Published online Jan 7, 2018. doi: 10.3748/wjg.v24.i1.87
Peer-review started: October 6, 2017
First decision: October 31, 2017
Revised: November 11, 2017
Accepted: November 27, 2017
Article in press: November 27, 2017
Published online: January 7, 2018
Processing time: 93 Days and 16.6 Hours
To investigate the relationship between histological mixed-type of early gastric cancer (EGC) in the mucosa and submucosa and lymph node metastasis (LNM).
This study included 298 patients who underwent gastrectomy for EGC between 2005 and 2012. Enrolled lesions were divided into groups of pure differentiated (pure D), pure undifferentiated (pure U), and mixed-type according to the proportion of the differentiated and undifferentiated components observed under a microscope. We reviewed the clinicopathological features, including age, sex, location, size, gross type, lymphovascular invasion, ulceration, and LNM, among the three groups. Furthermore, we evaluated the predictors of LNM in the mucosa-confined EGC.
Of the 298 patients, 165 (55.4%) had mucosa-confined EGC and 133 (44.6%) had submucosa-invasive EGC. Only 13 (7.9%) cases of mucosa-confined EGC and 30 (22.6%) cases of submucosa-invasive EGC were observed to have LNM. The submucosal invasion (OR = 4.58, 95%CI: 1.23-16.97, P = 0.023), pure U type (OR = 4.97, 95%CI: 1.21-20.39, P = 0.026), and mixed-type (OR = 5.84, 95%CI: 1.05-32.61, P = 0.044) were independent risk factors for LNM in EGC. The rate of LNM in mucosa-confined EGC was higher in the mixed-type group (P = 0.012) and pure U group (P = 0.010) than in the pure D group, but no significant difference was found between the mixed-type group and pure U group (P = 0.739). Similarly, the rate of LNM in the submucosa-invasive EGC was higher in the mixed-type (P = 0.012) and pure U group (P = 0.009) than in the pure D group but was not significantly different between the mixed-type and pure U group (P = 0.375). Multivariate logistic analysis showed that only female sex (OR = 5.83, 95%CI: 1.64-20.70, P = 0.028) and presence of lymphovascular invasion (OR = 13.18, 95%CI: 1.39-125.30, P = 0.020) were independent risk factors for LNM in mucosa-confined EGC, while histological type was not an independent risk factor for LNM in mucosa-confined EGC (P = 0.106).
For mucosal EGC, histological mixed-type is not an independent risk factor for LNM and could be managed in the same way as the undifferentiated type.
Core tip: This retrospective study investigated the relationship between the histological mixed-type of early gastric carcinoma (EGC) in the mucosa and submucosa and lymph node metastasis (LNM). We found that the rates of LNM in the histological mixed-type and the pure undifferentiated type were not significantly different in the mucosal or submucosal EGC. Furthermore, histological type was not an independent risk factor for LNM in mucosa-confined EGC. Hence, according to WHO classification, histological mixed-type and pure undifferentiated EGC could be managed in the same way, and curative endoscopic submucosal dissection is feasible for patients with histological mixed-type mucosa-confined EGC.
- Citation: Zhong Q, Sun Q, Xu GF, Fan XQ, Xu YY, Liu F, Song SY, Peng CY, Wang L. Differential analysis of lymph node metastasis in histological mixed-type early gastric carcinoma in the mucosa and submucosa. World J Gastroenterol 2018; 24(1): 87-95
- URL: https://www.wjgnet.com/1007-9327/full/v24/i1/87.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i1.87
Gastric carcinoma is the second leading cause of cancer-related death behind lung carcinoma, despite a worldwide decline in both its incidence and mortality since the late half of the twentieth century[1]. Endoscopic resection can be curative for selected cases of early gastric cancer (EGC)[2]. Endoscopic submucosal dissection (ESD), a recently developed technique, is a widely accepted treatment modality for EGC, including undifferentiated-type EGC within the expanded criteria (mucosal cancer measuring less than 2 cm without ulceration or lymphovascular tumor emboli). Some studies have shown that there is no risk of lymph node metastasis (LNM) in undifferentiated-type EGC, conforming to the expanded criteria for endoscopic resection[3-5]. The incidence of LNM is the most important factor when deciding on endoscopic resection in ECG[6]. Therefore, the criteria for ESD consist of factors related to the risk of LNM, including histological differentiation and tumor size.
The prognosis of EGC is mainly dependent on histological differentiation, which determines the extent of LNM and can potentially assist in selecting the most suitable treatment strategy[7-9]. However, gastric cancer tissues often present histological heterogeneity and comprise a mixture of several different cell types. Before a treatment has been selected, it is difficult for pathologists to accurately diagnose the histological type of the cancer tissue based on the histological differentiation state definitions as defined by the 14th Japanese classification of gastric carcinoma[10] or the 7th tumor-node-metastasis classification[11]. This is especially the case in mixed-type gastric cancer from several biopsy specimens. Approximately 5%-25% of gastric cancers are classified as having a histological mixed-type in previous studies[12,13], consisting of undifferentiated and differentiated components.
Recently, several studies have reported that mixed-type EGC was associated with aggressive clinical features as well as poor outcomes[12,14]. However, these studies did not consider the different biological behaviors of the mucosal mixed-type EGC and submucosal mixed-type EGC, although patients with submucosal EGC would undoubtedly be at a higher risk of LNM and poor prognosis than those with mucosal EGC. Therefore, the biological behavior of the histological mixed-type in mucosal EGC remains undetermined. In this study, we aimed to clarify the relationship of histological mixed-type EGC and the rate of LNM, and the feasibility of endoscopic resection for patients with mixed-type EGC in the mucosa.
A consecutive series of 298 patients diagnosed with EGC were examined. All patients, including 165 patients with EGC in the mucosa and 133 patients with EGC in the submucosa, underwent curative gastrectomy with lymph node dissection at the Department of Surgery at Nanjing Drum Tower Hospital from January 2005 to January 2012. Patients who were treated with chemotherapy before surgery were excluded from this study. Histopathological examination was performed by expert pathologists. Data on the clinicopathological factors, including age, sex, tumor location, macroscopic appearance, size, presence of stomach ulcer, infection with Helicobacter pylori (H. pylori), lymphovascular invasion, and presence of LNM, were obtained. After surgery, patients were told to visit our outpatient department for esophagogastroduodenoscopy and abdominal pelvic computed tomography, which were performed at 3- and 6-mo intervals during the first year and then annually thereafter. This study was approved by the Institutional Review Board of Nanjing Drum Tower Hospital.
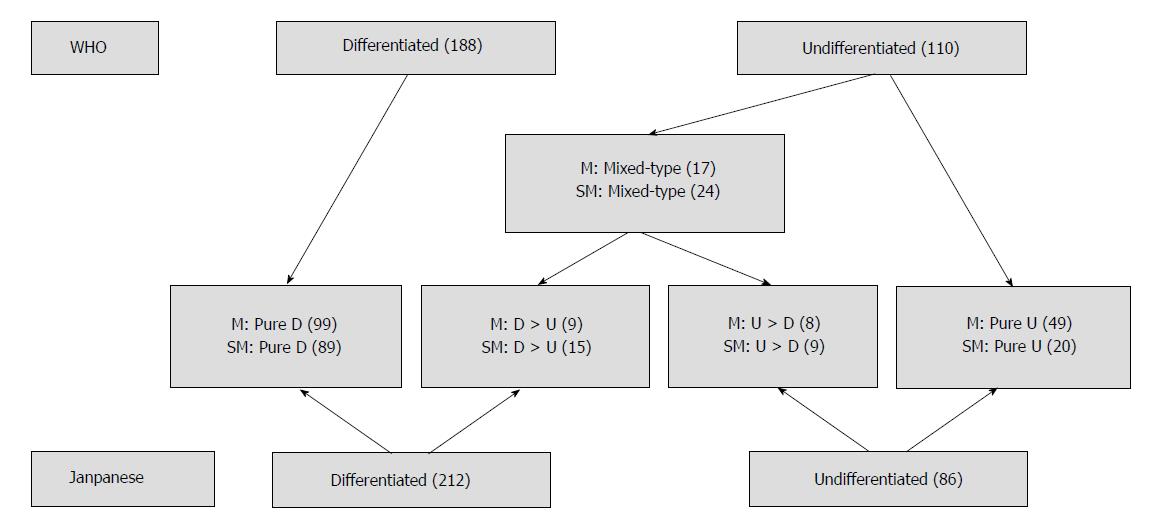
Patients were divided into three subgroups according to the histological features of their tumors: (1) a pure differentiated component with no undifferentiated component (pure D group); (2) a pure undifferentiated component with no differentiated component (pure U group); and (3) a histological mixed-type that consisted of both differentiated and undifferentiated components (mixed-type group). The histological mixed-type includes the differentiated-predominant mixed-type with undifferentiated component making up less 50% (D > U group) and the undifferentiated-predominant mixed-type with undifferentiated component making up more than 50% (U > D group). According to the Japanese classification of gastric carcinoma, the pure D and D > U groups were classified as the differentiated type of gastric cancer, whereas the U > D and pure U groups were classified as the undifferentiated type. On the other hand, according to the tumor-node-metastasis classification, only the pure D group was classified as the differentiated type, and the remaining three groups were classified as the undifferentiated type. The Japanese classification of gastric carcinoma[6] was used to classify the macroscopic type of the tumors as follows: elevated (I, IIa, I and IIa, IIa and IIb), flat (IIb), and depressed (IIc, IIc and III, III). According to the different histological classifications, the cases of EGC in the mucosa and submucosa are shown in Figure 1.
Analysis of variance was used to compare the mean values of the continuous variables among the three different histological subgroups, and the χ2 test was performed on the categorical variables. Bonferroni correction for multiple comparisons suggested differences between two of the three histological subgroups. Multivariate logistic regression analysis was performed to determine the predictors of LNM. The significant difference was set at an alpha level of 0.05. When the Bonferroni correction was applied, the significant difference was set at an alpha level of 0.017, which was 0.05/number of tests[15]. All statistical analyses were performed using SPSS software, version 22.0 (SPSS Inc., Chicago, IL, United States).
The study group consisted of 206 male patients and 92 female patients with a median age of 59.5 years (range, 18-86 years). Seventy-one patients had tumors in the upper third of the stomach and 227 in the middle or lower third. One hundred and six patients had an elevated gross type and 192 patients had a flat or depressed gross type. There were 165 patients with EGC in the mucosa and 133 patients with EGC in the submucosa. Histologically, patients were divided into the following four groups: 188 (63.1%) patients with the pure D histological type, 24 (7.0%) with the D > U histological type, 17 (5.7%) with the U > D histological type, and 69 (23.2%) with the pure U histological type. The overall prevalence of the histological mixed-type, including the D > U and U > D histological types, was 12.7% (41/298) in EGC. The overall incidence of LNM was 14.4% in EGC (Table 1). In a univariate analysis of EGC, LNM was associated with younger age (P = 0.005), female sex (P = 0.044), tumor size (P = 0.022), middle/lower location (P = 0.010), lymphovascular invasion (P = 0.013), flat/depressed gross type (P = 0.034), depth of submucosal invasion (P = 0.001), pure U type (P = 0.005), and mixed-type (P = 0.001). Given that the criteria for ESD treatment of EGC consist of the invasion depth, histological type, tumor size, and ulceration, it is reasonable and meaningful to take these four covariates into a multivariate analysis to assess their main effect on the rate of LNM. The multivariate analysis showed that submucosal invasion (OR = 4.58, 95%CI: 1.23-16.97, P = 0.023), pure U type (OR = 4.97, 95%CI: 1.21-20.39, P = 0.026), and mixed-type (OR = 5.84, 95%CI: 1.05-32.61, P = 0.044) were independent risk factors for LNM in EGC. There was no interaction effect between the invasion depth and histological type (P = 0.822) (Table 2).
| Variable | Value |
| Age (yr), median ± SD (range) | 59.5 ± 12.1 (18-86) |
| Gender | |
| Male | 206 (69.1) |
| Female | 92 (30.9) |
| Tumor size (cm), median ± SD (range) | 2.2 ± 1.2 (0.5-6.0) |
| Location | |
| U | 71 (23.8) |
| M/L | 227 (76.2) |
| Gross type | |
| Elevated | 106 (35.6) |
| Flat/depressed | 192 (64.4) |
| Depth of invasion | |
| M | 165 (55.4) |
| SM | 133 (44.6) |
| Histological type | |
| Pure D | 188 (63.1) |
| D > U | 24 (7.0) |
| U > D | 17 (5.7) |
| Pure U | 69 (23.2) |
| Lymph node metastasis | |
| Present | 43 (14.4) |
| Absent | 255 (85.6) |
| Variable | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age (yr) | 0.005 | |||||
| ≤ 60 | 1.00 | |||||
| > 60 | 0.37 | 0.18-0.74 | ||||
| Gender | 0.044 | |||||
| Male | 1.00 | |||||
| Female | 1.97 | 1.02-3.82 | ||||
| Size (cm) | 1.33 | 1.04-1.71 | 0.022 | 0.154 | ||
| Location | 0.010 | |||||
| U | 1.00 | |||||
| M/L | 4.85 | 1.45-16.19 | ||||
| LV invasion | < 0.001 | |||||
| Absent | 1.00 | |||||
| Present | 18.4 | 8.31-40.72 | ||||
| Gross type | 0.034 | |||||
| Elevated | 1.00 | |||||
| Flat/depressed | 2.32 | 1.07-5.04 | ||||
| Ulcer | 0.031 | 0.256 | ||||
| Absent | 1.00 | |||||
| Present | 2.15 | 1.07-4.31 | ||||
| H. pylori infection | 0.346 | |||||
| Absent | 1.00 | |||||
| Present | 1.46 | 0.67-3.19 | ||||
| Depth of invasion | 0.001 | 0.023 | ||||
| Mucosa | 1.00 | |||||
| Submucosa | 3.41 | 1.70-6.83 | 4.58 | 1.23-16.97 | ||
| Histological type | 0.001 | 0.054 | ||||
| Pure D | 1.00 | |||||
| Pure U | 2.99 | 1.39-6.44 | 0.005 | 4.97 | 1.21-20.39 | 0.026 |
| Mixed-type | 4.45 | 1.91-10.36 | 0.001 | 5.84 | 1.05-32.61 | 0.044 |
| Depth of invasion/histological type | 0.822 | |||||
Table 3 shows the relationship between the clinicopathological factors and histological types, which included the mixed-type, pure undifferentiated type, and pure differentiated type, in mucosal EGC. The distribution of age (P = 0.028), gender (P = 0.004), tumor location (P = 0.003), gross type (P < 0.001), ulceration (P = 0.015), H. pylori (P = 0.025), and LNM (P = 0.016) significantly differed among the three histological subgroups, while tumor size (P = 0.802) and lymphovascular invasion (P = 0.589) were similarly distributed among the three histological subgroups. Patients in the mixed-type group were more likely to have H. pylori infection than those in the pure U group (P = 0.015). Besides tumor size and lymphovascular invasion, age (P = 0.525), gender (P = 0.151), tumor location (P = 0.759), gross type (P = 0.507), and ulceration (P = 0.490) were similarly distributed between the mixed-type group and pure U group. Of note, the rate of LNM was similar between the mixed-type group and the pure U group (P = 0.739), although it was significantly higher in the mixed-type group (P = 0.012) and pure U group (P = 0.010) than in the pure D group. These data suggested that mixed-type group showed similar clinicopathologic features and aggressive behavior compared with the pure U group in mucosal EGC.
| Variable | MT, n = 17 | Pure U, n = 49 | Pure D, n = 99 | P value | P (Bonferroni corrected) |
| Age (yr) | 0.028 | MT vs PU, 0.525 | |||
| ≤ 60 | 10 (58.8) | 33 (67.3) | 44 (44.4) | MT vs PD, 0.272 | |
| > 60 | 7 (41.2) | 16 (32.7) | 55 (55.6) | PU vs PD, 0.009 | |
| Gender | 0.004 | MT vs PU, 0.151 | |||
| Male | 7 (41.2) | 30 (61.2) | 77 (77.8) | MT vs PD, 0.002 | |
| Female | 10 (58.8) | 19 (38.8) | 22 (22.2) | PU vs PD, 0.034 | |
| Tumor size (cm) | 2.1 ± 1.1 | 1.9 ± 1.2 | 1.9 ± 1.2 | 0.802 | |
| Location | 0.003 | MT vs PU, 0.759 | |||
| U | 1 (5.9) | 4 (8.2) | 29 (29.3) | MT vs PD, 0.042 | |
| M/L | 16 (94.1) | 45 (91.8) | 70 (70.7) | PU vs PD, 0.004 | |
| Gross type | < 0.001 | MT vs PU, 0.507 | |||
| Elevated | 4 (23.5) | 8 (16.3) | 51 (51.5) | MT vs PD, 0.033 | |
| Flat/depressed | 13 (76.5) | 41 (83.7) | 48 (48.5) | PU vs PD, < 0.001 | |
| Ulcer | 0.015 | MT vs PU, 0.490 | |||
| Absent | 6 (35.3) | 22 (44.9) | 64 (64.6) | MT vs PD, 0.022 | |
| Present | 11 (64.7) | 27 (55.1) | 35 (35.4) | PU vs PD, 0.022 | |
| H. pylori infection | 0.025 | MT vs PU, 0.015 | |||
| Absent | 1 (5.9) | 18 (36.7) | 22 (22.2) | MT vs PD, 0.119 | |
| Present | 16 (94.1) | 31 (63.3) | 77 (77.8) | PU vs PD, 0.061 | |
| LV invasion | 0.589 | ||||
| Absent | 17 (100.0) | 47 (95.9) | 97 (98.0) | ||
| Present | 0 (0) | 2 (4.1) | 2 (2.0) | ||
| LNM | 0.016 | MT vs PU, 0.739 | |||
| Absent | 14 (82.4) | 42 (85.7) | 96 (97.0) | MT vs PD, 0.012 | |
| Present | 3 (17.6) | 7 (14.3) | 3 (3.0) | PU vs PD, 0.010 |
Table 4 shows the relationship between the clinicopathological factors and histological types, which included the mixed type, pure undifferentiated type, and pure differentiated type in submucosal EGC. Distribution of age (P = 0.008), gender (P < 0.001), tumor location (P = 0.019), gross type (P = 0.028), and LNM (P = 0.008) significantly differed among the three histological subgroups, while tumor size (P = 0.166), ulceration (P = 0.369), H. pylori infection (P = 0.997), and lymphovascular invasion (P = 0.325) were similarly distributed among the three histological subgroups. Besides tumor size, ulceration, H. pylori infection, and lymphovascular invasion, age (P = 0.423), gender (P = 0.069), tumor location (P = 0.128), and gross type (P = 0.199) were similarly distributed between the mixed-type group and pure U group. The rate of LNM was higher in the mixed-type group (P = 0.012) and pure U group (P = 0.009) than in the pure D group, but was not significantly different between the mixed-type and pure U group (P = 0.375). These data suggested that the mixed-type group showed similar clinicopathologic features and aggressive behavior compared with the pure U group in submucosal EGC.
| Variable | MT, n = 24 | Pure U, n = 20 | Pure D, n = 89 | P value | P (Bonferroni corrected) |
| Age (yr) | 0.008 | MT vs PU, 0.423 | |||
| ≤ 60 | 14 (58.3) | 14 (70.0) | 32 (36.0) | MT vs PD, 0.048 | |
| > 60 | 10 (41.7) | 6 (30.0) | 57 (64.0) | PU vs PD, 0.005 | |
| Gender | < 0.001 | MT vs PU, 0.069 | |||
| Male | 15 (62.5) | 7 (35.0) | 70 (78.7) | MT vs PD, 0.104 | |
| Female | 9 (37.5) | 13 (65.0) | 19 (21.3) | PU vs PD, < 0.001 | |
| Size (cm) | 2.6 ± 1.3 | 2.8 ± 1.4 | 2.3 ± 1.1 | 0.166 | |
| Location | 0.019 | MT vs PU, 0.128 | |||
| U | 5 (20.8) | 1 (5.0) | 31 (34.8) | MT vs PD, 0.191 | |
| M/L | 19 (79.2) | 19 (95.0) | 58 (65.2) | PU vs PD, 0.008 | |
| Gross type | 0.028 | MT vs PU, 0.199 | |||
| Elevated | 6 (25.0) | 2 (10.0) | 35 (39.3) | MT vs PD, 0.195 | |
| Flat/depressed | 18 (75.0) | 18 (90.0) | 54 (60.7) | PU vs PD, 0.012 | |
| Ulcer | 0.369 | ||||
| Absent | 5 (20.8) | 7 (35.0) | 32 (36.0) | ||
| Present | 19 (79.2) | 13 (65.0) | 57 (64.0) | ||
| H. pylori infection | 0.997 | ||||
| Absent | 7 (29.2) | 6 (30.0) | 26 (29.2) | ||
| Present | 17 (70.8) | 14 (70.0) | 63 (70.8) | ||
| LV invasion | 0.325 | ||||
| Absent | 15 (62.5) | 15 (75.0) | 69 (77.5) | ||
| Present | 9 (37.5) | 5 (25.0) | 20 (22.5) | ||
| LNM | 0.008 | MT vs PU, 0.375 | |||
| Absent | 15 (62.5) | 12 (60.0 ) | 76 (85.4) | MT vs PD, 0.012 | |
| Present | 9 (37.5) | 8 (40.0) | 13 (14.6) | PU vs PD, 0.009 |
In a univariate analysis of mucosa-confined EGC, LNM was associated with pure undifferentiated type (P = 0.019), mixed-type (P = 0.026), female sex (P = 0.005), and presence of lymphovascular invasion (P = 0.013). However, in a multivariate analysis, only female sex (OR = 5.83, 95%CI: 1.64-20.70, P = 0.028) and presence of lymphovascular invasion (OR = 13.18, 95%CI: 1.39-125.30, P = 0.020) were independent risk factors for LNM of mucosa-confined EGC, while histological type was not an independent risk factor for LNM of mucosa-confined EGC (P = 0.106) (Table 5).
| Variable | Univariate analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age (yr) | 0.082 | |||||
| ≤ 60 | 1.00 | |||||
| > 60 | 0.31 | 0.08-1.16 | ||||
| Size (cm) | 1.36 | 0.90-2.06 | 0.145 | |||
| Gender | 0.005 | 0.028 | ||||
| Male | 1.00 | 1.00 | ||||
| Female | 5.89 | 1.72-20.17 | 5.83 | 1.64-20.70 | ||
| Location | 0.630 | |||||
| U | 1.00 | |||||
| M/L | 1.47 | 0.31-6.95 | ||||
| LV invasion | 0.013 | 0.020 | ||||
| Absent | 1.00 | 1.00 | ||||
| Present | 13.64 | 1.75-106.28 | 13.18 | 1.39-125.30 | ||
| Gross type | 0.253 | |||||
| Elevated | 1.00 | |||||
| Flat/depressed | 2.17 | 0.58-8.22 | ||||
| Ulcer | 0.199 | |||||
| Absent | 1.00 | |||||
| Present | 2.14 | 0.67-6.85 | ||||
| H. pylori infection | 0.244 | |||||
| Absent | 1.00 | |||||
| Present | 0.50 | 0.15-1.61 | ||||
| Histological type | 0.035 | 0.106 | ||||
| Pure D | 1.00 | |||||
| Pure U | 5.33 | 1.32-21.63 | 0.019 | 4.46 | 0.99-20.00 | 0.051 |
| Mixed-type | 6.86 | 1.26-37.77 | 0.026 | 5.31 | 0.86-33.01 | 0.073 |
Endoscopic resection is a curative modality for EGC and its indications have been expanded[16,17]. However, histological mixed-type is not categorized in the present criteria for ESD. In this study, we found that submucosal invasion, pure U type, and mixed-type were independent risk factors for LNM in EGC. However, the distribution of tumor size, location, gross type, ulceration, lymphovascular invasion, and LNM rate were similar between the mixed-type group and pure U group in mucosa-confined EGC, and histological mixed-type was not an independent risk factor for LNM in mucosal EGC.
EGC generally shows greater histological diversity than other types of cancer. Even tumors confined to the mucosa show histologic diversity, which tends to increase with deeper invasion and increased tumor diameter[18]. In addition, histological mixed-type has been reported to have a higher LNM rate and more aggressive behavior than other histological types[19]. New difficulties in determining the appropriate management for histological mixed-type EGC arose as a result of improvements in ESD, which allows en bloc resection of large superficial gastric lesions and precise histopathological evaluation[20]. In fact, there are two distinct groups of differentiated and undifferentiated mixed-type EGC. The first group shows both differentiated and undifferentiated histological components in the mucosa. The second group shows differentiated components confined in the mucosa and undifferentiated components confined in the submucosa. We hypothesized that these two groups had different prognoses and should be evaluated and managed independently. In our study, we found that submucosal invasion, pure U type, and mixed-type were independent risk factors for LNM in EGC. We next analyzed the clinicopathologic features of mucosal and submucosal EGC according to the histological type. The mixed-type group and pure U group showed similar clinicopathologic features and aggressive behavior in mucosal EGC, including the distribution of age, gender, tumor size, location, gross type, ulceration, lymphovascular invasion, and LNM rate. Furthermore, we validated that histological type was not an independent risk factor for LNM in mucosal EGC. Given the mucosal undifferentiated EGC has been included in the expanded criteria for ESD, we recommended that mucosal mixed-type EGC could be treated with ESD. For submucosal EGC, the mixed-type group also showed similar clinicopathologic features and aggressive behavior compared with the pure U group. Submucosal undifferentiated EGC has not been included in the criteria for ESD, due to the high frequency of LNM. Several studies have shown that mixed-type EGC with submucosal invasion carries a high risk of LNM, and endoscopic surgery should be limited to the differentiated type of invasive submucosal EGC without histological heterogeneity[20,21]. Our data supported that the LNM rate of submucosal EGC was significantly higher in the mixed-type group (9/24) and pure U group (8/20) than in the pure D group (13/89). In accordance with the 7th tumor-node-metastasis classification, the mixed-type group and pure U group, including undifferentiated components, were all undifferentiated EGC[22]. Hence, the decision criterion of histological mixed-type in tumor-node-metastasis classification is better than Japanese classification[23].
A study from Shimizu et al[23] showed that there was no significant difference in the clinicopathological factors between the D > U and U > D groups[23]. This means that the mixture of differentiated and undifferentiated components contributes to a worse prognosis, no matter the ratio of undifferentiated components to differentiated components. Actually, the pathogenesis and aggressiveness of mixed histology are unclear. Zheng et al[24] suggested that the mixed-type components might originate from stem cells with similar genetic traits, but follow different histogenic pathways. Park et al[25] found that mixed-type gastric carcinoma frequently showed an enhanced CpG island hypermethylation status, implicating enhanced CpG island promoter hypermethylation in the histogenesis of mixed-type carcinoma. Studies of the mucin phenotype have reported that some cases of differentiated gastric cancer with a gastric phenotype are transformed into undifferentiated gastric cancer during tumor growth and development, increasing the risk of LNM[26]. Although mixed-type carcinomas are thought to be more aggressive in some studies according to the Lauren classification[24,27], a recent study reported that mixed histology, according to WHO classification, showed no LNM within the criteria for endoscopic resection[13]. Our data supported that the rate of LNM was not significantly different in the mixed-type and pure undifferentiated EGC confined in the mucosa. Limited by the small sample sizes, our data showed considerably wide confidence interval of the OR ratio of lymphovascular invasion, in the univariate and multivariate analyses of mucosal EGC. Although similar data were reported in some studies[28,29], it should be confirmed by additional clinical research with larger sample sizes.
In conclusion, our results demonstrated that the histological mixed-type showed a similar low rate of LNM compared with the pure undifferentiated type in mucosa-confined EGC, and histological type was not an independent risk factor for LNM in mucosa-confined EGC. According to the tumor-node-metastasis classification, mucosal histological mixed-type and undifferentiated EGC could be managed in the same way, and curative ESD is feasible for patients with histological mixed-type EGC confined in the mucosa.
Endoscopic submucosal dissection (ESD) is a curative modality for mucosal-confined early gastric cancer (EGC) and its indications have been expanded. However, EGC tissues often present histological heterogeneity and comprise a mixture of several different cell types. The present criteria for ESD does not take consideration of the histological mixed-type EGC. The rate of lymph node metastasis (LNM) is the most important factor when deciding on endoscopic resection in ECG. Therefore, there is a current pressing need to clarify the relationship of histological mixed-type and the rate of LNM, and the feasibility of endoscopic resection for patients with histological mixed-type EGC confined in the mucosa.
According to the latest research, histological mixed-type EGC was associated with aggressive clinical features as well as poor outcomes, but these studies did not consider the different biological behaviors of the mucosal mixed-type EGC and submucosal mixed-type EGC. Given the mucosal undifferentiated EGC measuring less than 2 cm without ulceration has been included in the expanded criteria for ESD treatment, it is necessary to assess the feasibility of endoscopic resection for patients with histological mixed-type EGC confined in the mucosa.
In this study, we investigated the clinicopathologic features of EGC according to histological type classification to evaluate the biological behavior of mixed-type EGC and the predictive value of histological type on the rate of LNM in mucosa-confined EGC.
This study included 298 patients who underwent gastrectomy for EGC between 2005 and 2012. Enrolled lesions were divided into groups of pure differentiated (pure D), pure undifferentiated (pure U), and mixed-type according to the proportion of the differentiated and undifferentiated components observed under a microscope. We reviewed the clinicopathological features, including age, sex, location, size, gross type, lymphovascular invasion, ulceration, and LNM, among the three groups. Furthermore, we evaluated the risk factors for LNM in mucosal EGC.
Submucosal invasion, pure U type, and mixed-type were independent risk factors for LNM in EGC. The rate of LNM in mucosa-confined EGC was higher in the mixed-type group and pure U group than in the pure D group, but no significant difference was found between the mixed-type group and pure U group. Similarly, the rate of LNM in the submucosa-invasive EGC was higher in the mixed-type and pure U group than in the pure D group, but was not significantly different between the mixed-type and pure U group. Multivariate logistic analysis showed that histological type was not an independent risk factor for LNM in mucosa-confined EGC.
The distribution of tumor size, location, gross type, ulceration, lymphovascular invasion, and LNM rate were similar between the mixed-type group and pure undifferentiated group in mucosa-confined EGC, and the histological mixed-type was not an independent predictor of LNM in mucosa-confined EGC. According to the tumor-node-metastasis classification, the histological mixed-type and undifferentiated EGC could be managed in the same way, and curative ESD was feasible for patients with mucosal histological mixed-type EGC.
Endoscopic resection is a curative modality for EGC and its indications have been expanded. Our study indicated that the mucosal histological mixed-type EGC could be managed with curative ESD. With the gradual understanding of the pathogenesis and biological behavior of mixed-type EGC, more patients with mixed-type EGC would benefit from the ESD treatment.
| 1. | Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (2)] |
| 2. | Goto O, Fujishiro M, Kodashima S, Ono S, Omata M. Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy. 2009;41:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Shim CN, Lee SK. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: do we have enough data to support this? World J Gastroenterol. 2014;20:3938-3949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (2)] |
| 4. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 514] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 5. | Lee S, Choi KD, Han M, Na HK, Ahn JY, Jung KW, Lee JH, Kim DH, Song HJ, Lee GH. Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer meeting expanded indication including undifferentiated-type tumors: a criteria-based analysis. Gastric Cancer. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 370] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 7. | Komatsu S, Ichikawa D, Miyamae M, Shimizu H, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Kishimoto M, Otsuji E. Histological mixed-type as an independent prognostic factor in stage I gastric carcinoma. World J Gastroenterol. 2015;21:549-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, Sohn TS, Bae JM, Kim KM, Ahn JH. Long-Term Outcome of Endoscopic Resection vs. Surgery for Early Gastric Cancer: A Non-inferiority-Matched Cohort Study. Am J Gastroenterol. 2016;111:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 159] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 9. | Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Miwa S, Tsuneyama K, Takano Y. Pathobiological characteristics of intestinal and diffuse-type gastric carcinoma in Japan: an immunostaining study on the tissue microarray. J Clin Pathol. 2007;60:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2946] [Article Influence: 196.4] [Reference Citation Analysis (1)] |
| 11. | Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721-1724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 643] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 12. | Miyamae M, Komatsu S, Ichikawa D, Kosuga T, Kubota T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Kishimoto M. Histological mixed-type as an independent risk factor for nodal metastasis in submucosal gastric cancer. Tumour Biol. 2016;37:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Yoon HJ, Kim YH, Kim JH, Kim H, Kim H, Park JJ, Youn YH, Park H, Kim JW, Hyung WJ. Are new criteria for mixed histology necessary for endoscopic resection in early gastric cancer? Pathol Res Pract. 2016;212:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Hwang CS, Ahn S, Lee BE, Lee SJ, Kim A, Choi CI, Kim DH, Jeon TY, Kim GH, Song GA. Risk of lymph node metastasis in mixed-type early gastric cancer determined by the extent of the poorly differentiated component. World J Gastroenterol. 2016;22:4020-4026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Blankenburg M, Boekens H, Hechler T, Maier C, Krumova E, Scherens A, Magerl W, Aksu F, Zernikow B. Reference values for quantitative sensory testing in children and adolescents: developmental and gender differences of somatosensory perception. Pain. 2010;149:76-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 16. | Ahn JY, Jung HY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Song HJ, Lee GH. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc. 2011;74:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 17. | Jeon HK, Lee SJ, Kim GH, Park DY, Lee BE, Song GA. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: short- and long-term outcomes. Surg Endosc. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Luinetti O, Fiocca R, Villani L, Alberizzi P, Ranzani GN, Solcia E. Genetic pattern, histological structure, and cellular phenotype in early and advanced gastric cancers: evidence for structure-related genetic subsets and for loss of glandular structure during progression of some tumors. Hum Pathol. 1998;29:702-709. [PubMed] |
| 19. | Takizawa K, Ono H, Kakushima N, Tanaka M, Hasuike N, Matsubayashi H, Yamagichi Y, Bando E, Terashima M, Kusafuka K. Risk of lymph node metastases from intramucosal gastric cancer in relation to histological types: how to manage the mixed histological type for endoscopic submucosal dissection. Gastric Cancer. 2013;16:531-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 20. | Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009;41:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Mita T, Shimoda T. Risk factors for lymph node metastasis of submucosal invasive differentiated type gastric carcinoma: clinical significance of histological heterogeneity. J Gastroenterol. 2001;36:661-668. [PubMed] |
| 22. | Wittekind C. [2010 TNM system: on the 7th edition of TNM classification of malignant tumors]. Pathologe. 2010;31:331-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Shimizu H, Ichikawa D, Komatsu S, Okamoto K, Shiozaki A, Fujiwara H, Murayama Y, Kuriu Y, Ikoma H, Nakanishi M. The decision criterion of histological mixed type in “T1/T2” gastric carcinoma--comparison between TNM classification and Japanese Classification of Gastric Cancer. J Surg Oncol. 2012;105:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Zheng HC, Li XH, Hara T, Masuda S, Yang XH, Guan YF, Takano Y. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch. 2008;452:525-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Park SY, Kook MC, Kim YW, Cho NY, Kim TY, Kang GH. Mixed-type gastric cancer and its association with high-frequency CpG island hypermethylation. Virchows Arch. 2010;456:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Saito A, Shimoda T, Nakanishi Y, Ochiai A, Toda G. Histologic heterogeneity and mucin phenotypic expression in early gastric cancer. Pathol Int. 2001;51:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, Sohn TS, Bae JM, Kim KM, Yeon S. Early gastric cancer with a mixed-type Lauren classification is more aggressive and exhibits greater lymph node metastasis. J Gastroenterol. 2017;52:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Lee JH, Choi IJ, Han HS, Kim YW, Ryu KW, Yoon HM, Eom BW, Kim CG, Lee JY, Cho SJ. Risk of lymph node metastasis in differentiated type mucosal early gastric cancer mixed with minor undifferentiated type histology. Ann Surg Oncol. 2015;22:1813-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 29. | Huo ZB, Chen SB, Zhang J, Li H, Wu DC, Zhai TS, Luan SF. Risk clinicopathological factors for lymph node metastasis in poorly differentiated early gastric cancer and their impact on laparoscopic wedge resection. World J Gastroenterol. 2012;18:6489-6493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Goral V, Vinh-Hung V, Vorobjova T S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Li D