Published online Mar 7, 2017. doi: 10.3748/wjg.v23.i9.1602
Peer-review started: September 22, 2016
First decision: December 19, 2016
Revised: December 27, 2016
Accepted: January 17, 2017
Article in press: January 17, 2017
Published online: March 7, 2017
Processing time: 165 Days and 15.8 Hours
To explore whether copy number variations (CNVs) of toll-like receptor 7 (TLR7) are associated with susceptibility to chronic hepatitis B virus (HBV) infection.
This study included 623 patients (495 males and 128 females) with chronic hepatitis B virus infection (CHB) and 300 patients (135 females and 165 males) with acute hepatitis B virus infection (AHB) as controls. All CHB patients were further categorized according to disease progression after HBV infection (CHB, liver cirrhosis, or hepatocellular carcinoma). Copy numbers of the TLR7 gene were measured using the AccuCopy method. χ2 tests were used to evaluate the association between TLR7 CNVs and infection type. P values, odds ratios, and 95% confidence intervals (CIs) were used to estimate the effects of risk.
Among male patients, there were significant differences between the AHB group and CHB group in the distribution of TLR7 CNVs. Low copy number of TLR7 was significantly associated with chronic HBV infection (OR = 0.329, 95%CI: 0.229-0.473, P < 0.001). Difference in TLR7 copy number was also found between AHB and CHB female patients, with low copy number again associated with an increased risk of chronic HBV infection (OR = 0.292, 95%CI: 0.173-0.492, P < 0.001). However, there were no significant differences in TLR7 copy number among the three types of chronic HBV infection (CHB, liver cirrhosis, or hepatocellular carcinoma). In addition, there was no association between TLR7 copy number and titer of the HBV e antigen.
Low TLR7 copy number is a risk factor for chronic HBV infection but is not associated with later stages of disease progression.
Core tip: Differences in patient genetic backgrounds may influence the quality of the immune response and may result in different hepatitis B virus (HBV) infection outcomes. Toll-like receptor 7 (TLR7) is involved in the sensing of viruses and priming of the subsequent immune response. We investigated the association between copy number at the TLR7 locus and genetic susceptibility to chronic HBV infection. Comparison of individuals with chronic and acute HBV revealed that low TLR7 copy number was associated with chronic but not acute HBV in both males and females, though it was not associated with subsequent disease progression.
- Citation: Li F, Li X, Zou GZ, Gao YF, Ye J. Association between TLR7 copy number variations and hepatitis B virus infection outcome in Chinese. World J Gastroenterol 2017; 23(9): 1602-1607
- URL: https://www.wjgnet.com/1007-9327/full/v23/i9/1602.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i9.1602
The outcome of hepatitis B virus (HBV) infection is influenced by not only the virus but also the host's immune response and genetic diversity, including single nucleotide polymorphism (SNPs) and gene copy number variants (CNVs)[1-3]. Many studies support the idea that host genetic susceptibility plays an important role in determining the outcome of HBV infection[4]. Previous studies have shown that several SNP sites are associated with the development of chronic HBV infection and the progression of disease[5-7].
CNVs arise when individuals carry different numbers of copies of the same DNA sequence. These may be the result of deletions or duplications and insertions, and they typically cover about 15% of the human genome in each individual. CNVs are a common source of variation in the human genome and are known to play an important role in complex genetic diseases. In addition to affecting the copy number (CN) of a DNA sequence[8,9], CNVs may affect gene expression through dosage and position effects, which may further influence an individual's disease susceptibility and progression[9-11]. In humans, CNVs have so far been identified as a hereditable source of susceptibility to complex diseases such as systemic lupus erythematosus (SLE), type 1 diabetes, HBV infection, and Alzheimer's disease[8,12].
Toll-like receptors (TLRs) function as critical pattern recognition receptors (PRRs). TLRs recognize foreign pathogens as well as necrosis of endogenous substances, and play a role in both the innate and acquired immune responses[13-15]. TLRs are widely expressed in various tissues, with individual receptors exhibiting specific cell distributions. TLR7 is a highly conserved gene on the X chromosome[16,17], and its product is expressed primarily in dendritic cells (pDC), B cells, and macrophages. TLR7 recognizes single-stranded RNA through the MyD88-dependent signaling pathway, inducing pro-inflammatory factors and the production of type I interferon to mediate the body's non-specific immune response. Activation of TLR7 initiates downstream signaling cascades through transcription factors such as interferon regulatory factor 7. This induces production of pro-inflammatory cytokines and chemokines, which are involved in various HBV infection outcomes, including spontaneous clearance and viral persistence. The role of TLR7 CNVs in the outcome of HBV infection has not yet been investigated. In the present study, we categorized the groups according to gender, analyzed the TLR7 CNVs, and found that there is indeed an association between the CN of the TLR7 gene and genetic susceptibility to chronic HBV infection.
A total of 923 Han Chinese individuals with HBV infection were enrolled in this study from 2010 to 2015. Following recruitment, all subjects gave informed consent for genetic analysis. Among the patients, 623 (495 males and 128 females) suffered from chronic HBV (CHB) infection. The remaining 300 (135 females and 165 males) were individuals with acute, self-limiting HBV (AHB) clearance and served as the control group. Average ages were 44.41 ± 15.32 years in the CHB group and 45.26 ± 15.54 years in the AHB group. All CHB patients fulfilled the diagnostic criteria of being positive for hepatitis B surface antigen (HBsAg) for a period of at least 6 mo, with serum HBV DNA level > 1000 copies/mL. AHB patients were positive for hepatitis B surface antibody and hepatitis B core antibody but negative for HBsAg, and had no history of HBV vaccination. AHB controls were age-matched with CHB cases (t = 0.823, P > 0.05). The 623 CHB patients were further classified according to disease progression: 253 patients had CHB only, 248 had liver cirrhosis (LC), and 122 had hepatocellular carcinoma (HCC). None of these patients had received anti-HBV therapy or had overlapping infections of hepatitis A, C, D, E, or G. CHB patients were also free from drug-induced hepatitis, alcoholic liver disease, fatty liver disease, and pregnancy. All of them have given consent for this study.
Peripheral blood was collected from all subjects in vacuum blood tubes containing EDTA-K2. Genomic DNA was isolated from 2 mL whole blood using a Qiagen kit according to the manufacturer's instructions (Qiagen, Hilden, Germany) and was stored at -20 °C before CNV detection.
Quantification of copy number at the TLR7 locus using AccuCopy assay
CNs of the TLR7 gene were measured using the AccuCopy method (Genesky Biotechnologies Inc., Shanghai, China). The basic molecular principle of AccuCopy's competitive PCR amplification was illustrated by Du et al[18]. Forward and reverse primers of target segments are listed in Table 1.
Competitive DNAs for the two references and 12 target segments were designed and synthesized as double-stranded DNA by Genesky Biotechnologies. Sequences of synthesized competitive DNAs were identical to their reference sequences in the human reference genome except for an introduced 2-bp deletion. The synthesized competitive DNAs for target and reference segments were first mixed with a defined amount of genomic DNA from each patient and then subjected to multiplex fluorescence competitive PCR amplification, which simultaneously amplified all reference and target segments from both the sample DNA and competitive DNA using multiple fluorescence-labeled primer pairs.
Thermal cycling conditions for multiplex competitive PCR amplification were based on the manufacturer's instructions. PCR products were diluted 20-fold before being loaded on to an ABI 3130XL (Applied Biosystems, Carlsbad, CA, United States) genetic analyzer for capillary electrophoresis. Raw data were analyzed by GeneMapper 4.0 (Applied Biosystems), and height data for all specific peaks were exported into a Microsoft Excel file where the peak ratio of sample DNA to competitive DNA (S/C) for each segment was calculated. After normalization using the reference segment's peak ratio, the CN ratio of each target segment to the reference was determined by the target's peak ratio divided by the reference's peak ratio.
The distributions of TLR7 CNs were compared between CHB patients and AHB control subjects using χ2 test. P values, odds ratios (ORs), and 95% confidence intervals (CIs) were estimated using SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, United States).
TLR7 gene CNs were analyzed and quantified in triplicate in 623 Chinese CHB patients and 300 Chinese AHB patients. Baseline patient characteristics are shown in Table 2. While there were no significant age differences between AHB and CHB patients (including CHB, LC and HCC groups) (t = 0.823, P = 0.944 > 0.05), there was a difference if the CHB group was divided into CHB, LC and HCC groups (F = 83.216, P < 0.01).
| n | Males/females | Age (yr) | 95%CI | |
| AHB | 300 | 165/135 | 45.26 ± 15.54 | 43.94-47.03 |
| CHB | 253 | 198/55 | 33.98 ± 12.07 | 32.49-35.48 |
| LC | 248 | 196/52 | 50.26 ± 13.29 | 48.60-51.92 |
| HCC | 122 | 101/21 | 53.72 ± 12.50 | 51.48-55.96 |
Frequency distribution of TLR7 gene copy number
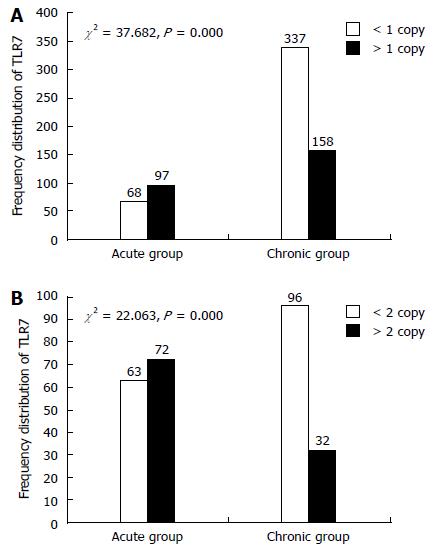
A reference category was chosen based on the median TLR7 CN obtained in the female and male control groups (CN = 2 for females and CN = 1 for males), as previously described[19]. Based on the reference category, the risk of disease progression associated with the absolute TLR7 CN was estimated by comparing cases and controls that were categorized as CN > 2 or CN ≤ 2 for females and as CN > 1 or CN ≤ 1 for males. The frequency distributions of TLR7 CNs of the different patient groups are shown in Figure 1.
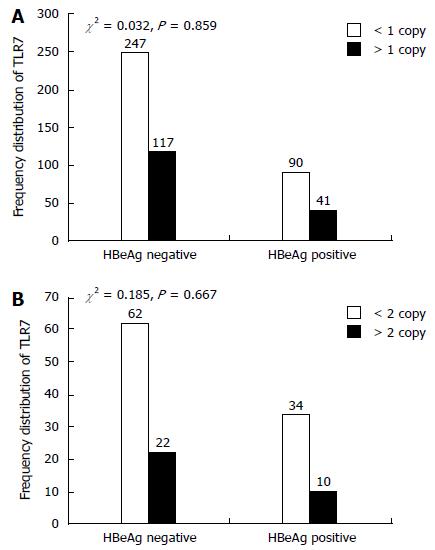
We examined the single-locus association between TLR7 CN and susceptibility to chronic HBV infection. TLR7 CNs were significantly different between the acute and chronic group in both male and female patients (males: χ2 = 37.682, P < 0.001; females: χ2 = 22.063, P < 0.001). As shown in Table 3, low TLR7 CN was significantly associated with chronic HBV infection in males (OR = 0.329, 95%CI: 0.229-0.473) and females (OR = 0.292, 95%CI: 0.173-0.492). We also compared the TLR7 CN distributions of the CHB, LC, and HCC groups, but there were no significant differences among the three groups (Table 4). HBeAg can play a role of immune regulation which is associated with the outcome of HBV infection. E antigen was used as an important index of HBV replication and infection. We divided patients into two groups according to e-antigen titers (0-1 IU/mL vs > 1 IU/mL). However, titer levels did not vary significantly among patient groups (Figure 2).
| Gender | CN | Chronic group | Acute group | OR | 95%CI: | P value |
| Male | < 1 | 337 | 68 | 0.33 | 0.23-0.47 | < 0.001 |
| > 1 | 158 | 97 | ||||
| Female | < 2 | 96 | 63 | 0.29 | 0.17-0.49 | < 0.001 |
| > 2 | 32 | 72 |
| Gender | CN | CHB | LC | HCC | χ2 | P value |
| Male | < 1 | 137 | 137 | 63 | 1.923 | 0.382 |
| > 1 | 61 | 59 | 38 | |||
| Female | < 2 | 39 | 42 | 15 | 1.557 | 0.459 |
| > 2 | 16 | 10 | 6 |
The disease spectrum of HBV infection ranges from acute HBV infection to chronic HBV carrier to chronic hepatitis, liver cirrhosis, and HCC. Many studies have provided evidence to support the idea that host genetic variation and immunity play important roles in determining the outcome of HBV infection[20]. The gene for Toll-like receptor TLR7 is located on the X chromosome and so experiences sex-linked inheritance. Therefore, analysis of TLR7 CNVs suggested that the data should be separated according to gender. TLR7 CNVs have been previously found to be associated with SLE[20], while Lund et al[21] found that TLR7 is involved in the body's antiviral immune response. In addition, some researchers believe that TLR7 represents a new sensor of viral infections[22]. However, its role in HBV infection has not been previously determined. We therefore designed this study to explore the association between TLR7 CNVs and chronic HBV susceptibility. In a previous study, we found that up-regulation of TLR7 is essential for serological clearance of hepatitis B surface antigen in HBV infection. The results in the current study revealed that low TLR7 CNs were significantly associated with chronic HBV infection in both males and females, suggesting that elimination of HBsAg is more difficult with reduced levels of TLR7. This indicates that a reduction in TLR7 CN is a risk factor for chronic HBV in Han Chinese patients. Hui et al[23] found that increased expression of TLR7 in the recovery phase may be involved in the clearance of HBsAg in patients with CHB. García-Ortiz et al[19] found that TLR7 mRNA levels correlated significantly with TLR7 CN, suggesting that an increase in TLR7 gene dosage results in the up-regulation of TLR7 mRNA expression in humans as well as in murine models of lupus. Moreover, Xu et al[24] found that PBMCs and pDCs from CHB patients exhibited diminished capacities to produce IFN-α in response to TLR7 activation. The fact that TLR7 is expressed in pDCs rather than in mDCs indicates that pDCs are potentially involved in fighting against viral infections. Therefore, reduced expression of TLRs in pDCs of CHB patients may lead to functional defects, as demonstrated by diminished production of IFN-α, resulting in persistent HBV infection. We also found that the replication system is adapted to generate high levels of virions without stimulating the innate immune system. Secreted viral proteins (HBsAg and HBeAg) suppress innate responses through inhibition of TLR signaling, which leads to a weak adaptive immune response with an exhausted phenotype that is incapable of inducing viral elimination[25]. Our experiment aims to study the relationship between the two indicators at a gene level. We found no association between TLR7 copy number and titer of the HBV e antigen, which implied that there was no correlation at a gene level between the two indicators.
In conclusion, this was the first study to independently analyze the relationship between TLR7 CNVs and susceptibility to chronic HBV. Our findings indicate that low copy numbers of the TLR7 gene may represent a risk factor for chronic HBV infection. Further replication and functional studies are necessary to confirm these results. In particular, as the biological functions of TLR7 CNVs in HBV infection have not been fully elucidated, further research is required to determine the mechanistic basis by which TLR7 CNVs affect the immune response.
More and more research suggest that the outcome of hepatitis B virus (HBV) infection is influenced by not only the virus but also the host's immune response and genetic diversity, including SNPs and gene copy number variations (CNVs). TLRs recognize foreign pathogens as well as necrosis of endogenous substances, and play a role in both the innate and acquired immune responses, but the role of Toll-like receptor 7 (TLR7) CNVs in the outcome of HBV infection has not yet been investigated.
In humans, CNVs have so far been identified as a hereditable source of susceptibility to complex diseases such as systemic lupus erythematosus type 1 diabetes, HBV infection, and Alzheimer's disease. Some researchers believe that TLR7 represents a new sensor of viral infections. However, its role in HBV infection has not been previously determined.
In this study, the authors found that there were significant differences between the acute patients and chronic patients in TLR7 copy number, with low copy numbers of TLR7 associated with chronic HBV infection in both males and females. This study identified a significant risk factor for chronic hepatitis B infection.
This study sheds light on the major underlying mechanisms of hepatitis B, which may have future clinical implications.
This is a really interesting study since TLR7 is involved in outcome of viral infections such as hepatitis C virus, HBV or human immunodeficiency virus, but no evidence is available about the association of CNV in TLR7 and HBV. The authors compared TLR7 CNVs in patients with acute HBV infection and chronic infection and among patients with chronic HBV classified according to disease progression.
| 1. | Ramezani A, Hasanjani Roshan MR, Kalantar E, Eslamifar A, Banifazl M, Taeb J, Aghakhani A, Gachkar L, Velayati AA. Association of human leukocyte antigen polymorphism with outcomes of hepatitis B virus infection. J Gastroenterol Hepatol. 2008;23:1716-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, Hasnian SS, Leung N, Lesmana L, Phiet PH. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356-1361. [PubMed] |
| 3. | Wei XQ, Guo YW, Liu JJ, Wen ZF, Yang SJ, Yao JL. The significance of Toll-like receptor 4 (TLR4) expression in patients with chronic hepatitis B. Clin Invest Med. 2008;31:E123-E130. [PubMed] |
| 4. | Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508-117, 1508-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 5. | Karra VK, Gumma PK, Chowdhury SJ, Ruttala R, Polipalli SK, Chakravarti A, Kar P. IL-18 polymorphisms in hepatitis B virus related liver disease. Cytokine. 2015;73:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Kimkong I, Chankaew J, Kunanopparat A, Hirankarn N, Tangkijvanich P. Gene polymorphisms of interleukin 28B and the risk to chronic hepatitis B virus infection in Thai. Tissue Antigens. 2015;85:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Hou SH, Hu J, Zhang Y, Li QL, Guo JJ. Effects of interaction between genetic variants in human leukocyte antigen DQ and granulysin genes in Chinese Han subjects infected with hepatitis B virus. Microbiol Immunol. 2015;59:209-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Swaminathan S, Kim S, Shen L, Risacher SL, Foroud T, Pankratz N, Potkin SG, Huentelman MJ, Craig DW, Weiner MW. Genomic Copy Number Analysis in Alzheimer's Disease and Mild Cognitive Impairment: An ADNI Study. Int J Alzheimers Dis. 2011;2011:729478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G. Characterization of Hepatitis B Virus(HBV) -Specific T-Cell Dysfunction in chronic HBV infection. J Virol. 2007;81:4215-4225. |
| 10. | Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, Nibbs RJ, Freedman BI, Quinones MP, Bamshad MJ. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 865] [Cited by in RCA: 841] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 11. | Shiow LR, Paris K, Akana MC, Cyster JG, Sorensen RU, Puck JM. Severe combined immunodeficiency (SCID) and attention deficit hyperactivity disorder (ADHD) associated with a Coronin-1A mutation and a chromosome 16p11.2 deletion. Clin Immunol. 2009;131:24-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Grayson BL, Smith ME, Thomas JW, Wang L, Dexheimer P, Jeffrey J, Fain PR, Nanduri P, Eisenbarth GS, Aune TM. Genome-wide analysis of copy number variation in type 1 diabetes. PLoS One. 2010;5:e15393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Chang ZL. Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm Res. 2010;59:791-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Kondo Y, Ueno Y, Shimosegawa T. Toll-like receptors signaling contributes to immunopathogenesis of HBV infection. Gastroenterol Res Pract. 2011;2011:810939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 15. | Hirsch I, Caux C, Hasan U, Bendriss-Vermare N, Olive D. Impaired Toll-like receptor 7 and 9 signaling: from chronic viral infections to cancer. Trends Immunol. 2010;31:391-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2469] [Cited by in RCA: 2581] [Article Influence: 117.3] [Reference Citation Analysis (0)] |
| 17. | Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2734] [Cited by in RCA: 2913] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 18. | Du R, Lu C, Jiang Z, Li S, Ma R, An H, Xu M, An Y, Xia Y, Jin L. Efficient typing of copy number variations in a segmental duplication-mediated rearrangement hotspot using multiplex competitive amplification. J Hum Genet. 2012;57:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | García-Ortiz H, Velázquez-Cruz R, Espinosa-Rosales F, Jiménez-Morales S, Baca V, Orozco L. Association of TLR7 copy number variation with susceptibility to childhood-onset systemic lupus erythematosus in Mexican population. Ann Rheum Dis. 2010;69:1861-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Grio R, Curti A, Piacentino R, Cellura A, Malara D, Geranio R, Porpiglia M. Anomalous uterine hemorrhages in the climacteric. Their etiopathogenesis. Minerva Ginecol. 1998;50:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 326] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 21. | Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101:5598-5603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1343] [Cited by in RCA: 1420] [Article Influence: 64.5] [Reference Citation Analysis (1)] |
| 22. | Crozat K, Beutler B. TLR7: A new sensor of viral infection. Proc Natl Acad Sci USA. 2004;101:6835-6836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Hui CK, Zhang HY, Lee NP, Yueng YH, Lu L, Leung N, Wong BCY, Lo CM, Fan ST, Luk JM. Upregulation of toll-like receptor7 is essential for serological clearance of hepatitis B surface antigen chronic hepatitis B virus(HBV)infection. Hepatology. 2005;42:707A-708A. |
| 24. | Xu N, Yao HP, Lv GC, Chen Z. Downregulation of TLR7/9 leads to deficient production of IFN-α from plasmacytoid dendritic cells in chronic hepatitis B. Inflamm Res. 2012;61:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Thursz M. Basis of HBV persistence and new treatment options. Hepatol Int. 2014;8 Suppl 2:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Citores MJ, Roltgen K S- Editor: Yu J L- Editor: Ma JY E- Editor: Wang CH