O. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495:524-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 254] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 18. | Meireles AM, Shiau CE, Guenther CA, Sidik H, Kingsley DM, Talbot WS. The phosphate exporter xpr1b is required for differentiation of tissue-resident macrophages. Cell Rep. 2014;8:1659-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Colonna M. Interkeukin-34, a cytokine crucial for the differentiation and maintenance of tissue resident macrophages and Langerhans cells. Eur J Immunol. 2014;44:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | David M, Jonathan B, David BR, Ivan MR. Mononuclear Phagocytes in Immune Defense. 8 ed. Philadelphia, PA, US: Elsevier Saunders 2013; 125-126. |
| 21. | Gren ST, Grip O. Role of Monocytes and Intestinal Macrophages in Crohn’s Disease and Ulcerative Colitis. Inflamm Bowel Dis. 2016;22:1992-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 22. | Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71-82. [PubMed] |
| 23. | Bain CC, Mowat AM. Intestinal macrophages - specialised adaptation to a unique environment. Eur J Immunol. 2011;41:2494-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985;161:475-489. [PubMed] |
| 25. | Kolaczkowska E, Arnold B, Opdenakker G. Gelatinase B/MMP-9 as an inflammatory marker enzyme in mouse zymosan peritonitis: comparison of phase-specific and cell-specific production by mast cells, macrophages and neutrophils. Immunobiology. 2008;213:109-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Riquelme P, Amodio G, Macedo C, Moreau A, Obermajer N, Brochhausen C, Ahrens N, Kekarainen T, Fändrich F, Cuturi C. DHRS9 Is a Stable Marker of Human Regulatory Macrophages. Transplantation. 2017;101:2731-2738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Dhakal M, Hardaway JC, Guloglu FB, Miller MM, Hoeman CM, Zaghouani AA, Wan X, Rowland LM, Cascio JA, Sherman MP. IL-13Rα1 is a surface marker for M2 macrophages influencing their differentiation and function. Eur J Immunol. 2014;44:842-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Sanyal R, Polyak MJ, Zuccolo J, Puri M, Deng L, Roberts L, Zuba A, Storek J, Luider JM, Sundberg EM. MS4A4A: a novel cell surface marker for M2 macrophages and plasma cells. Immunol Cell Biol. 2017;95:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | David M, Jonathan B, David BR, Ivan MR. Antigen Presentation. Immunology. 8 ed. Philadelphia, PA, US: Elsevier Saunders 2013; 143-153. |
| 30. | Gerber HA, Morris B, Trevella W. The role of gut-associated lymphoid tissues in the generation of immunoglobulin-bearing lymphocytes in sheep. Aust J Exp Biol Med Sci. 1986;64:201-213. [PubMed] |
| 31. | Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr. 2005;93 Suppl 1:S41-S48. [PubMed] |
| 32. | Donaldson DS, Else KJ, Mabbott NA. The Gut-Associated Lymphoid Tissues in the Small Intestine, Not the Large Intestine, Play a Major Role in Oral Prion Disease Pathogenesis. J Virol. 2015;89:9532-9547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 34. | Kajiwara E, Shigeta A, Horiuchi H, Matsuda H, Furusawa S. Development of Peyer’s patch and cecal tonsil in gut-associated lymphoid tissues in the chicken embryo. J Vet Med Sci. 2003;65:607-614. [PubMed] |
| 35. | Roy MJ, Varvayanis M. Development of dome epithelium in gut-associated lymphoid tissues: association of IgA with M cells. Cell Tissue Res. 1987;248:645-651. [PubMed] |
| 36. | Ratcliffe MJ. B cell development in gut associated lymphoid tissues. Vet Immunol Immunopathol. 2002;87:337-340. [PubMed] |
| 37. | Kawanishi H, Saltzman L, Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. II. Terminal differentiation of postswitch sIgA-bearing Peyer’s patch B cells. J Exp Med. 1983;158:649-669. [PubMed] |
| 38. | Kawanishi H, Strober W. T cell regulation of IgA immunoglobulin production in gut-associated lymphoid tissues. Mol Immunol. 1983;20:917-930. [PubMed] |
| 39. | Weisz-Carrington P, Grimes SR Jr, Lamm ME. Gut-associated lymphoid tissue as source of an IgA immune response in respiratory tissues after oral immunization and intrabronchial challenge. Cell Immunol. 1987;106:132-138. [PubMed] |
| 40. | Moro I, Komiyama K, Kusama K, Iwase T, Asano M, Takenouchi N. [Molecular aspects of secretory IgA (S-IgA) in gut-associated lymphoid tissues]. Nihon Rinsho. 1996;54:1155-1161. [PubMed] |
| 41. | Pabst O, Bernhardt G, Förster R. The impact of cell-bound antigen transport on mucosal tolerance induction. J Leukoc Biol. 2007;82:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7233] [Cited by in RCA: 7026] [Article Influence: 390.3] [Reference Citation Analysis (0)] |
| 43. | Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2525] [Article Influence: 109.8] [Reference Citation Analysis (0)] |
| 44. | Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 632] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 45. | Cordeiro-da-Silva A, Tavares J, Araújo N, Cerqueira F, Tomás A, Kong Thoo Lin P, Ouaissi A. Immunological alterations induced by polyamine derivatives on murine splenocytes and human mononuclear cells. Int Immunopharmacol. 2004;4:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549-555. [PubMed] |
| 47. | Sun S, Cui Y, Ren K, Quan M, Song Z, Zou H, Li D, Zheng Y, Cao J. 8-bromo-7-methoxychrysin Reversed M2 Polarization of Tumor-associated Macrophages Induced by Liver Cancer Stem-like Cells. Anticancer Agents Med Chem. 2017;17:286-293. [PubMed] |
| 48. | Simões RL, De-Brito NM, Cunha-Costa H, Morandi V, Fierro IM, Roitt IM, Barja-Fidalgo C. Lipoxin A4 selectively programs the profile of M2 tumor-associated macrophages which favour control of tumor progression. Int J Cancer. 2017;140:346-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Kimura Y, Sumiyoshi M, Baba K. Antitumor and Antimetastatic Activity of Synthetic Hydroxystilbenes Through Inhibition of Lymphangiogenesis and M2 Macrophage Differentiation of Tumor-associated Macrophages. Anticancer Res. 2016;36:137-148. [PubMed] |
| 50. | Melancon MP, Lu W, Huang Q, Thapa P, Zhou D, Ng C, Li C. Targeted imaging of tumor-associated M2 macrophages using a macromolecular contrast agent PG-Gd-NIR813. Biomaterials. 2010;31:6567-6573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 52. | Van Ginderachter JA, Meerschaut S, Liu Y, Brys L, De Groeve K, Hassanzadeh Ghassabeh G, Raes G, De Baetselier P. Peroxisome proliferator-activated receptor gamma (PPARgamma) ligands reverse CTL suppression by alternatively activated (M2) macrophages in cancer. Blood. 2006;108:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1138] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 54. | Mantovani A, Sozzani S, Locati M, Schioppa T, Saccani A, Allavena P, Sica A. Infiltration of tumours by macrophages and dendritic cells: tumour-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Novartis Found Symp. 2004;256:137-145; discussion 146-148, 259-269. [PubMed] |
| 55. | Chen Y, Zhang S, Wang Q, Zhang X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncol. 2017;10:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 369] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 56. | Beider K, Bitner H, Leiba M, Gutwein O, Koren-Michowitz M, Ostrovsky O, Abraham M, Wald H, Galun E, Peled A. Multiple myeloma cells recruit tumor-supportive macrophages through the CXCR4/CXCL12 axis and promote their polarization toward the M2 phenotype. Oncotarget. 2014;5:11283-11296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 57. | Maier C, Ramming A, Bergmann C, Weinkam R, Kittan N, Schett G, Distler JHW, Beyer C. Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin-6 from M2 macrophages. Ann Rheum Dis. 2017;76:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 58. | Vannella KM, Barron L, Borthwick LA, Kindrachuk KN, Narasimhan PB, Hart KM, Thompson RW, White S, Cheever AW, Ramalingam TR. Incomplete deletion of IL-4Rα by LysM(Cre) reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis. PLoS Pathog. 2014;10:e1004372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Bility MT, Cheng L, Zhang Z, Luan Y, Li F, Chi L, Zhang L, Tu Z, Gao Y, Fu Y. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10:e1004032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 60. | Braga TT, Correa-Costa M, Guise YF, Castoldi A, de Oliveira CD, Hyane MI, Cenedeze MA, Teixeira SA, Muscara MN, Perez KR. MyD88 signaling pathway is involved in renal fibrosis by favoring a TH2 immune response and activating alternative M2 macrophages. Mol Med. 2012;18:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Braune J, Weyer U, Hobusch C, Mauer J, Brüning JC, Bechmann I, Gericke M. IL-6 Regulates M2 Polarization and Local Proliferation of Adipose Tissue Macrophages in Obesity. J Immunol. 2017;198:2927-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 62. | Camell C, Smith CW. Dietary oleic acid increases m2 macrophages in the mesenteric adipose tissue. PLoS One. 2013;8:e75147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 294] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 64. | Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58:2574-2582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 578] [Article Influence: 34.0] [Reference Citation Analysis (7)] |
| 65. | Aron-Wisnewsky J, Tordjman J, Poitou C, Darakhshan F, Hugol D, Basdevant A, Aissat A, Guerre-Millo M, Clément K. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab. 2009;94:4619-4623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 66. | Zasłona Z, Przybranowski S, Wilke C, van Rooijen N, Teitz-Tennenbaum S, Osterholzer JJ, Wilkinson JE, Moore BB, Peters-Golden M. Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma. J Immunol. 2014;193:4245-4253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 67. | Madore AM, Perron S, Turmel V, Laviolette M, Bissonnette EY, Laprise C. Alveolar macrophages in allergic asthma: an expression signature characterized by heat shock protein pathways. Hum Immunol. 2010;71:144-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Vissers JL, van Esch BC, Hofman GA, van Oosterhout AJ. Macrophages induce an allergen-specific and long-term suppression in a mouse asthma model. Eur Respir J. 2005;26:1040-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Lee TH, Poston R, Godard P, Bousquet J. Macrophages and allergic asthma. Clin Exp Allergy. 1991;21 Suppl 1:22-23. [PubMed] |
| 70. | Godard P, Damon M, Chanez P, Michel FB. Releasability of airway macrophages in bronchial asthma. Int Arch Allergy Appl Immunol. 1991;95:97-101. [PubMed] |
| 71. | Cochain C, Zernecke A. Macrophages in vascular inflammation and atherosclerosis. Pflugers Arch. 2017;469:485-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 72. | Neele AE, Van den Bossche J, Hoeksema MA, de Winther MP. Epigenetic pathways in macrophages emerge as novel targets in atherosclerosis. Eur J Pharmacol. 2015;763:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 73. | Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2133] [Cited by in RCA: 2103] [Article Influence: 140.2] [Reference Citation Analysis (0)] |
| 74. | Lucas AD, Greaves DR. Atherosclerosis: role of chemokines and macrophages. Expert Rev Mol Med. 2001;3:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Daoud AS, Fritz KE, Jarmolych J, Frank AS. Role of macrophages in regression of atherosclerosis. Ann N Y Acad Sci. 1985;454:101-114. [PubMed] |
| 76. | Wolman M, Gaton E. [Macrophages and smooth muscle cells in the pathogenesis of atherosclerosis]. Harefuah. 1976;90:400-402. [PubMed] |
| 77. | Portillo JC, Lopez Corcino Y, Miao Y, Tang J, Sheibani N, Kern TS, Dubyak GR, Subauste CS. CD40 in Retinal Müller Cells Induces P2X7-Dependent Cytokine Expression in Macrophages/Microglia in Diabetic Mice and Development of Early Experimental Diabetic Retinopathy. Diabetes. 2017;66:483-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 78. | Davies MH, Eubanks JP, Powers MR. Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol Vis. 2006;12:467-477. [PubMed] |
| 79. | Naug HL, Browning J, Gole GA, Gobé G. Vitreal macrophages express vascular endothelial growth factor in oxygen-induced retinopathy. Clin Exp Ophthalmol. 2000;28:48-52. [PubMed] |
| 80. | Esser P, Heimann K, Wiedemann P. Macrophages in proliferative vitreoretinopathy and proliferative diabetic retinopathy: differentiation of subpopulations. Br J Ophthalmol. 1993;77:731-733. [PubMed] |
| 81. | Spoettl T, Hausmann M, Menzel K, Piberger H, Herfarth H, Schoelmerich J, Bataille F, Rogler G. Role of soluble factors and three-dimensional culture in in vitro differentiation of intestinal macrophages. World J Gastroenterol. 2007;13:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 82. | Mowat AM, Bain CC. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun. 2011;3:550-564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 83. | Smith PD, Smythies LE, Shen R, Greenwell-Wild T, Gliozzi M, Wahl SM. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 84. | Sheikh SZ, Plevy SE. The role of the macrophage in sentinel responses in intestinal immunity. Curr Opin Gastroenterol. 2010;26:578-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Bar-On L, Zigmond E, Jung S. Management of gut inflammation through the manipulation of intestinal dendritic cells and macrophages? Semin Immunol. 2011;23:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM, Milling SW. Intestinal CD103(-) dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 2013;6:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 87. | Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101-3114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 515] [Cited by in RCA: 543] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 88. | Kelsall B. Recent progress in understanding the phenotype and function of intestinal dendritic cells and macrophages. Mucosal Immunol. 2008;1:460-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 89. | Pabst O, Bernhardt G. The puzzle of intestinal lamina propria dendritic cells and macrophages. Eur J Immunol. 2010;40:2107-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 90. | Cerovic V, Bain CC, Mowat AM, Milling SW. Intestinal macrophages and dendritic cells: what’s the difference? Trends Immunol. 2014;35:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 189] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 91. | Wehner S, Engel DR. Resident macrophages in the healthy and inflamed intestinal muscularis externa. Pflugers Arch. 2017;469:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Mahida YR, Wu KC, Jewell DP. Respiratory burst activity of intestinal macrophages in normal and inflammatory bowel disease. Gut. 1989;30:1362-1370. [PubMed] |
| 93. | Rugtveit J, Haraldsen G, Høgåsen AK, Bakka A, Brandtzaeg P, Scott H. Respiratory burst of intestinal macrophages in inflammatory bowel disease is mainly caused by CD14+L1+ monocyte derived cells. Gut. 1995;37:367-373. [PubMed] |
| 94. | Goode EC, Warburton RC, Gelson WT, Watson AJ. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. Gastroenterology. 2013;145:1481-1484. [PubMed] |
| 95. | Platt AM, Bain CC, Bordon Y, Sester DP, Mowat AM. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J Immunol. 2010;184:6843-6854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 96. | Kang GD, Kim DH. Poncirin and its metabolite ponciretin attenuate colitis in mice by inhibiting LPS binding on TLR4 of macrophages and correcting Th17/Treg imbalance. J Ethnopharmacol. 2016;189:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 97. | Lee IA, Park YJ, Joh EH, Kim DH. Soyasaponin Ab ameliorates colitis by inhibiting the binding of lipopolysaccharide (LPS) to Toll-like receptor (TLR)4 on macrophages. J Agric Food Chem. 2011;59:13165-13172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Qian Z, Wu Z, Huang L, Qiu H, Wang L, Li L, Yao L, Kang K, Qu J, Wu Y. Mulberry fruit prevents LPS-induced NF-κB/pERK/MAPK signals in macrophages and suppresses acute colitis and colorectal tumorigenesis in mice. Sci Rep. 2015;5:17348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 99. | Regan T, Nally K, Carmody R, Houston A, Shanahan F, Macsharry J, Brint E. Identification of TLR10 as a key mediator of the inflammatory response to Listeria monocytogenes in intestinal epithelial cells and macrophages. J Immunol. 2013;191:6084-6092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 100. | Wehner S, Buchholz BM, Schuchtrup S, Rocke A, Schaefer N, Lysson M, Hirner A, Kalff JC. Mechanical strain and TLR4 synergistically induce cell-specific inflammatory gene expression in intestinal smooth muscle cells and peritoneal macrophages. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1187-G1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Little MC, Hurst RJ, Else KJ. Dynamic changes in macrophage activation and proliferation during the development and resolution of intestinal inflammation. J Immunol. 2014;193:4684-4695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 102. | Seo SU, Kuffa P, Kitamoto S, Nagao-Kitamoto H, Rousseau J, Kim YG, Núñez G, Kamada N. Intestinal macrophages arising from CCR2(+) monocytes control pathogen infection by activating innate lymphoid cells. Nat Commun. 2015;6:8010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van Rooijen N, Fallon PG. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178:4557-4566. [PubMed] |
| 104. | Hayashi A, Sato T, Kamada N, Mikami Y, Matsuoka K, Hisamatsu T, Hibi T, Roers A, Yagita H, Ohteki T. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe. 2013;13:711-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 254] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 105. | Jamontt J, Petit S, Clark N, Parkinson SJ, Smith P. Nucleotide-binding oligomerization domain 2 signaling promotes hyperresponsive macrophages and colitis in IL-10-deficient mice. J Immunol. 2013;190:2948-2958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 106. | Leung G, Wang A, Fernando M, Phan VC, McKay DM. Bone marrow-derived alternatively activated macrophages reduce colitis without promoting fibrosis: participation of IL-10. Am J Physiol Gastrointest Liver Physiol. 2013;304:G781-G792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Krause P, Morris V, Greenbaum JA, Park Y, Bjoerheden U, Mikulski Z, Muffley T, Shui JW, Kim G, Cheroutre H. IL-10-producing intestinal macrophages prevent excessive antibacterial innate immunity by limiting IL-23 synthesis. Nat Commun. 2015;6:7055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 108. | Li B, Gurung P, Malireddi RK, Vogel P, Kanneganti TD, Geiger TL. IL-10 engages macrophages to shift Th17 cytokine dependency and pathogenicity during T-cell-mediated colitis. Nat Commun. 2015;6:6131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Liu W, Zhang S, Gu S, Sang L, Dai C. Mesenchymal stem cells recruit macrophages to alleviate experimental colitis through TGFβ1. Cell Physiol Biochem. 2015;35:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 110. | MohanKumar K, Namachivayam K, Chapalamadugu KC, Garzon SA, Premkumar MH, Tipparaju SM, Maheshwari A. Smad7 interrupts TGF-β signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr Res. 2016;79:951-961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 111. | Sun W, Tadmori I, Yang L, Delgado M, Ganea D. Vasoactive intestinal peptide (VIP) inhibits TGF-beta1 production in murine macrophages. J Neuroimmunol. 2000;107:88-99. [PubMed] |
| 112. | Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galán JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 346] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 113. | Delgado M, Munoz-Elias EJ, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide enhance IL-10 production by murine macrophages: in vitro and in vivo studies. J Immunol. 1999;162:1707-1716. [PubMed] |
| 114. | Weinhage T, Däbritz J, Brockhausen A, Wirth T, Brückner M, Belz M, Foell D, Varga G. Granulocyte Macrophage Colony-Stimulating Factor-Activated CD39+/CD73+ Murine Monocytes Modulate Intestinal Inflammation via Induction of Regulatory T Cells. Cell Mol Gastroenterol Hepatol. 2015;1:433-449.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 115. | Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 116. | Kamada N, Hisamatsu T, Okamoto S, Chinen H, Kobayashi T, Sato T, Sakuraba A, Kitazume MT, Sugita A, Koganei K. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 328] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 117. | Arnold IC, Mathisen S, Schulthess J, Danne C, Hegazy AN, Powrie F. CD11c(+) monocyte/macrophages promote chronic Helicobacter hepaticus-induced intestinal inflammation through the production of IL-23. Mucosal Immunol. 2016;9:352-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 118. | Simon JM, Davis JP, Lee SE, Schaner MR, Gipson GR, Weiser M, Sartor RB, Herfarth HH, Rahbar R, Sadiq TS. Alterations to chromatin in intestinal macrophages link IL-10 deficiency to inappropriate inflammatory responses. Eur J Immunol. 2016;46:1912-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 119. | Boudakov I, Liu J, Fan N, Gulay P, Wong K, Gorczynski RM. Mice lacking CD200R1 show absence of suppression of lipopolysaccharide-induced tumor necrosis factor-alpha and mixed leukocyte culture responses by CD200. Transplantation. 2007;84:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 120. | Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 368] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 121. | Cipriani G, Gibbons SJ, Kashyap PC, Farrugia G. Intrinsic Gastrointestinal Macrophages: Their Phenotype and Role in Gastrointestinal Motility. Cell Mol Gastroenterol Hepatol. 2016;2:120-130.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 122. | Obata Y, Pachnis V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology. 2016;151:836-844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 123. | Asano K, Takahashi N, Ushiki M, Monya M, Aihara F, Kuboki E, Moriyama S, Iida M, Kitamura H, Qiu CH. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat Commun. 2015;6:7802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 124. | Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2322] [Cited by in RCA: 2290] [Article Influence: 143.1] [Reference Citation Analysis (0)] |
| 125. | Haribhai D, Ziegelbauer J, Jia S, Upchurch K, Yan K, Schmitt EG, Salzman NH, Simpson P, Hessner MJ, Chatila TA. Alternatively Activated Macrophages Boost Induced Regulatory T and Th17 Cell Responses during Immunotherapy for Colitis. J Immunol. 2016;196:3305-3317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 126. | Biagioli M, Carino A, Cipriani S, Francisci D, Marchianò S, Scarpelli P, Sorcini D, Zampella A, Fiorucci S. The Bile Acid Receptor GPBAR1 Regulates the M1/M2 Phenotype of Intestinal Macrophages and Activation of GPBAR1 Rescues Mice from Murine Colitis. J Immunol. 2017;199:718-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 127. | Rogler G, Hausmann M, Vogl D, Aschenbrenner E, Andus T, Falk W, Andreesen R, Schölmerich J, Gross V. Isolation and phenotypic characterization of colonic macrophages. Clin Exp Immunol. 1998;112:205-215. [PubMed] |
| 128. | Däbritz J. Granulocyte macrophage colony-stimulating factor and the intestinal innate immune cell homeostasis in Crohn’s disease. Am J Physiol Gastrointest Liver Physiol. 2014;306:G455-G465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 129. | Meshkibaf S, Martins AJ, Henry GT, Kim SO. Protective role of G-CSF in dextran sulfate sodium-induced acute colitis through generating gut-homing macrophages. Cytokine. 2016;78:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 130. | Nakata K, Inagawa H, Nishizawa T, Honda T, Kohchi C, Tonomoto Y, Yoshimura H, Nagasue N, Natori S, Terada H. Inherent potential for production of tumor necrosis factor-alpha by human intestinal macrophages. Int J Colorectal Dis. 2006;21:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 131. | Khalil M, Babes A, Lakra R, Försch S, Reeh PW, Wirtz S, Becker C, Neurath MF, Engel MA. Transient receptor potential melastatin 8 ion channel in macrophages modulates colitis through a balance-shift in TNF-alpha and interleukin-10 production. Mucosal Immunol. 2016;9:1500-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 132. | Monteleone G, Boirivant M, Pallone F, MacDonald TT. TGF-beta1 and Smad7 in the regulation of IBD. Mucosal Immunol. 2008;1 Suppl 1:S50-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 133. | Cosín-Roger J, Ortiz-Masiá D, Calatayud S, Hernández C, Alvarez A, Hinojosa J, Esplugues JV, Barrachina MD. M2 macrophages activate WNT signaling pathway in epithelial cells: relevance in ulcerative colitis. PLoS One. 2013;8:e78128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 134. | Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 674] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 135. | Mizuno S, Mikami Y, Kamada N, Handa T, Hayashi A, Sato T, Matsuoka K, Matano M, Ohta Y, Sugita A. Cross-talk between RORγt+ innate lymphoid cells and intestinal macrophages induces mucosal IL-22 production in Crohn’s disease. Inflamm Bowel Dis. 2014;20:1426-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 136. | Che F, Heng X, Zhang H, Su Q, Zhang B, Chen Y, Zhang Z, Du Y, Wang L. Novel B7-H4-mediated crosstalk between human non-Hodgkin lymphoma cells and tumor-associated macrophages leads to immune evasion via secretion of IL-6 and IL-10. Cancer Immunol Immunother. 2017;66:717-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 137. | Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy. 2017;9:289-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 279] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 138. | Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune microenvironment of gliomas. Lab Invest. 2017;97:498-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 415] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 139. | Mantovani A, Allavena P. The interaction of anticancer therapies with tumor-associated macrophages. J Exp Med. 2015;212:435-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 517] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 140. | Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer. 2010;10:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (1)] |
| 141. | Zhou Q, Xian M, Xiang S, Xiang D, Shao X, Wang J, Cao J, Yang X, Yang B, Ying M. All-Trans Retinoic Acid Prevents Osteosarcoma Metastasis by Inhibiting M2 Polarization of Tumor-Associated Macrophages. Cancer Immunol Res. 2017;5:547-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 142. | Yamaguchi T, Fushida S, Yamamoto Y, Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I, Munesue S. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer. 2016;19:1052-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 214] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 143. | Yamada K, Uchiyama A, Uehara A, Perera B, Ogino S, Yokoyama Y, Takeuchi Y, Udey MC, Ishikawa O, Motegi S. MFG-E8 Drives Melanoma Growth by Stimulating Mesenchymal Stromal Cell-Induced Angiogenesis and M2 Polarization of Tumor-Associated Macrophages. Cancer Res. 2016;76:4283-4292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 144. | Wahl LM, Kleinman HK. Tumor-associated macrophages as targets for cancer therapy. J Natl Cancer Inst. 1998;90:1583-1584. [PubMed] |
| 145. | Andón FT, Digifico E, Maeda A, Erreni M, Mantovani A, Alonso MJ, Allavena P. Targeting tumor associated macrophages: The new challenge for nanomedicine. Semin Immunol. 2017;34:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 146. | Kawahara A, Hattori S, Akiba J, Nakashima K, Taira T, Watari K, Hosoi F, Uba M, Basaki Y, Koufuji K. Infiltration of thymidine phosphorylase-positive macrophages is closely associated with tumor angiogenesis and survival in intestinal type gastric cancer. Oncol Rep. 2010;24:405-415. [PubMed] |
| 147. | Kratochvill F, Neale G, Haverkamp JM, Van de Velde LA, Smith AM, Kawauchi D, McEvoy J, Roussel MF, Dyer MA, Qualls JE. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015;12:1902-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 148. | McClellan JL, Davis JM, Steiner JL, Enos RT, Jung SH, Carson JA, Pena MM, Carnevale KA, Berger FG, Murphy EA. Linking tumor-associated macrophages, inflammation, and intestinal tumorigenesis: role of MCP-1. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1087-G1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |