Published online Mar 7, 2017. doi: 10.3748/wjg.v23.i9.1657
Peer-review started: October 26, 2016
First decision: December 1, 2016
Revised: December 28, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: March 7, 2017
Processing time: 131 Days and 14.3 Hours
To investigated the usefulness of a novel slim type ball-tipped FlushKnife (FlushKnife-BTS) over ball-tipped FlushKnife (FlushKnife-BT) in functional experiments and clinical practice.
In order to evaluate the functionality of FlushKnife-BTS, water aspiration speed, resistance to knife insertion through the scope, and waterjet flushing speed were compared between FlushKnife-BTS and BT. In clinical practice, esophageal endoscopic submucosal dissection (ESD) performed using FlushKnife-BTS or BT by an experienced endoscopist between October 2015 and January 2016 were retrospectively reviewed. The treatment speed and frequency of removing and reinserting the knife to aspirate fluid and air during ESD sessions were analyzed.
Functional experiments revealed that water aspiration speed by the endoscope equipped with a 2.8-mm working channel with FlushKnife-BTS was 7.7-fold faster than that with conventional FlushKnife-BT. Resistance to knife insertion inside the scope with a 2.8-mm working channel was reduced by 40% with FlushKnife-BTS. The waterjet flushing speed was faster with the use of FlushKnife-BT. In clinical practice, a comparison of 6 and 7 ESD using FlushKnife-BT and BTS, respectively, revealed that the median treatment speed was 25.5 mm2/min (range 19.6-30.3) in the BT group and 44.2 mm2/min (range 15.5-55.4) in the BTS group (P = 0.0633). However, the median treatment speed was significantly faster with FlushKnife-BTS when the resection size was larger than 1000 m2 (n = 4, median 24.2 mm2/min, range 19.6-27.7 vs n = 4, median 47.4 mm2/min, range 44.2-55.4, P = 0.0209). The frequency of knife replacement was less in the BTS group (median 1.76 times in one hour, range 0-5.45) than in the BT group (7.02 times in one hour, range 4.23-15) (P = 0.0065).
Our results indicate that FlushKnife-BTS enhances the performance of ESD, particularly for large lesions, by improving air and fluid aspiration and knife insertion during ESD and reducing the frequency of knife removal and reinsertion.
Core tip: Devices utilized in endoscopic submucosal dissection (ESD) play an important role in facilitating the safe and effective procedure. A novel slim type ball-tipped FlushKnife (FlushKnife-BTS) has been developed to enhance the performance of aspiration and insertion of the knife through the scope. We herein investigated the usefulness of FlushKnife-BTS over FlushKnife-BT in functional experiments and clinical practice. FlushKnife-BTS showed a faster water aspiration speed, reduced resistance to knife insertion, a faster treatment speed when the resection size was large, and low frequency of knife replacement. Our results indicate that FlushKnife-BTS supports the efficient performance of ESD, particularly for large lesions.
- Citation: Ohara Y, Toyonaga T, Hoshi N, Tanaka S, Baba S, Takihara H, Kawara F, Ishida T, Morita Y, Umegaki E, Azuma T. Usefulness of a novel slim type FlushKnife-BT over conventional FlushKnife-BT in esophageal endoscopic submucosal dissection. World J Gastroenterol 2017; 23(9): 1657-1665
- URL: https://www.wjgnet.com/1007-9327/full/v23/i9/1657.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i9.1657
Endoscopic submucosal dissection (ESD) is a standard treatment for early-stage tumors in the digestive tract[1-6]. Many devices have been developed to safely and efficiently facilitate this procedure[7-14]. One of these devices is FlushKnife and the subsequently developed ball-tipped FlushKnife (FlushKnife-BT) (DK2618JN, DK2618JB; Fujifilm Medical Co., Ltd., Tokyo, Japan)[13,14]. Some of the features and functions of these knives are advantageous such as injection, irrigation by waterjets, dissection, hemostasis, and vessel sealing[15]. However, when they are used with endoscopes equipped with a working channel of 2.8 mm, difficulties are associated with aspirating fluid and air in the working space and finely controlling the knife length due to limited space with friction resistance in the channel with a knife with a diameter of 2.7 mm.
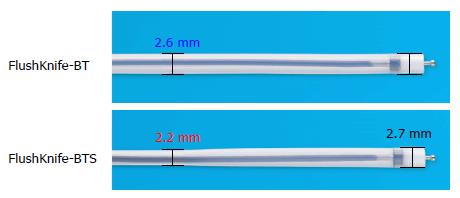
Therefore, a novel slim type FlushKnife BT (FlushKnife-BTS, DK2620JBS; Fujifilm) was developed to provide more space in the working channel, (Figure 1). FlushKnife-BTS is characterized by its slim sheath (2.2 mm), but same sized sheath tip (2.7 mm, approximately 30 mm long) as the conventional knife, which maintains stable maneuverability.
In an attempt to clarify whether the performance of ESD is better with FlushKnife-BTS than with FlushKnife-BT, we retrospectively investigated the usefulness of FlushKnife-BTS over FlushKnife-BT in functional experiments and clinical practice. In the clinical practice, we included esophageal ESD because esophagus is a narrow tract compared to stomach and colorectum, which procedure is affected easily by air inflation, and was thought to be a good candidate for first investigation into the efficiency of FlushKnife-BTS.
Water aspiration speed with the knife inserted in the scope, resistance to knife insertion inside the scope, and waterjet flushing speed were compared between FlushKnife-BTS and FlushKnife-BT. Regarding the speed of water absorption, a total of 200 mL water in a graduated cylinder was aspirated using the 2.8-mm scope and 3.2-mm channel with FlushKnife-BT or FlushKnife-BTS inserted, and the amount of water aspirated in 10 s was measured. The experiment was repeated 9 times. Resistance to the insertion of Flushknife-BT or Flushknife-BTS inside the scope was measured with various endoscopic angles. A measuring instrument named force gage FGP-5 produced by NIDEC-SHIMPO CORPORATION was equipped with the FlushKnife-BTS and FlushKnife-BT, inserted into the endoscope equipped with a working channel of 2.8 mm and the resistance during the insertion was determined. The experiment was repeated 3 times. The waterjet flushing speeds of the knives were measured at three different water pressure settings 9 times using waterjet equipment (JW-2; Fujifilm, waterjet volume; min/mid/max 80/135/190 mL/min).
All cases that underwent esophageal ESD performed using FlushKnife-BTS or FlushKnife-BT at Kobe University Hospital and Kishiwada Tokushukai Hospital between October 2015 and January 2016 were retrospectively reviewed. Of these, cases that underwent ESD performed by an experienced endoscopist (T.T.) were analyzed in the study.
Indications for esophageal ESD were defined according to the esophageal ESD guidelines issued by the Japan Esophageal Society[16]: (1) absolute criteria; lesions that do not exceed the mucosal layer (T1a), those remaining within the mucosal epithelium (EP) or the lamina propria mucosae (LPM), and (2) relative criteria; lesions reaching the muscularis mucosae (MM) or slightly infiltrating the submucosa (up to 200 μm, T1b-SM1). ESD was not recommended for a 10%-15% risk of lymph node metastasis with lesions filling the relative criteria; however, it was performed on patients who were too frail to tolerate more invasive surgical approaches due to comorbidities, those who requested a diagnostic endoscopic treatment before surgery, or those who requested ESD with chemoradiation.
All patients underwent an initial screening examination including esophagogastroduodenoscopy with magnified narrow band imaging and computed tomography in order to evaluate submucosal invasion and lymph node metastasis. When submucosal invasion was suspected, endoscopic ultrasonography was performed.
Patients were mainly sedated using dexmedetomidine hydrochloride, flunitrazepam, and pentazocine. ESD was performed with a single-channel endoscope equipped with a working channel of 2.8 mm (GIF-Q240, H290Z; Olympus Corporation, Tokyo, Japan). ERBE VIO 300 D high performance cautery (Erbe Elektromedizin GmbH, Tubingen, Germany) was utilized in all cases. Carbon dioxide insufflation was routinely used.
The knife was removed and reinserted to aspirate fluid and air when required, to clean the endoscope lens for better visualization, and/or when the thread-traction method was needed[17].
The patient and lesion characteristics including sex, age, lesion site, circumference of the resected area, major axis diameter of the resected specimen, size of resected area, major axis diameter of the tumor, histology of the tumor and depth of the tumor were investigated.
Furthermore, the procedure time, treatment speed, frequency of knife removal and reinsertion for aspiration, en bloc resection rate, and adverse events were assessed and compared between the FlushKnife-BTS (BTS) group and FlushKnife-BT (BT) group.
The procedure time was defined as the time between the first submucosal injection and completion of dissection. The treatment speed was calculated by dividing the area of the resected specimen by the procedure time (cm2/min). The approximate oval area (cm2) of the resected specimen was calculated as follows; 3.14 × 0.25 × long axis diameter × short axis diameter.
Perforation and postoperative bleeding were counted as adverse events related to the procedure.
Postoperative bleeding was recorded if one of the following conditions was identified: (1) bleeding that required endoscopic hemostatic treatment; and (2) bleeding with a reduction in total hemoglobin of more than 2 g/dL from the preoperative level. Perforation was diagnosed by endoscopic findings during ESD or by the presence of free air on computed tomography.
All patients were informed of the risks and benefits of ESD, and provided written informed consent to undergo the procedure. This study was approved by the Ethics Committees of Kobe University Hospital (No. 160051) and Kishiwada Tokushukai Hospital (No. 28-10).
The Mann-Whitney U test was used to compare continuous variables, and the χ2 test or Fisher's exact probability test was used to compare categorical variables. P < 0.05 was considered significant. All statistical analyses were performed using JMP version 10 (SAS Institute Inc., Cary, NC, United States).
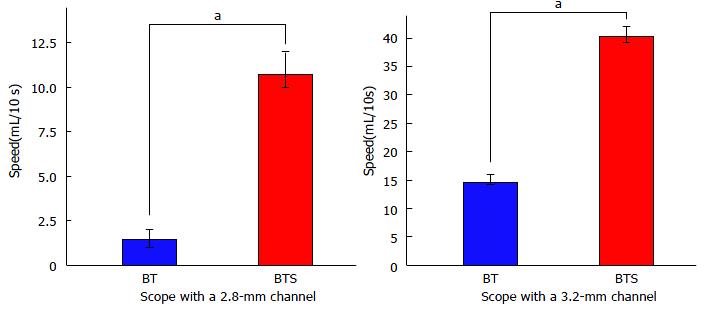
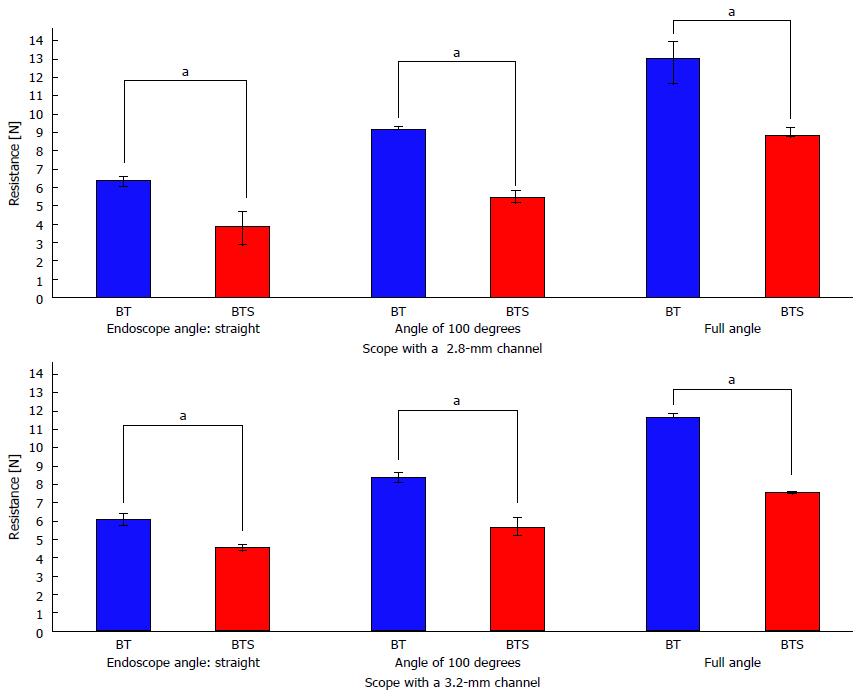
The results of the functional experiments revealed that water aspiration speed by the endoscope equipped with a 2.8-mm working channel with FlushKnife-BTS inserted was 7.7-fold faster than that with FlushKnife-BT (Figure 2). Even when using an endoscope with a 3.2-mm working channel, water aspiration speed was 2.7-fold faster with FlushKnife-BTS than with FlushKnife-BT. Resistance to knife insertion through the 2.8-mm working channel in a straight endoscopic position was 40% less using the new slim knife than the conventional knife (Figure 3). Reductions in resistance were detected using both scopes with 2.8-mm and 3.2-mm working channels and at any endoscopic angle. Waterjet flushing speed was lower with FlushKnife-BTS than with FlushKnife-BT at all water pressure settings tested (Figure 4). This difference became larger with increases in water pressure.
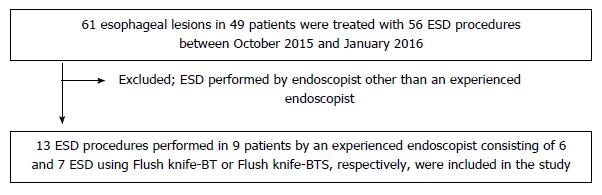
During the study period, 61 esophageal lesions in 49 patients were treated with 56 ESD procedures. Of these, 13 ESD procedures performed in 9 patients, consisting of 6 and 7 ESD using FlushKnife-BT and FlushKnife-BTS, respectively, were completed by an experienced endoscopist and these cases were included in the study (Figure 5).
The characteristics of the patients and lesions are shown in Table 1. There were 3 males and 1 female in the BT group, and 4 males and 1 female in the BTS group. Two patients in the BT group and one patient in the BTS group had multiple lesions that were treated separately. Ages ranged between 68 and 78 years with a median age of 73.5 years in the BT group, and between 57 and 74 years with a median age of 57 years in the BTS group (P = 0.0235). Lesion sites (Ut/Mt/Lt) were 0/4/2 and 0/6/1, respectively (P = 0.559).
| FlushKnife-BT (n = 6) | FlushKnife-BTS (n = 7) | P vaule1 | |
| Male/Female | 32/12 | 42/1 | 0.559 |
| Age, median (range), years | 73.5 (68-78) | 57 (57-74) | 0.0235 |
| Lesion site Ut/Mt/Lt | 0/4/2 | 0/6/1 | 0.559 |
| Circumference of the resected area, median (range) | 68% (25-100) | 75% (50-92) | 0.599 |
| Major axis diameter of the resected specimen, median (range), mm | 39.5 (30-55) | 40 (31-60) | 0.277 |
| Resected area, median (range), mm² | 1117 (636-2547) | 1193 (511-2418) | 0.943 |
| Major axis diameter of the tumor3, median (range), mm | 33 (19-42) | 32 (25-50) | 0.616 |
| Histology of the tumor HGIN/SCC | 0/6 | 0/7 | 0.000 |
| Depth of the tumor EP/LPM/MM | 0/5/1 | 1/5/1 | 0.629 |
The circumference of the resected area ranged between 25% and 100% with a median of 68% in the BT group, and between 50% and 92% with a median of 75% in the BTS group (P = 0.599).
The median major axis diameters of resected specimens were 39.5 mm (range 30-55) and 40 mm (range 31-60) respectively (P = 0.277) in BT and BTS groups, respectively.
The median resected area was 1117 mm2 (range 636-2547) in the BT group and 1193 mm2 (range 511-2418) in the BTS group (P = 0.943).
The median major axis diameter of tumors was 33 mm (range 19-42) in the BT group and 32 mm (range 25-50) in the BTS group (P = 0.616). In the case of multiple lesions resected together, the maximum distance between the edges of each tumor was defined as the tumor size.
The histology of tumors revealed SCC in all cases. The depths of tumors (EP/LPM/MM) were 0/5/1 in the BT group and 1/5/1 in the BTS group (P = 0.629). The outcomes of ESD are shown in Table 2. The thread-traction method was performed on 3 out of 6 cases in the BT group and 2 out of 7 cases in the BTS group (P = 0.592). Median procedure times were 48 min (range 24-106) and 33 (range 27-51) min in the BT and BTS groups, respectively (P = 0.389).
| FlushKnife-BT (n = 6) | FlushKnife-BTS (n = 7) | P vaule1 | |
| Use of the thread-traction method, yes/no | 3/3 | 2/5 | 0.592 |
| Procedure time, median (range), min | 48 (24-106) | 33 (27-51) | 0.389 |
| Treatment speed, median (range), mm2/min | 25.5 (19.6-30.3) | 44.2 (15.5-55.4) | 0.0633 |
| Number of times the knife was replaced, median (range), times | 5.5 (4-10) | 1 (0-3) | 0.0025 |
| Frequency of knife replacement, median (range), times/hour | 7.02 (4.23-15) | 1.76 (0-5.45) | 0.0065 |
| En bloc resection rate | 6/6 (100%) | 7/7 (100%) | 0.000 |
| Adverse events, Perforation/muscle injury/postoperative bleeding | 0/1/0 | 0/0/0 | 0.462 |
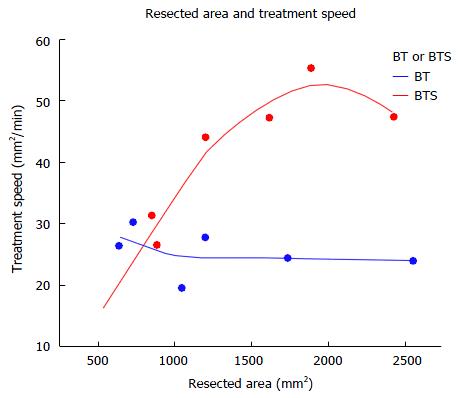
The median treatment speed was 25.5 mm2/min (range 19.6-30.3) in the BT group and 44.2 mm2/min (range 15.5-55.4) in the BTS group (P = 0.0633). However, it was significantly faster with FlushKnife-BTS when the resection size of ESD was larger than 1000 m2 (n = 4, median 24.2, range 19.6-27.7 vs n = 4, median 47.4 mm2/min, range 44.2-55.4, P = 0.0209) (Table 3). The relationship between the resected area and treatment speed is shown in Figure 6; the treatment speed was faster with FlushKnife-BTS when the resected size was large, but was similar to that with FlushKnife-BT when it was small.
| FlushKnife-BT (n = 4) | FlushKnife-BTS (n = 4) | P vaule | |
| Treatment speed, median (range), mm2/min | 24.4 (19.6-27.7) | 47.4 (44.2-55.4) | 0.0209 |
The number of times the knife was replaced was 5.5 (range 4-10) and 1 (range 0-3) in the BT and BTS groups, respectively (P = 0.0025).
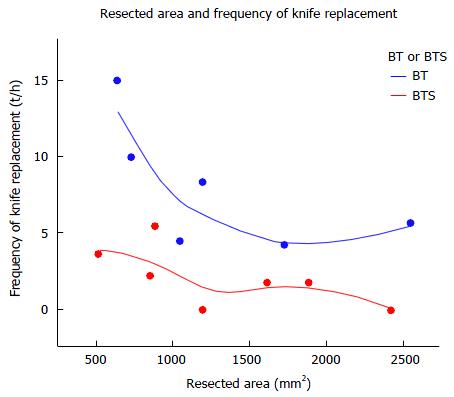
The frequency of knife replacement was lower (median 1.76, range 0-5.45 vs median 7.02, range 4.23-15 times in one hour, P = 0.0065) with FlushKnife-BTS than with FlushKnife-BT. Figure 7 shows the relationship between the resected area and frequency of knife replacement, and demonstrates a reduced frequency of knife replacement with FlushKnife-BTS regardless of the resected size and slightly reduced frequency as the resected size became larger. En bloc resection rates were 100% and muscle injury was detected in one case in the BT group.
ESD is the first-line therapeutic option for superficial gastrointestinal tract tumors[1,6,18-20]. The devices used in ESD are important for performing the procedure safely. A large number of devices have been developed to date[8-10,12,13]. FlushKnife-BT is one of the most frequently used knives and has advantageous functions including injection, irrigation by waterjets, dissection, and vessel sealing[14]. However, when this knife is used with endoscopes equipped with a 2.8-mm channel, difficulties are associated with the aspiration of fluid and mucus in the working space during the procedure and finely controlling the knife length because of its diameter of 2.7 mm. Uncontrolled fluid and mucus pooling and air inflation/deflation may complicate ESD. In order to precisely dissect the appropriate plane between the vessel network in the submucosa and muscle layer[21], subtle endoscope movements in addition to adjustments in knife length are needed. Therefore, smooth aspiration and delicate knife control are essential for performing this procedure safely and efficiently.
In an attempt to overcome these limitations, we developed a novel slim type FlushKnife-BT.
Functional experiments revealed that fluid aspiration speed by the endoscope with a 2.8-mm working channel with FlushKnife-BTS inserted was 7.7-fold faster than that with the conventional knife. Resistance to the insertion of the knife inside the scope with a 2.8-mm working channel was 40% less with the new knife than with the conventional knife.
In clinical practice, though the number of the patients was small, increase was achieved in the treatment speed with FlushKnife-BTS when large resection was required, but remained the same as that with the conventional knife when the resected size was small. The frequency of knife replacement was less with FlushKnife-BTS than with FlushKnife-BT regardless of the resected size.
The faster aspiration of bubbles, air, mucus, and fluid by FlushKnife-BTS contributed to a clear field of view, which may have, in turn, reduced the frequency of knife removal from the working channel.
The reason why treatment speed only improved with FlushKnife-BTS when the resection size was large may be that, in ESD of a small resection size, the effects by a reduced frequency of knife replacement, smooth knife insertion, and fine knife control with FlushKnife-BTS were not clearly reflected due to the short procedure time and fewer knife replacements, but became more evident as the resection size became larger and the procedure time increased. Therefore, FlushKnife-BTS is considered to exhibit its effectiveness when large resection is needed.
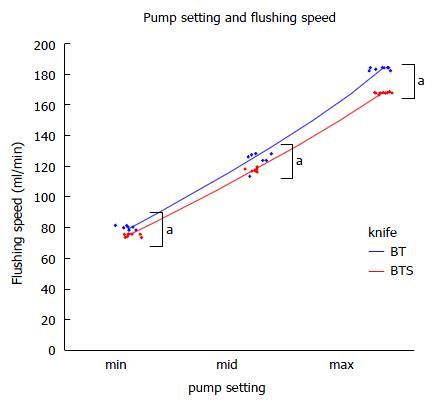
Waterjet flushing speed was slower with FlushKnife-BTS in functional experiments. This result was expected due to the difference in the diameters of the two knives. However, this did not markedly affect the clinical practice of ESD in our analysis. Since FlushKnife-BT offers a high-flow flushing function, the speed reductions observed with FlushKnife-BTS do not appear to be of clinical importance. Moreover, the mid pump setting is typically used in clinical practice and may be resolved by turning up the setting to its maximum where necessary.
Although the safety and efficacy of ESD in the esophagus have already been reported[2,6,22], intraoperative perforation, muscle layer damage, and bleeding may occur because of the anatomically thin wall and narrow working space. Moreover, postoperative esophageal stricture is one of the main complications associated with large esophageal ESD[23,24]. Muscle layer damage with entire circumferential esophageal ESD has been linked to refractory post-ESD stenosis[25]. Hence, ESD in the esophagus requires a highly skilled endoscopic technique and careful operation, and the provision of a more comfortable environment for ESD will contribute to reductions in complications. Based on the results presented above, FlushKnife-BTS is considered to contribute to safer esophageal ESD.
Though the present clinical study focused on only esophageal ESD because esophagus is the narrow tract in which inflated air inside affects the procedure easily, further investigation including ESD in other organs such as stomach and colorectum would be desired in the near future.
The limitations of this study include its retrospective design and small patient population. Furthermore, the procedure was only performed by one endoscopist. Therefore, the generalizability of the results obtained remains unclear and, thus, further studies with more cases undergoing ESD performed by other endoscopists including less experienced operators are warranted. However, our results still support FlushKnife-BTS creating better conditions for and contributing to the efficient performance of ESD.
In conclusion, our results demonstrate that FlushKnife-BTS supports the efficient performance of ESD, particularly for large lesions, by improving air and fluid aspiration and allowing for smooth knife insertion without frequent knife removal and reinsertion during ESD.
Endoscopic submucosal dissection (ESD) has been widely accepted as a treatment for early-stage tumors in the digestive tract. Devices utilized in ESD play an important role in facilitating the safe and effective performance of this procedure. A novel slim type ball-tipped FlushKnife (FlushKnife-BTS) has been developed to enhance the performance of aspiration and insertion of the knife through the scope.
This study investigated the usefulness of FlushKnife-BTS over FlushKnife-BT in functional experiments and clinical practice and is the first report comparing the conventional ESD knife and the developed new one.
This study indicated that FlushKnife-BTS enhances the performance of ESD, particularly for large lesions, by improving air and fluid aspiration and knife insertion during ESD and reducing the frequency of knife removal and reinsertion.
This study suggested that FlushKnife-BTS supports the efficient performance of ESD, particularly for large lesions.
FlushKnife-BTS is a novel slim type FlushKnife BT that has been developed to enhance the performance of aspiration and insertion of the knife through the scope.
Theoretically, the new device facilitates use in a standard scope with 2.8 mm of working channel. The paper compares a new and an older device with respect to the ability of insertion and suction in the laboratory as well as the resection speed by the measurement of the mm2 per minute in esophageal lesions in clinical practice.
| 1. | Iizuka T, Kikuchi D, Hoteya S, Nakamura M, Yamashita S, Mitani T, Takeda H, Yahagi N. Clinical advantage of endoscopic submucosal dissection over endoscopic mucosal resection for early mesopharyngeal and hypopharyngeal cancers. Endoscopy. 2011;43:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: a meta-analysis. Dig Dis Sci. 2014;59:1862-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 351] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 5. | Toyonaga T, Man-i M, Fujita T, East JE, Nishino E, Ono W, Morita Y, Sanuki T, Yoshida M, Kutsumi H. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for laterally spreading tumors of the colorectum. Endoscopy. 2010;42:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Toyonaga T, Man-i M, East JE, Nishino E, Ono W, Hirooka T, Ueda C, Iwata Y, Sugiyama T, Dozaiku T. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc. 2013;27:1000-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1162] [Article Influence: 46.5] [Reference Citation Analysis (5)] |
| 8. | Gotoda T. A large endoscopic resection by endoscopic submucosal dissection procedure for early gastric cancer. Clin Gastroenterol Hepatol. 2005;3:S71-73. [RCA] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis. 2005;6:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 461] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 11. | Kodashima S, Fujishiro M, Yahagi N, Kakushima N, Ichinose M, Omata M. Endoscopic submucosal dissection for gastric neoplasia: experience with the flex-knife. Acta Gastroenterol Belg. 2006;69:224-229. [PubMed] |
| 12. | Akahoshi K, Akahane H, Murata A, Akiba H, Oya M. Endoscopic submucosal dissection using a novel grasping type scissors forceps. Endoscopy. 2007;39:1103-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Takeuchi Y, Uedo N, Ishihara R, Iishi H, Kizu T, Inoue T, Chatani R, Hanaoka N, Taniguchi T, Kawada N. Efficacy of an endo-knife with a water-jet function (Flushknife) for endoscopic submucosal dissection of superficial colorectal neoplasms. Am J Gastroenterol. 2010;105:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Toyonaga T, Man-I M, Fujita T, Nishino E, Ono W, Morita Y, Sanuki T, Masuda A, Yoshida M, Kutsumi H. The performance of a novel ball-tipped Flush knife for endoscopic submucosal dissection: a case-control study. Aliment Pharmacol Ther. 2010;32:908-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Tanaka S, Toyonaga T, Morita Y. Endoscopic vessel sealing: a novel endoscopic precoagulation technique for blood vessels during endoscopic submucosal dissection. Dig Endosc. 2013;25:341-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, Shimada H, Takiuchi H, Toh Y, Doki Y. Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 17. | Koike Y, Hirasawa D, Fujita N, Maeda Y, Ohira T, Harada Y, Suzuki K, Yamagata T, Tanaka M. Usefulness of the thread-traction method in esophageal endoscopic submucosal dissection: randomized controlled trial. Dig Endosc. 2015;27:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Tsujii Y, Nishida T, Nishiyama O, Yamamoto K, Kawai N, Yamaguchi S, Yamada T, Yoshio T, Kitamura S, Nakamura T. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy. 2015;47:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 19. | Shin KY, Jeon SW, Cho KB, Park KS, Kim ES, Park CK, Chung YJ, Kwon JG, Jung JT, Kim EY. Clinical outcomes of the endoscopic submucosal dissection of early gastric cancer are comparable between absolute and new expanded criteria. Gut Liver. 2015;9:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Yamamoto Y, Yoshizawa N, Tomida H, Fujisaki J, Igarashi M. Therapeutic outcomes of endoscopic resection for superficial non-ampullary duodenal tumor. Dig Endosc. 2014;26 Suppl 2:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Toyonaga T, Nishino E, Man-I M, East JE, Azuma T. Principles of quality controlled endoscopic submucosal dissection with appropriate dissection level and high quality resected specimen. Clin Endosc. 2012;45:362-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Yamashina T, Ishihara R, Uedo N, Nagai K, Matsui F, Kawada N, Oota T, Kanzaki H, Hanafusa M, Yamamoto S. Safety and curative ability of endoscopic submucosal dissection for superficial esophageal cancers at least 50 mm in diameter. Dig Endosc. 2012;24:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 24. | Shi Q, Ju H, Yao LQ, Zhou PH, Xu MD, Chen T, Zhou JM, Chen TY, Zhong YS. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy. 2014;46:640-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 25. | Miwata T, Oka S, Tanaka S, Kagemoto K, Sanomura Y, Urabe Y, Hiyama T, Chayama K. Risk factors for esophageal stenosis after entire circumferential endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Surg Endosc. 2016;30:4049-4056. [PubMed] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bordas JM S- Editor: Qi Y L- Editor: A E- Editor: Liu WX