Published online Mar 7, 2017. doi: 10.3748/wjg.v23.i9.1594
Peer-review started: July 13, 2016
First decision: September 28, 2016
Revised: October 9, 2016
Accepted: February 7, 2017
Article in press: February 8, 2017
Published online: March 7, 2017
Processing time: 241 Days and 23.5 Hours
To examine the role of microRNA 1181 (miR-1181) in invasion and proliferation in pancreatic cancer.
We analyzed the expression of miR-1181 in several pancreatic cancer cell lines and generated stable MIA-PaCa-2 and PANC-1 cell lines with up-regulated miR-1181 expression using an adenovirus delivery system. We then investigated miR-1181's effect on invasion and proliferation of pancreatic cancer cells by transwell assay, wound healing assay, cell counting kit-8 assay and colony-forming assay, and explored any underlying mechanisms by western bolt. Beyond that, we observed the change of the PANC-1 cell's cytoskeleton by immunofluorescence staining.
Our data showed that miR-1181 was relatively down-regulated in pancreatic cancer cell lines compared with normal pancreatic ductal epithelial cells. And miR-1181 inhibited the migration, invasion and proliferation activities of MIA-PaCa-2 and PANC-1 cells. Notably, after over-expressing of miR-1181 in PANC-1 cells, F-actin depolymerized. Immunofluorescence staining shows decreased F-actin and β-tubulin expression in PANC-1 cells over-expressing miR-1181 compared with the control cells. Furthermore, we found that over-expressing miR-1181 inhibited the expression of signal transducer and activator of transcription 3 (STAT3) while knocking-down miR-1181 up-regulated the expression of STAT3. Knocking-down miR-1181 promoted the invasion and proliferation of pancreatic cancer cells. And inhibition of STAT3 blocked the promotion effects of knocking-down miR-1181 on proliferation and invasion in pancreatic cancer.
Together our findings suggest that miR-1181 may be involved in pancreatic cancer cell invasion and proliferation by targeting STAT3 and indicate that miR-1181 may be a potential therapeutic agent for pancreatic cancer.
Core tip: We found that miR-1181 may be involved in pancreatic cancer cell invasion and proliferation by targeting signal transducer and activator of transcription 3. Our findings suggest that miR-1181 may be a potential therapeutic agent for pancreatic cancer.
- Citation: Wang J, Guo XJ, Ding YM, Jiang JX. miR-1181 inhibits invasion and proliferation via STAT3 in pancreatic cancer. World J Gastroenterol 2017; 23(9): 1594-1601
- URL: https://www.wjgnet.com/1007-9327/full/v23/i9/1594.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i9.1594
Pancreatic cancer is a highly lethal disease, with a mortality that closely parallels incidence. Most patients with pancreatic cancer remain asymptomatic until the disease reaches an advanced stage. Surgical resection is currently regarded as the only potentially curative treatment[1-3]. Therefore, there is an urgent need to design novel strategies for achieving better treatment outcome in pancreatic cancer patients. High invasion and rapid proliferation are the two main features of pancreatic cancer tumors[4]. Understanding the molecular mechanisms that collaboratively regulate pancreatic cancer cell invasion and proliferation is expected to provide new insights into the development of novel and effective therapies for pancreatic cancer.
MicroRNAs (miRNAs) are noncoding 17- to 25-nucleotide-long RNAs that posttranscriptionally regulate the expression of multiple genes[5]. miRNA dysregulation is implicated in the development and progression of nearly all tumor types[6-8]. Recent evidence has indicated that some miRNAs can function as tumor suppressors[9]. We previously found that miR1181 inhibited CSC phenotypes by directly suppressing SOX2 and STAT3 in pancreatic cancer cells[10]. But we have not elucidated miR-1181's role on proliferation and invasion of pancreatic cancer cells, which was important malignant biological behavior of pancreatic cancer. And CSCs regulator always play an important role in the process of growth and invasion. As a result, we hypothesized that miR-1181 may suppress the invasion and proliferation of pancreatic cancer and explored it.
The HPDE, BxPC-3, AsPC-1, MIA-PaCa-2 and PANC-1 cell lines were purchased and authenticated though STR typing from ATCC. MIA-PaCa-2 and PANC-1 cells were grown in DMEM medium (Gibco, NY, United States) supplemented with 10% fetal bovine serum (Gibco), 100 U/mL penicillin G and 100 μg/ml streptomycin (Sigma, MO, United States) at 37 °C in a humidified 5% CO2 incubator. HPDE, BxPC-3 and AsPC-1 cells were grown in 1640 medium (Gibco) supplemented with 10% fetal bovine serum, 100 U/mL penicillin G and 100 μg/ml streptomycin at 37 °C in a humidified 5% CO2 incubator.
Harvesting, tissue processing, and reactions were conducted according to standard procedures. RNA was reverse-transcribed to cDNA using the ReverTra Ace qPCR RT Kit (TransGen, China). Quantitative real-time PCR was performed using the SYBR Green Realtime PCR Master Mix (TransGen), according to the manufacturer's instructions; and primers were shown in Table 1.
| Gene name | Sequences (forward and reverse) | |
| GAPDH | F | TGACTTCAACAGCGACACCCA |
| R | CACCCTGTTGCTGTAGCCAAA | |
| STAT3 | F | AGTGACCAGGCAGAAGATGC |
| R | CTCTTCCAGTCAGCCAGCTC |
We purchased human miR-1181 over-expressing (miR-1181U), knocking-down (miR-1181D) and negative-control (NC) adenovirus from Genechem (Shanghai, China). All transfections were carried out according to manufacturers' instructions.
Transwell assay: Cell invasion and migration were assessed using 24-well Corning Costar inserts with 8-μm pores. The upper surface of each insert was coated with Matrigel (BD, NJ, United States; diluted 1: 8) for 6 h in an incubator. Cells (1 × 105) were added to upper chambers and incubated at 37 °C; migration was assessed at 12 h and invasion at 24 h. Non-migrating and non-invading cells were removed with cotton buds from the top chambers. Cells remaining in bottom chambers were fixed with 100% methanol, stained with 0.1% crystal violet in 2% ethanol, and quantified visually in nine random fields using bright-field optics. Experiments were performed in triplicate and data are reported as mean ± SD of cell numbers.
Wound healing assay: Cells (5 × 106 per well) were cultured in 6-well plates for 24 h. Cell layers were subsequently scratched with sterile plastic tips, washed with PBS twice, cultured for 24 h with medium containing 1% FBS, and photographed under an Olympus BX51 microscope.
Cells grown on coverslips were incubated overnight at 4 °C with primary antibody against β-tubulin (CST; 1:100), followed by incubation for 45 min at 37 °C with CY3-conjugated goat anti-rabbit antibody (Boster, Wuhan, China). F-Actin distribution was detected using rhodamine phalloidin (Sigma-Aldrich) according to the manufacturer's protocol. Slides were counterstained with DAPI to visualize the cell nuclei, photographed using a LEICA LCSSP2 confocal laser scanning microscope and analyzed by ZEN 2009 (Carl Zeiss, Baden-Wurttemberg, Germany).
Cells were seeded in 96-well culture plates (2 × 104 cells/100 μL/well) and grown in the incubator. We then added 10 μL of CCK-8 (Dojindo, Tokyo, Japan) solution to each well. Plates were incubated for 1 h in the incubator and the absorbance was monitored at 450 nm using a microplate reader.
Cells were isolated by Trypsin-EDTA and seeded into 6-well plates at 1 × 103 cells per well in a final volume of 2 mL culture media. Cells were allowed to grow at 37 °C for 1 wk. After three washes with PBS, cells were fixed with 4% paraformaldehyde for 20 min and stained with 0.1% crystal violet for 30 min. Total colonies were counted from seven random fields under the microscope. Assays were repeated at least three times.
We purchased siRNAs from RiboBio (Guangzhou, China). Cells were seeded in 6-well plates at 50% confluence without antibiotics on the day before transfection. Transfection with si-STAT3 or miRNA negative control #22 (NC) (Ribobio, Guangzhou, China) was performed using Lipofectamine 2000 reagent (Invitrogen, NY, United States). Transfection complexes were prepared according to the manufacturer's instructions. All transfections were carried out according to manufacturers' instructions.
Polyvinylidene difluoride membranes containing electrophoretically separated proteins from PANC-1 and MIA-PaCa-2 cells were incubated with rabbit anti-STAT3 (CST, United States; 1:200), rabbit anti-p-STAT3 (CST, United States; 1:200), and mouse anti-GAPDH (Boster), followed by incubation with peroxidase-conjugated goat anti-rabbit IgG secondary antibody (CST; 1:2000) and visualized by enhanced chemiluminescence (Boster).
All values are presented as means ± SD. Significant differences were determined using SPSS 17.0 software (SPSS, Chicago, IL, United States). Student's t-test was used to determine statistical differences. P < 0.05 was considered significant.
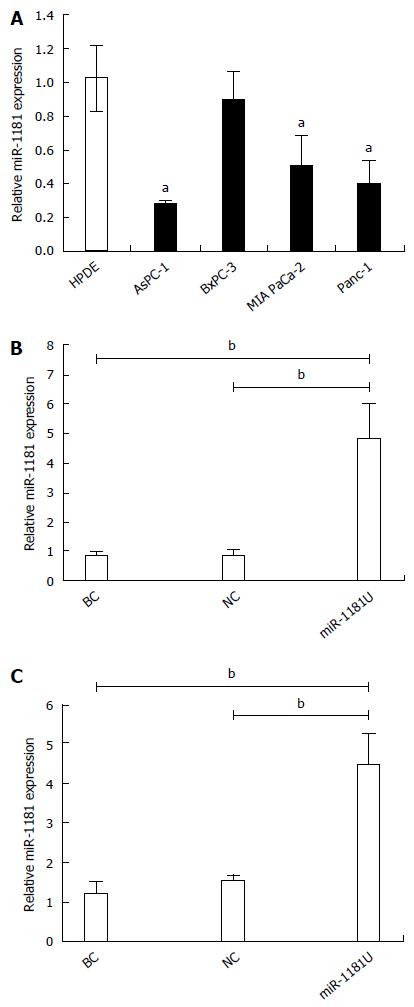
We first analyzed the expression of miR-1181 in several pancreatic cancer cell lines, including MIA-PaCa-2, BxPC-3, AsPC-1 and PANC-1, by qRT-PCR (Figure 1A). We found that the expression level of miR-1181 is downregulated in pancreatic cancer cell lines compared with levels in the human pancreatic duct epithelial (HPDE) cell line. We therefore generated stable MIA-PaCa-2 and PANC-1 cell lines with up-regulated miR-1181 expression using an adenovirus delivery system. Up-regulated miR-1181 (miR-1181U) was verified by PCR analyses (Figure 1B and C).
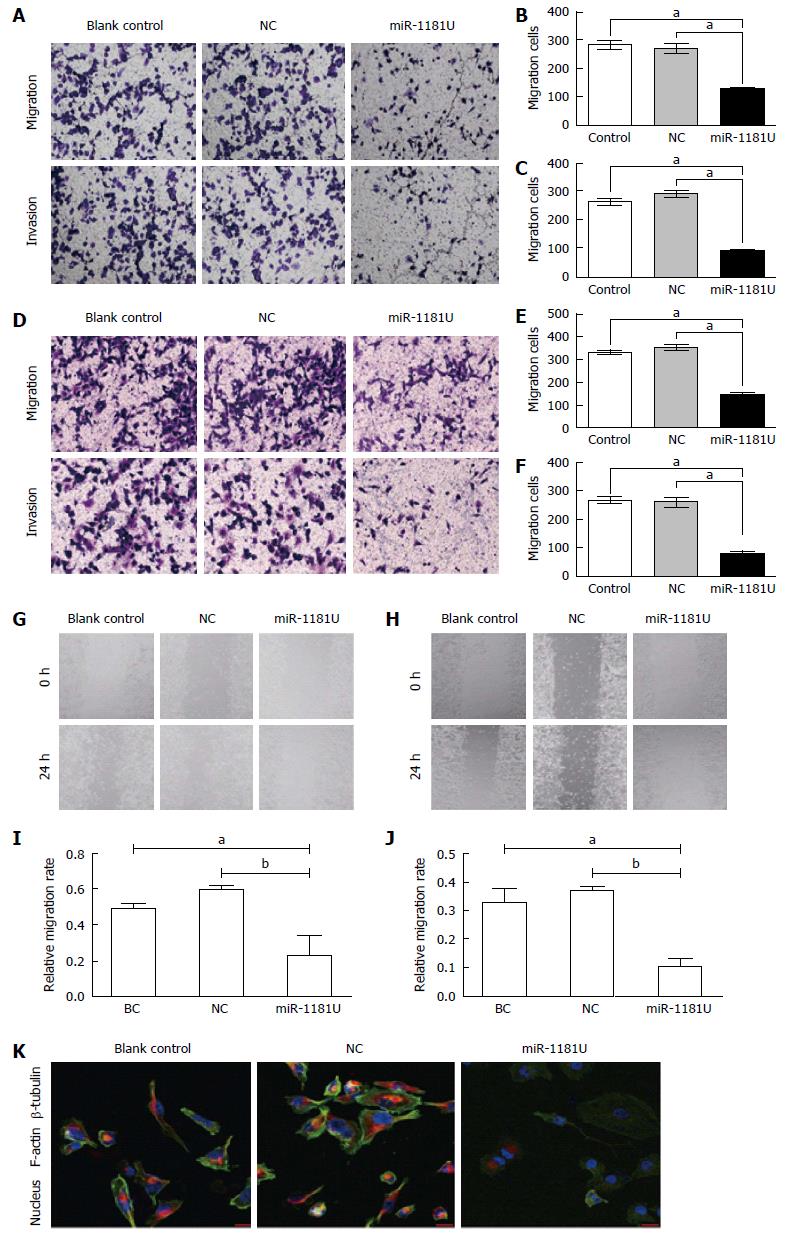
To examine whether up-regulated miR-1181 affect MIA-PaCa-2 and PANC-1 cell migration, we performed transwell assays to examine whether miR-1181 affect MIA PaCa-2 and PANC-1 cell migration and invasion. We found that over-expression of miR-1181 inhibited MIA-PaCa-2 and PANC-1 cell migration and invasion (Figure 2A-F). We next performed wound healing assays. Our results showed that overexpression of miR-1181 inhibited both MIA-PaCa-2 and PANC-1 cell migration (Figure 2G-J). As expected, knockdown of miR-1181 resulted in suppressed invasiveness of MIA-PaCa-2 and PANC-1 cells. Together these data indicate that miR-1181 inhibits migration and invasion of MIA-PaCa-2 and PANC-1 cells in vitro. Notably, after over-expressing of miR-1181 in PANC-1 cells, F-actin depolymerized. Immunofluorescence staining shows decreased F-actin and β-tubulin expression in PANC-1 cells over-expressing miR-1181 compared with the control cells (Figure 2K).
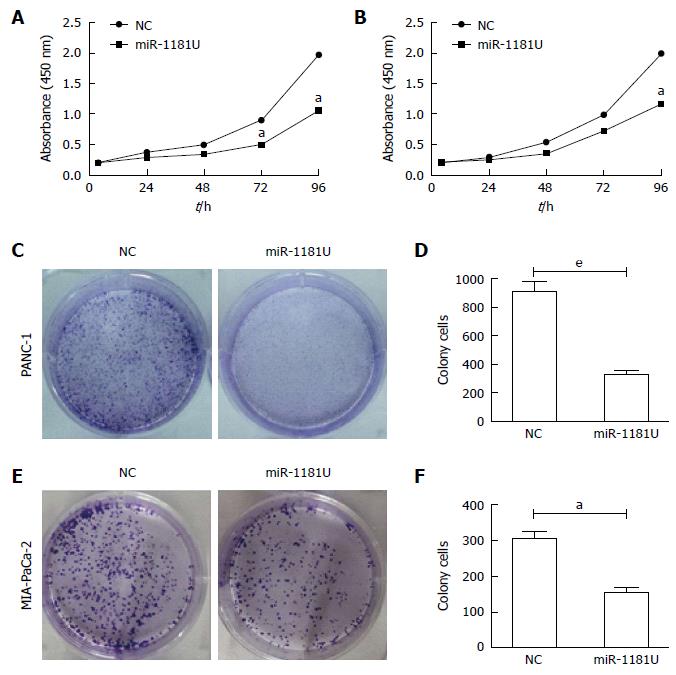
To examine whether miR-1181 expression affected MIA-PaCa-2 and PANC-1 cells proliferation, we next performed CCK-8 assays and colony forming assays. Our results showed that over-expression of miR-1181 inhibited MIA-PaCa-2 and PANC-1 cell proliferation in both CCK-8 assays (Figure 3A and B) and colony-forming assays (Figure 3C-F).
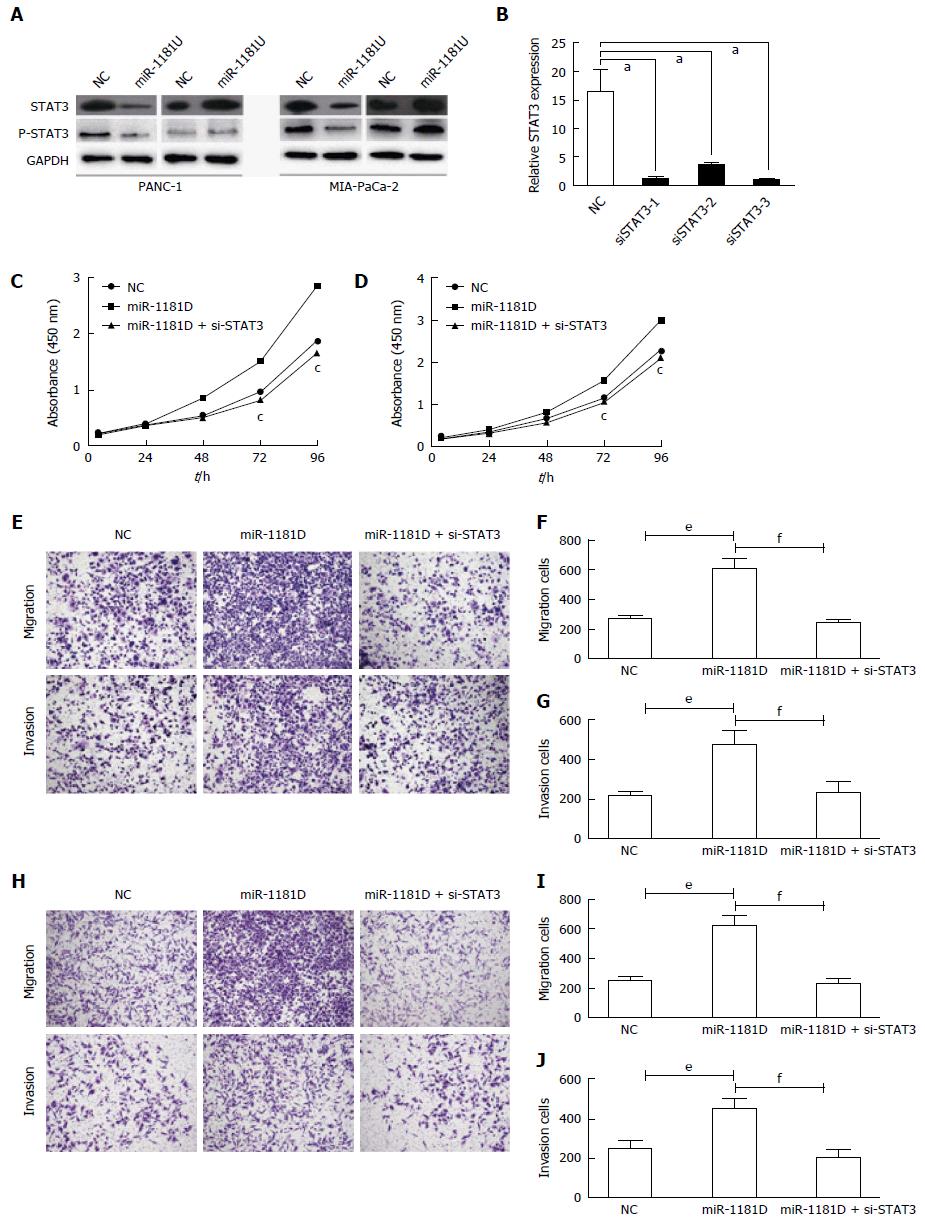
We previously found that miR-1181 directly targets STAT3 and inhibits STAT3 transactivity. It was found that STAT3 activation may promote the progression of pancreatic cancer. Thus, we next examined whether miR-1181 promoted pancreatic cell invasion and proliferation though the STAT3 pathway. Firstly, we found that over-expressing miR-1181 inhibited the expression of STAT3 and p-STAT3 while knocking-down miR-1181 up-regulated the expression of STAT3 and p-STAT3 in western blot assay (Figure 4A). Next we generated stable MIA-PaCa-2 and PANC-1 cell lines with knocked-down miR-1181 expression using an adenovirus delivery system. We transfected knocked-down miR-1181 MIA-PaCa-2 and PANC-1 cells with siRNA targeting STAT3 and examined cell proliferation and invasion (Figure 4C-J). Our results showed that inhibition of STAT3 by siRNA blocked the promotion effects of knocking-down miR-1181 on proliferation and invasion.
This study demonstrated that miR-1181 expression is markedly downregulated in pancreatic cancer cells and that upregulation of miR-1181 could suppress the invasion and proliferation of pancreatic cancer cells. Moreover, our results suggest that miR-1181 inhibits invasion and proliferation through targeting STAT3 in pancreatic cancer cells. In conclusion, our results indicate that miR-1181 plays an important role in suppressing invasion and proliferation in pancreatic cancer though targeting STAT3.
The Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway was originally discovered in the context of interferon-α (IFNα)-, IFNγ- and intereukin-6 (IL-6)-mediated downstream signaling pathways[11]. Among the seven members of the STAT protein family, STAT3 and STAT5 are the most important for cancer progression[12,13]. Although both STAT3 and STAT5 contribute to tumor cell proliferation and survival, a notable feature of STAT3 as a promising target for cancer therapy is that it also has a crucial role in stromal cells, including immune cells, which are recruited to the tumor microenvironment to promote tumor progression. Importantly, STAT3 activation also functions as a potent immune checkpoint for multiple anti-tumor immune responses. Evidence continues to accumulate that STAT3 activation is a promising molecular target for cancer therapies[14-17]. The STAT3 signaling pathway is especially important for various aspects of neoplasia, including proliferation, drug resistance, and survival of cancer cells through constitutive phosphorylation of STAT3[18-20]. Although numerous STAT3 inhibitors have been developed, and several STAT3 inhibitors have completed Phase I/II clinical trials, targeting STAT3 as a cancer therapy remains frustratingly elusive compared to targeting RTKs[18,21,22]. Numerous inhibitors for specific targets in signaling pathways have been applied in clinical use, but the success of these targeted cancer therapies has been limited by the development of resistance to these inhibitors. Therefore, there is an urgent demand to reassess the ongoing strategies to develop clinically useful drugs.
We previously found that miR-1181 inhibited stemness and tumorigenicity via targeting STAT3 and SOX2. Another study demonstrated that miR-1181 promoted mesenchymal-epithelial transition in ovarian cancer cells and its expression was significantly downregulated by ARK5[22].
In conclusion, here we found that miR-1181 may be involved in pancreatic cancer cell invasion and proliferation by targeting STAT3. Our findings suggest that miR-1181 may be a potential therapeutic agent for pancreatic cancer.
MicroRNA (miRNA) dysregulation is implicated in the development and progression of nearly all tumor types. Recent evidence has indicated that some miRNAs can function as tumor suppressors. The authors thus investigated whether miR-1181 suppressed the invasion and proliferation of pancreatic cancer.
The authors previously found that miR-1181 plays an important role in inhibiting stemness and tumorigenicity of pancreatic cancer stem cells via targeting multiple key cancer stem cell regulators, including STAT3 and SOX2. And miR-1181 promoted mesenchymal-epithelial transition in ovarian cancer cells and its expression was significantly downregulated by ARK5.
The authors found that miR-1181 may be involved in pancreatic cancer cell invasion and proliferation by targeting STAT3.
these findings suggest that miR-1181 may be a potential therapeutic agent for pancreatic cancer.
miRNAs are noncoding 17- to 25-nucleotide-long RNAs that posttranscriptionally regulate the expression of multiple genes. miRNA dysregulation is implicated in the development and progression of nearly all tumor types. Recent evidence has indicated that some miRNAs can function as tumor suppressors.
The manuscript by Wang and co-workers analyzes the effects of miR-1181 on invasion and proliferation of pancreatic cancer cells with special emphasis on STAT-3. The manuscript is well written and the experimental procedure are sound and valid.
| 1. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1784] [Article Influence: 178.4] [Reference Citation Analysis (1)] |
| 2. | Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 686] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 3. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2224] [Article Influence: 139.0] [Reference Citation Analysis (2)] |
| 4. | Sirri E, Castro FA, Kieschke J, Jansen L, Emrich K, Gondos A, Holleczek B, Katalinic A, Urbschat I, Vohmann C. Recent Trends in Survival of Patients With Pancreatic Cancer in Germany and the United States. Pancreas. 2016;45:908-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Hayes CN, Chayama K. MicroRNAs as Biomarkers for Liver Disease and Hepatocellular Carcinoma. Int J Mol Sci. 2016;17:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 7. | Cohen A, Burgos-Aceves MA, Smith Y. Estrogen repression of microRNA as a potential cause of cancer. Biomed Pharmacother. 2016;78:234-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Raimondi L, De Luca A, Morelli E, Giavaresi G, Tagliaferri P, Tassone P, Amodio N. MicroRNAs: Novel Crossroads between Myeloma Cells and the Bone Marrow Microenvironment. Biomed Res Int. 2016;2016:6504593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Li Y, Sarkar FH. MicroRNA Targeted Therapeutic Approach for Pancreatic Cancer. Int J Biol Sci. 2016;12:326-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Jiang J, Li Z, Yu C, Chen M, Tian S, Sun C. MiR-1181 inhibits stem cell-like phenotypes and suppresses SOX2 and STAT3 in human pancreatic cancer. Cancer Lett. 2015;356:962-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Zhao C, Li H, Lin HJ, Yang S, Lin J, Liang G. Feedback Activation of STAT3 as a Cancer Drug-Resistance Mechanism. Trends Pharmacol Sci. 2016;37:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 12. | Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1338] [Cited by in RCA: 1710] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 13. | Bosch-Barrera J, Menendez JA. Silibinin and STAT3: A natural way of targeting transcription factors for cancer therapy. Cancer Treat Rev. 2015;41:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Sun X, Zhang J. STAT3 Decoy ODN Therapy for Cancer. Methods Mol Biol. 2015;1317:167-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Yeh JE, Frank DA. STAT3-Interacting Proteins as Modulators of Transcription Factor Function: Implications to Targeted Cancer Therapy. ChemMedChem. 2016;11:795-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Tan FH, Putoczki TL, Stylli SS, Luwor RB. The role of STAT3 signaling in mediating tumor resistance to cancer therapy. Curr Drug Targets. 2014;15:1341-1353. [PubMed] |
| 17. | Shen N, Huang X, Li J. Upregulation of miR-129-5p affects laryngeal cancer cell proliferation, invasiveness, and migration by affecting STAT3 expression. Tumour Biol. 2016;37:1789-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Wang S, An W, Yao Y, Chen R, Zheng X, Yang W, Zhao Y, Hu X, Jiang E, Bie Y. Interleukin 37 Expression Inhibits STAT3 to Suppress the Proliferation and Invasion of Human Cervical Cancer Cells. J Cancer. 2015;6:962-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Lin J, Li Q, Chen H, Lin H, Lai Z, Peng J. Hedyotis diffusa Willd. extract suppresses proliferation and induces apoptosis via IL-6-inducible STAT3 pathway inactivation in human colorectal cancer cells. Oncol Lett. 2015;9:1962-1970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Liu J, Mao Y, Zhang D, Hao S, Zhang Z, Li Z, Li B. MiR-143 inhibits tumor cell proliferation and invasion by targeting STAT3 in esophageal squamous cell carcinoma. Cancer Lett. 2016;373:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Zhang F, Wang Z, Yuan J, Wei X, Tian R, Niu R. RNAi-mediated silencing of Anxa2 inhibits breast cancer cell proliferation by downregulating cyclin D1 in STAT3-dependent pathway. Breast Cancer Res Treat. 2015;153:263-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Zhang HY, Li JH, Li G, Wang SR. Activation of ARK5/miR-1181/HOXA10 axis promotes epithelial-mesenchymal transition in ovarian cancer. Oncol Rep. 2015;34:1193-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kleeff J S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH