Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1458
Peer-review started: August 27, 2016
First decision: September 20, 2016
Revised: November 21, 2016
Accepted: January 18, 2017
Article in press: January 18, 2017
Published online: February 28, 2017
Processing time: 185 Days and 19.1 Hours
To characterize natural history of cryptogenic cirrhosis (CC) and compare its clinical features and outcomes to those of hepatitis C virus (HCV)-related cirrhosis.
A prospective cohort of 102 consecutive patients at their first diagnosis of CC were enrolled in this study. The clinical data and outcomes were compared to an age- and Child-Pugh class-matched cohort of 110 patients with HCV-related cirrhosis. Diagnosis of cirrhosis was based on compatible clinical and laboratory parameters, ultrasound/endoscopic parameters and, whenever possible, on histological grounds and transient elastography. All cases of cirrhosis without a definite etiology were enrolled in the CC group. The parameters assessed were: (1) severity of liver disease at the time of first diagnosis; (2) liver decompensation during follow-up; (3) hepatocellular carcinoma (HCC); (4) orthotopic liver transplantation; and (5) death. The independent associated factors were evaluated by multiple logistic regression analysis, and survival and its determinants by the Kaplan-Meier model, log-rank test and Cox regression.
At the first observation, median age was 66 and 65 years and male gender was 36% and 58% for CC and HCV cirrhosis, respectively. CC showed Child-Pugh class A/B/C of 47%/31%/22%, respectively. Compared to HCV cirrhosis, CC exhibited a significantly higher prevalence of metabolic syndrome (12% vs 54%, respectively), overweight/obesity, high BMI, impaired glucose tolerance, high blood pressure, dyslipidemia, hyperuricemia, cardiovascular diseases, extrahepatic cancer, and gallstones. Over a median period of 42 mo of follow-up, liver decompensation, HCC development and death for CC and HCV-related cirrhosis were 60.8%, and 54.4%, 16.7% and 17.2%, 39.2% and 30%, respectively. The median survival was 60 mo for CC. Independent predictors of death were age and Child-Pugh class at diagnosis. CC showed an approximately twofold higher incidence of HCC in Child-Pugh class A.
Undiagnosed nonalcoholic fatty liver disease has an etiologic role in CC that is associated with a poor prognosis, early HCC development, high risk of cardiovascular disease and extrahepatic cancer.
Core tip: We evaluated the features and outcomes of cryptogenic cirrhosis (CC) compared to age- and Child-Pugh class-matched hepatitis C virus-related cirrhosis at baseline and over a 42-mo follow-up. At diagnosis, the median age of CC was 66 years and Child-Pugh classes A/B/C were 47%/31%/22%, respectively. Among CC cases higher prevalences of metabolic syndrome, overweight/obesity, high BMI, impaired glucose metabolism, high blood pressure, dyslipidemia, hyperuricemia, cardiovascular diseases, extrahepatic cancer, and gallstones were observed. Although in the two groups we detected a similar incidence of liver decompensation, hepatocellular carcinoma (HCC) and death, an earlier development of HCC was observed in CC. Age and Child-Pugh were predictors of death. Most CC cases are the consequence of undiagnosed nonalcoholic fatty liver disease.
- Citation: Rinaldi L, Nascimbeni F, Giordano M, Masetti C, Guerrera B, Amelia A, Fascione MC, Ballestri S, Romagnoli D, Zampino R, Nevola R, Baldelli E, Iuliano N, Rosato V, Lonardo A, Adinolfi LE. Clinical features and natural history of cryptogenic cirrhosis compared to hepatitis C virus-related cirrhosis. World J Gastroenterol 2017; 23(8): 1458-1468
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1458.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1458
Liver cirrhosis is a major health burden worldwide ranking among the top ten causes of years of life lost in high-income countries[1]. More than 1000000 of deaths worldwide were reported due to cirrhosis in 2010[2]. The number of cirrhotic patients and the related complications and mortality rates are rising[3]. Cirrhosis is the shared, end-stage result of etiologically diverse chronic liver diseases, with a different geographic distribution, which can follow an indolent course and remains asymptomatic until complications or be discovered incidentally at necropsy[4]. In other cases, asymptomatic cirrhosis is usually detected incidentally with laboratory tests or imaging studies performed for unrelated reasons[4]. Although the clinical manifestations of cirrhosis are stereotypic irrespective of its etiology, the identification of the causes is important to define specific therapeutic and surveillance strategies. 3A substantial number of cases of cirrhosis remain of unknown origin and are therefore designated as “cryptogenic cirrhosis” (CC), which accounts for up to 30% of cases of cirrhosis and about 10% of liver transplants[5,6].
There are no standardized diagnostic criteria for CC and it is best defined by exclusion. Nevertheless, a proportion of CC cases are deemed to result from the progression of previously unrecognized non-alcoholic steatohepatitis (NASH)[7-9]. Models of the natural history of cirrhosis are based on findings in individuals with viral or alcoholic cirrhosis[10,11] and the outcome of CC is far less characterized than cirrhosis with a definite etiology. This is of importance given that the natural history of NASH-cirrhosis offers clues to preventing cardiometabolic risk and hepatocellular carcinoma (HCC)[12,13].
On these grounds and given the paucity of published data, we aimed at characterizing the clinical features and long-term course of CC in a case series of Italian patients consecutively observed at two Italian tertiary liver units. To do so, the features and outcome of CC were compared with those of matched hepatitis C virus (HCV)-related cirrhosis cases.
In this prospective cohort study we enrolled consecutive patients receiving for the first time a diagnosis of cirrhosis of unknown origin, defined as CC, at two Italian tertiary hepatology centers, the University of Naples and the University of Modena and Reggio Emilia, from January 2008 to June 2015. During the same period, we also enrolled, as control group, an age- and child-Pugh class matched cohort of patients with a diagnosis of HCV-related cirrhosis.
The diagnosis of cirrhosis was based on compatible clinic and laboratory parameters (hepatic encephalopathy, jaundice, ascites, platelet < 90000 mm3, albumin < 2.5 g/dL), ultrasound/endoscopic parameters (coarse-pattern, irregular liver surface, clear-cut evidence of portal hypertension such as splenomegaly and esophago-gastric varices)[5] and, whenever possible, on histological ground. In addition, in a subset of patients we performed transient elastography by Fibroscan® as a further diagnostic support, and cut-off values of liver stiffness > 12.5 kPa were considered consistent with liver cirrhosis[14].
The criteria for the exclusion of diagnosis of CC were: cases with histological clear-cut features of fatty changes and cirrhosis with definite etiology such as, excessive alcohol consumption (defined as ≥ 30 g of alcohol per day for men and 20 g per day for women); chronic use of hepatotoxic drugs (based on medical history); HCV antibody positivity; hepatitis B surface antigen positivity; autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis or genetic liver diseases such as hemochromatosis, alpha1-antitrypsin deficiency or Wilson’s disease (based on clinical grounds, appropriate serum bio-markers or imaging findings)[15].
For HCV-related cirrhosis the diagnosis was based on the presence of serum HCV-Ab and HCV RNA.
The following outcomes were assessed: (1) severity of liver disease at the time of first diagnosis; (2) liver decompensation (i.e., at least 1 episode of ascites, encephalopathy, or variceal bleeding either at presentation or during follow-up; (3) HCC, diagnosed according to current guidelines[16]; (4) orthotopic liver transplantation (OLT); and (5) death. At time of first diagnosis, the cirrhosis stage was assessed by Child-Pugh score. Death was considered to be liver-related if resulting from liver decompensation/hepatorenal syndrome and/or spontaneous bacterial peritonitis and/or variceal bleeding and/or HCC and/or OLT complications. Survival was calculated starting from the first diagnosis of cirrhosis. Extra-hepatic malignancies and cardiovascular events, particularly the presence of coronary artery disease, were recorded. In order to address a potential source of bias, we compared the prevalence of coronary artery disease in the CC cohorts with the HCV-related cirrhosis cohort and an age-matched (1:3) pathological control [652 patients hospitalized in the same period for non-liver conditions such as chronic obstructive pulmonary disease, acute pneumonia, type 2 diabetes (T2D), renal diseases]. Past coronary artery disease was diagnosed based on clinical charts (laboratory analysis, ECG, cardiac ultrasonography, coronary angiography).
All data were collected on the basis of “a priori” codification of parameters on a computerized database which was regularly updated during follow-up. The Ethics Boards of the two participating hospitals approved the study protocol.
The main metabolic features were defined as follows: T2D from a previous diagnosis, use of anti-diabetic medications, fasting glucose ≥ 126 mg/dL or HbA1c ≥ 6.5%, impaired fasting glucose (IFG) with blood glucose ≥ 100 mg/dL, central obesity with BMI ≥ 30 kg/m2 and/or waist circumference ≥ 94 cm in men and ≥ 80 cm in women; overweight with BMI ≥ 25 kg/m2 and < 30 kg/m2, high blood pressure by a previous diagnosis or blood pressure ≥ 130/85 mmHg or anti-hypertensive medications. Dyslipidemia was based on a previous diagnosis of dyslipidemia, use of lipid-lowering drugs, triglycerides ≥ 150 mg/dL, and/or serum total cholesterol ≥ 200 mg/dL, and/or HDL < 50 mg/dL in women and < 40 mg/dL in men. According to modified IDF criteria of the AHA/NHBLI[17], metabolic syndrome was diagnosed in the presence of at least 3 of these metabolic risk factors: T2D/IFG, central obesity/overweight, hypertension and dyslipidemia. Hyperuricemia was defined by serum uric acid ≥ 6 mg/dL in women and ≥ 7 mg/dL in men.
Quantitative variables were expressed as mean ± SD or median (range), as appropriate, and categorical variables as percentage. Numerical variables were compared using the Mann-Whitney U test. χ2 and Fisher’s exact tests were used for qualitative data when appropriate. Two-sided P values of less than 0.05 were considered statistically significant.
Multiple logistic regression analysis was performed to determine the independent determinants of outcomes variables (liver decompensation and HCC).
Overall survival was evaluated by Kaplan-Meier model. The log-rank test was used to compare overall survival and the Cox regression to identify factors associated with overall survival. Statistical tests were performed using SPSS 17.0 software (SPSS, Inc, Chicago, IL, United States).
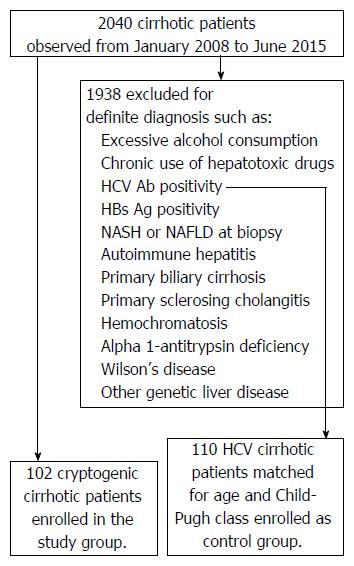
Based on inclusion and exclusion criteria, 102 patients with diagnosis of CC were enrolled in this study: 70 from Naples and 32 from Modena. They account for approximately 4%-5% of all cases of cirrhosis observed in the same period in our Units (Figure 1). Comparison of CC patients according to enrollment center revealed no significant differences as far as demographic, biochemical, and clinical characteristics were concerned (data not shown). Accordingly, data from these 2 cohorts were processed together. As a control group, 110 matched patients with HCV-related cirrhosis were enrolled.
The demographic, clinical, and laboratory characteristics of the studied subjects (CC and HCV cirrhosis) are summarized in Table 1, and the prevalences of metabolic features is shown in Figure 2. The mean age at diagnosis of CC patients was 66 ± 11 years [median (range): 66 (38-84) years]. There was a majority of women (63.7%, F:M = 1.8:1), whereas, in HCV cohort there was a higher prevalence of male (F:M = 1:3). Among CC patients, 14.1% were ex-smokers and 15.3% were active smokers. 11.8% consumed low amount of alcohol (< 30 g of alcohol per day for men and < 20 g per day for women); similar values were observed for HCV cirrhotic patients (data not sowed).
| Cryptogenic cirrhosis | Hepatitis C virus cirrhosis | P value | |
| No. of patients | 102 | 110 | |
| Age at diagnosis (yr), median | 66 (38-84) | 65 (48-86) | NS |
| Male sex (M:F ratio) | 36 (1:1.8) | 58 (3:1) | < 0.01 |
| BMI (mean ± SD) | 30 ± 5.7 | 27.1 ± 3.9 | < 0.05 |
| Low alcohol intake | 11.8% | 12.8% | NS |
| Past and current smokers | 29.4% | 27.2% | NS |
| AST (U/L), mean ± SD | 47 ± 43 | 122 ± 88 | < 0.01 |
| ALT (U/L), mean ± SD | 35 ± 25 | 102 ± 63 | < 0.01 |
| Υ-GT (U/L), mean ± SD | 126 ± 124 | 82 ± 61 | < 0.05 |
| Alph (U/L), mean ± SD | 172 ± 181 | 198 ± 180 | NS |
| Total bilirubin (mg/dL), mean ± SD | 2.8 ± 5.2 | 2.6 ± 4.2 | NS |
| Albumin (g/dL), mean ± SD | 3.4 ± 0.8 | 3.4 ± 0.9 | NS |
| INR, mean ± SD | 1.3 ± 0.6 | 1.4 ± 0.8 | NS |
| Platelets (103/mm3), mean ± SD | 120 ± 80 | 115 ± 60 | NS |
| Creatininemia (mg/dL), mean ± SD | 1.1 ± 0.7 | 1.1 ± 0.6 | NS |
| Ferritin (ng/mL), mean ± SD | 163 ± 153 | 186 ± 162 | NS |
| α-fetoprotein (ng/mL), median | 3.4 (0.6-3300) | 36 (2-600) | NS |
| Child-Pugh class A/B/C (%) | 47/31/22 | 48/29/23 | NS |
| HBsAb/HBcAb positive | 27.5% | 42.7% | < 0.03 |
| Extrahepatic tumors | 12.6% | 4.4% | NS |
Aminotransferases were normal or slightly elevated in CC; mean ALT values were 1.5-fold higher than cut-off levels for both men and women, whereas, they were significantly higher in HCV-related cirrhosis cases.
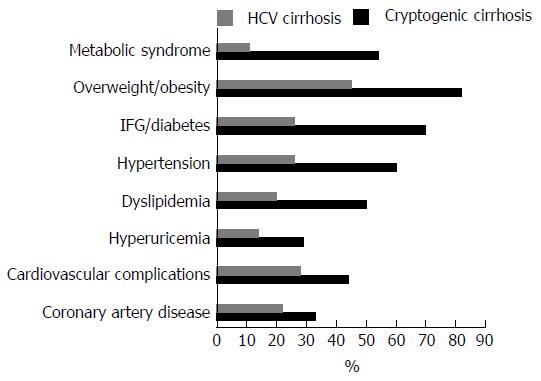
The majority of patients were overweight/obese (82.3%) and the mean BMI was 30.0 ± 5.7 kg/m2; 70% had IFG/T2D, 55.9% had high blood pressure and 50.0% had dyslipidemia 52.9% of patients had full-blown MS. We found hyperuricemia in 29.2%, 14.4% had hypothyroidism and 58.7% had gallstones or had previously undergone cholecystectomy for gallstones. As showed in Figure 2 these metabolic conditions were significantly lesser in HCV patients.
Cholelithiasis or cholecystectomy were present in 58.7% of CC and in 24.2% of HCV cirrhosis (P < 0.01). Hypothyroidism was present in CC and HCV cirrhosis in 14.4% and 5.6% (P < 0.01), respectively. There was a history of extrahepatic tumors in 12.4% of CC patients and in 5% of HCV patients; 43.3% of patients had a positive history for cardiovascular complications, in the majority of cases coronary artery disease. Interestingly, the prevalence of coronary artery disease in our CC cohort was significantly higher than that found in two age-matched control groups (P = 0.002). Coronary artery disease was present in 33.3% of CC patients vs 21.6% in the cohort of HCV cirrhotic patients used as a control for liver disease vs 12.0% in a heterogeneous cohort of 652 patients hospitalized for reasons other than liver diseases [median age 66 (47-86), 48% men].
Twenty-eight patients (27.5%) had anti-HBV antibodies: 21 were HBsAb positive and 7 only HBcAb positive. All but one of these patients were serum HBV DNA negative. The HBV DNA positive patient was excluded from the study. A higher prevalence of anti-HBV-Ab was observed in the HCV cohort (42, 7%).
At the first observation, 46.7% of patients had Child-Pugh class A cirrhosis, 31.1% were Child-Pugh B and 22.2% were Child-Pugh C; 67.7% of CC had esophageal varices, a prevalence similar to that observed in HCV-related cirrhosis.
Table 2 shows the metabolic characteristics of patients with CC. More than 88% of patients were overweight/obese and a full metabolic syndrome was observed in 52.9%. In patients with metabolic syndrome, higher prevalences of female, obesity and IFG or diabetes were present. A minority of patients (11.8%) was lean and without component of metabolic syndrome. The lean subgroup showed a significant lower prevalence of cardiovascular diseases than the overweight/obese patients.
| With full metabolic syndrome, n = 54 (52.9%) | Without full metabolic syndrome, n = 36 (35.8%) | Without components of metabolic syndrome, n = 12 (11.8%) | |
| Age (yr), median | 66 (48-84) | 66 (42-84) | 70 (38-81) |
| Female sex | 70.4% | 58.3% | 50%a |
| Obesity | 69% | 39% | 0%a |
| Overweight | 27% | 55.5% | 0%a |
| IFG/T2D | 81.8% | 61% | 33%a |
| Ascites | 50% | 61.3% | 55% |
| HCC | 20% | 14% | 18% |
| Cardiovascular diseases | 48% | 41.6% | 25%a |
The median follow-up period was of 42 mo (range 10-96 mo) for CC and 40.8 mo (range 10-95 mo) for HCV-related cirrhosis (Table 3).
| Cryptogenic cirrhosis | Hepatitis C virus cirrhosis | |
| Follow up, median, mo (range) | 42 (10-96) | 40.8 (10-95) |
| Liver decompensation | 60.8% | 55.4% |
| Ascites | 54.9% | 51.8% |
| Encephalopathy | 25.5% | 21.8% |
| Esophageal variceal bleeding | 6.9% | 6.3% |
| Hepatocellular carcinoma | 16.7% | 17.2% |
| Orthotopic liver transplantation | 1.0% | 3.6% |
| Death | 39.2% | 30.0% |
| Liver-related | 77.5% | 78.8% |
| Cardiovascular events | 10.0% | 9.1% |
| Non-liver-related cancer | 7.5% | 3.0% |
| Other causes | 5.0% | 9.1% |
| Time to death, median, mo (range) | 26 (1-96) | 28 (4-86) |
Liver decompensation: During follow up, 60.7% of patients with CC developed liver decompensation: ascites occurred in 54.9%, encephalopathy in 25.5% and esophageal variceal bleeding in 6.9% (Table 3). A similar incidence of ascites, encephalopathy and variceal bleeding was observed in the HCV cohort (Table 3). A similar incidence of liver decompensation for patients with a full metabolic syndrome, those with a component of metabolic syndrome and lean.
Liver transplantation: Only 1 patient with CC and 4 (3.6%) HCV cirrhotic patients, underwent OLT (Table 3). The patient with CC died after liver transplantation.
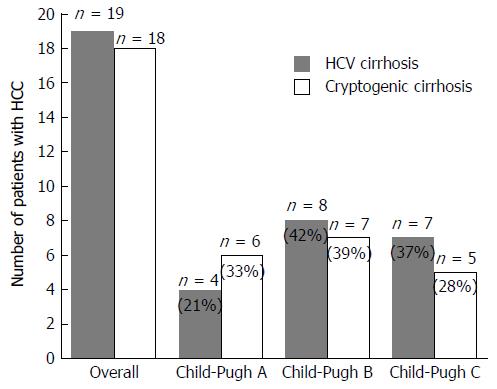
HCC development: Overall, among CC, 18 patients (17.6%) developed HCC, in 6 of them HCC and cirrhosis were diagnosed at the same time and in 12 during follow up (Table 4). Of the HCV cirrhotic patients, 19 (17.2%) had HCC, 4 of whom at enrollment and 15 developing during follow up (Table 4). The cumulative incidence of HCC developing during follow up was 3.5% per year for CC and 4.5% per year for HCV cirrhosis. The median age of patients with HCC was 73 years for CC and 72 for HCV; the male:female ratio was 1.5:1 and 3:1 for CC and HCV, respectively. The Child-Pugh classification was: A, 39%; B, 39%; C, 22% with a Child A:B/C ratio of 0.50 for CC, and A, 21%; B, 42%; C, 37% with a Child A:B/C ratio of 0.27 for HCV cirrhosis (Figure 3). The prevalence of HBV markers (HBsAb/HBcAb) in CC and HCV was 55.5% and 57,7%, respectively. Of the CC patients, HCC was found in 16 patients (20.5%) with one or more metabolic components, and in 2 lean patients (17%) (Table 2).
| Cryptogenic cirrhosis | HCV cirrhosis | |
| HCC at first diagnosis | 6 (5.8%) | 4 (3.6%) |
| HCC during follow-up | 12 (12.5%) | 15 (14.1%) |
| HCC overall | 17.6% | 17.2% |
| Annual rate of incidence | 3.5% | 4.5% |
| Age, median, yr (range) | 73 (63-82) | 72 (60-80) |
| Male sex (M:F ratio) | 52% (1.5:1) | 58% (3:1) |
| Child-Pugh A/B/C (%) | 39/39/22 | 21/42/37 |
| Ratio Child-Pugh A:(B-C) | 0.5 | 0.27 |
| α-fetoprotein (ng/mL), median (range) | 48 (6-800) | 66 (6-680) |
Survival: Death in CC and HCV cirrhosis occurred in 40 patients (39.2%) and 33 patients (30%), respectively (Table 3). The vast majority of deaths in both CC and HCV cirrhosis were liver-related (Table 3).
The mortality rate for CC patients with full metabolic syndrome, with a component of metabolic syndrome and the lean was 42.5%, 36.1% and 33.3%, respectively.
The median time to death after cirrhosis detection was 26 mo for CC and 28 mo for HCV-related cirrhosis (Table 3).
The median survival in CC was 60 mo (95%CI: 42-78); the calculated probability of overall survival was 86%, 67% and 35% at 12, 36 and 120 mo, respectively.
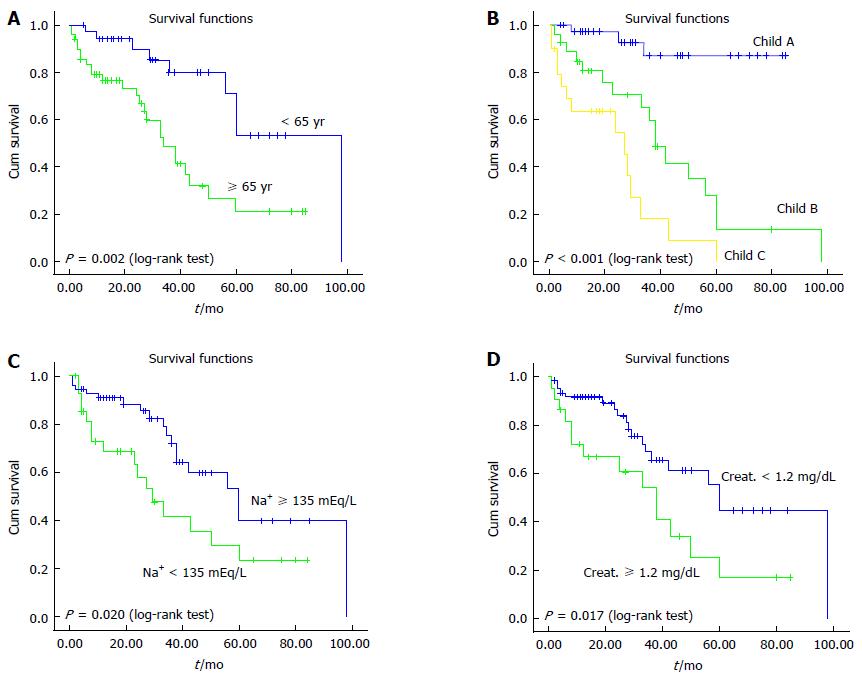
Predictors of outcome in CC: Table 5 shows the predictors of both cirrhosis decompensation and development of HCC. By univariate analysis, the predictors for liver decompensation were age and Child-Pugh class at the first observation, and baseline serum sodium and creatinine levels. Multivariate analysis showed that Child-Pugh class at presentation was the only independent factors associated with liver decompensation. The only predictors for HCC were baseline serum sodium and creatinine levels; hyponatremia was found to be independently associated with HCC also at multivariate analysis.
| Outcome | Univariate analysis, OR (95%CI) | P value | Multivariate analysis, OR (95%CI) | P value |
| Liver decompensation | ||||
| Age at diagnosis | 1.06 (1.01-1.11) | < 0.01 | 0.97 (0.90-1.05) | NS |
| Male sex | 1.00 (0.41-2.40) | NS | 0.85 (0.18-4.12) | NS |
| Child B vs A | 40.0 (8.03-199) | < 0.001 | 111 (11-1101) | < 0.001 |
| Child C vs A | 60.8 (7.2-513) | < 0.001 | 102 (7-1480) | < 0.001 |
| Renal impairment1 | 3.75 (1.14-12.3) | < 0.023 | 2.90 (0.40-21.1) | NS |
| Hyponatremia2 | 4.45 (1.50-13.2) | < 0.005 | 2.89 (0.48-17.4) | NS |
| Hepatocellular carcinoma | ||||
| Age at diagnosis | 1.04 (0.99-1.10) | NS | 1.03 (0.95-1.12) | NS |
| Male sex | 2.30 (0.79-6.70) | NS | 2.38 (0.67-8.44) | NS |
| Child B vs A | 1.09 (0.31-3.84) | NS | 0.62 (0.13-2.86) | NS |
| Child C vs A | 1.67 (0.46-6.10) | NS | 0.76 (0.14-4.04) | NS |
| Renal impairment1 | 3.62 (1.18-11.0) | < 0.019 | 2.37 (0.52-10.9) | NS |
| Hyponatremia2 | 4.37 (1.18-13.15) | < 0.006 | 4.11 (1.14-14.9) | < 0.031 |
Age and Child-Pugh class at presentation, and the baseline serum sodium and creatinine levels were the variables significantly associated with survival. Multivariate Cox regression analysis confirmed that age and Child-Pugh class at presentation were independent predictors of survival. Sex, BMI, T2D, dyslipidemia and cardiovascular complications did not emerge as significant predictors of any outcomes. Variables significantly associated with liver-related outcomes are summarized in Table 6. Figure 4 shows comparison of survival according to age, Child-Pugh class at presentation and baseline serum sodium and creatinine levels.
| Outcome | Univariate analysis, HR (95%CI) | P value | Multivariate analysis, HR (95%CI) | P value |
| Overall mortality | ||||
| Age at diagnosis | 1.07 (1.03-1.10) | < 0.001 | 1.11 (1.04-1.18) | 0.001 |
| Male sex | 1.10 (0.55-2.20) | NS | 1.07 (0.46-2.48) | NS |
| Child B vs A | 9.75 (2.85-33.32) | < 0.001 | 8.35 (2.16-32.29) | < 0.002 |
| Child C vs A | 23.62 (6.64-84.05) | < 0.005 | 34.64 (7.5-160.7) | < 0.001 |
| Renal impairment1 | 2.63 (1.31-5.30) | < 0.007 | 0.65 (0.28-1.54) | NS |
| Hyponatremia2 | 2.66 (1.34-27) | < 0.005 | 0.84 (0.36-1.95) | NS |
This study evaluated the features of CC and associated factors in a cohort at their first diagnosis, as well as developments during follow up. Five major characteristic were identified: (1) CC cases had a high prevalence of metabolic syndrome and oncologic/cardiovascular co-morbidities; (2) CC patients without full-blown metabolic syndrome had, in a high proportion, at least one or more metabolic derangements and only a minority of patients did not have any metabolic alterations; (3) CC was associated with a poor prognosis, and with a significant occurrence of liver decompensation and HCC; (4) age, advanced liver damage and kidney impairment were predictors of liver decompensation and death in CC; and (5) a high proportion of HCC was observed in an early stage of CC.
The CC cohort was compared to age- and child-Pugh-matched HCV-related cirrhosis cohort. CC showed a higher prevalence of female sex, of metabolic features, of cardiovascular diseases, extrahepatic malignancy, and lower serum levels of aminotransferases. However, during follow up, there were no differences in the overall incidence of liver decompensation, development of HCC and survival. Of concern, the incidence of HCC in CC child-Pugh class A was 1.8 times higher than that observed in patients with HCV cirrhosis.
Our series recapitulates the demographic and clinical sketch of CC, a disease with a high prevalence of females in their sixties, associated with slightly elevated aminotransferases values and metabolic derangements[7-9,18-20]. The prevalence of body weight excess and IFG/T2D in our cohort, however, ranks among the highest ever reported in a CC case series[8,9,18,19,21]. It is important to highlight that T2D often develops as complication of cirrhosis per se and, therefore, it may be argued that it results from, rather than precedes cirrhosis[22]. Nevertheless, the concurrence of either the full-blown metabolic syndrome (54%) or its individual features (in particular overweight/obesity), which are not classically found in cirrhosis due to other etiologies, and the high rate of cardiovascular co-morbidities, are in agreement with a primarily metabolic etiology in a substantial number of the cases of cirrhosis observed in our study. Supporting this view, nearly one-third of our patients had hyperuricemia, another cardio-metabolic risk factor associated with a higher risk of cirrhosis[23], and with development and progression of nonalcoholic fatty liver disease (NAFLD)[24,25], which is more common in CC patients compared to cirrhosis due to identifiable etiologies[19]. Further suggesting a pathogenic link of NAFLD with CC, a high proportion of our patients had either hypothyroidism or gallstones/cholecystectomy, which may be additional clues to NAFLD as a predisposing factor to CC[26-29] such as first suggested by Ludwig in 1980[30]. Thus, the overall clinic-pathologic analysis of our cohort of patients at their first diagnosis of CC seems to indicate that the leading cause include previously unrecognized NAFLD.
We also found that only a small number of patients with CC (about 12% of our population) were lean and did not have any components of metabolic syndrome. These patients were characterized by an older age and lower prevalence of T2D and cardiovascular diseases and thus had different features from those of other dysmetabolic CC patients. It is not possible to clearly establish the putative underlying etiology of cirrhosis in this subset of patients, but occult alcohol consumption or viral, autoimmune or genetic factors may account for the development of CC. However, the clinical outcome such as development of ascites, HCC and death was similar for the overweight/obese patients and the HCV-related cirrhosis groups, suggesting that, irrespective of underlying mechanisms, once cirrhosis is established it follows a similar natural course.
The natural history of CC, in term of morbidity and mortality, has been explored only marginally, and with conflicting results[8,21,31,32]. At variance with previous studies that had suggested a mild course of disease[31-35], our cohort of CC patients had a high rate of complications of cirrhosis such as liver failure and HCC that were similar to those observed in the HCV-related cirrhosis cases. In agreement with some of the previous studies[8,18,21], such complications may arise as the inaugural manifestation of liver diseases. Of concern, we confirm that the hepatocarcinogenic potential of CC is not negligible[21,35,36]. Indeed, the cumulative incidence per year of HCC was 3.5%, slightly higher than those reported in two previous studies, 2.6% and 2.7%, respectively[32,36]; this incidence was slightly lower than that observed (4.5%) in HCV-related cirrhosis cohort. Overall, our results are in agreement with other studies[18,37,38] , which reported a substantial risk of HCC developing in CC.
The estimated long-term prognosis of our patients was poor; the overall median survival was of about 5 years and only one-third of patients survived at 10 years. This prognosis is much more severe than that reported for metabolic-cirrhosis[29,34,35], and is in agreement with two previous studies on CC[34,39]. Although mortality was overwhelmingly liver-related, our cohort had a high prevalence of cancer and cardiovascular diseases. The prevalence of coronary artery disease in our cohort was significantly higher than that found in two age-matched groups of patients at high cardiovascular risk owing to either traditional (T2D, kidney and lung diseases) or emerging (HCV cirrhotic patients) risk factors[40]. Finally, in our study, a single patient underwent OLT and died of complications after surgery. Owing to age and co-morbidities, patients with NASH-cirrhosis and CC are less likely to receive OLT[32], have a high risk of NASH or cryptogenic liver disease recurrence after OLT[41], and a higher rate of postoperative complications and mortality than other etiologies[42].
In accordance with a previous study on CC[21], our study showed that age at presentation and severity of liver and kidney tests predicted liver failure and death. No association was found between BMI, T2D and/or other metabolic features with liver-related outcomes. This could be the result of the limited number of events and/or of the high prevalence of metabolic factors.
Of interest, was the observation that no condition predict the development of HCC and that a high proportion was observed in the early phase of cirrhosis. Previous, studies have shown consistently that HCC in NAFLD patients may develop even without cirrhosis, is diagnosed late and, therefore, has a poor prognosis[37,38]. The role of genetic factors in the development of HCC has consistently been suggested by several studies[43-45].
Our study has a number of points of strength in that we have characterized the features of a cohort of cirrhotic patients with a previously unknown liver disease as well as the predictors of the outcomes. Considering the scarcity of published data on such population, we have reason to believe that our findings may help to identify these patients earlier, possibly before the development of cirrhosis, with the aim of preventing and rapidly treating hepatic and extrahepatic complications, thus improving survival and quality of life for these individuals.
As our study was conducted at two tertiary centers, this may have created a selection bias. In addition, it is a cohort study including previously unknown cirrhotic patients with an advanced stage of liver disease and, therefore, so the natural history of CC might not have been faithfully characterized. Moreover, this study specifically identifies risk factors and natural history of CC in the center and south of Italy and our findings may not apply to other ethnicities or geographical area. Finally, the number of patients may be relatively too small to identify all predictors of the outcome. However, we highlight that the size of our cohort was not inferior to those previously published case-series on CC[8,21,31,32].
In conclusion, the data of this study confirm that CC likely results from the progression of unrecognised NASH in a large proportion of cases; hence it may be better defined as “metabolic cirrhosis” in most cases. The subset of lean patients with CC, however, may suggest non-metabolic risk factors, and this group therefore deserves further investigation. The predictors of hepatic complications and death in patients with CC are similar to those of cirrhosis due to HCV. Patients with CC have a high risk of developing severe liver complications and a poor prognosis owing to liver-related death and cardiovascular and oncologic co-morbidities. The risk of HCC developing in CC, even in the early phase, is not negligible, and thus warrants the setting up of surveillance programs for early detection of NAFLD-NASH in patients with dysmetabolic features.
Cryptogenic cirrhosis (CC) are often incidentally discovered or diagnosed in the late stage, are poor characterized as well as natural history is not well known.
CC have a high clinical impact considering that account for up to 30% of cases of cirrhosis and about 10% of liver transplantsand thus, there is a need to know their characteristics and outcomes. This study investigated the clinical features at first diagnosis, the outcomes and associated conditions of CC.
The results showed that CC are associated with metabolic features, poor prognosis, hepatocellular carcinoma (HCC) development in early stage, cardiovascular diseases and extrahepatic cancer risk. The data suggest an etiologic role of undiagnosed nonalcoholic fatty liver disease in a high percentage of cases of CC.
The data are utilizing in the clinical setting to propose screening strategies for early detection of liver impairment in dysmetabolic subjects and early screening for HCC.
CC is cirrhosis of unknown etiology, in particular with no history of alcoholism or previous infective hepatitis. At present, in a large proportion of cases, CC may be best-defined “metabolic cirrhosis”.
This is an interesting manuscript by Rinaldi et al regarding the natural history of CC compared to hepatitis C virus-related cirrhosis. The manuscript is well-structured, the methodology and the sample size seem appropriate, and overall the topic is relevant for the field.
| 1. | GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 629] [Reference Citation Analysis (1)] |
| 2. | Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, Naghavi M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 720] [Cited by in RCA: 761] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 3. | Hsiang JC, Bai WW, Raos Z, Stableforth W, Upton A, Selvaratnam S, Gane EJ, Gerred SJ. Epidemiology, disease burden and outcomes of cirrhosis in a large secondary care hospital in South Auckland, New Zealand. Intern Med J. 2015;45:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Runyon BA. A Primer on Detecting Cirrhosis and Caring for These Patients without Causing Harm. Int J Hepatol. 2011;2011:801983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1357] [Article Influence: 113.1] [Reference Citation Analysis (0)] |
| 6. | Clark JM, Diehl AM. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000-3004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 288] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74-80. [PubMed] |
| 8. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 751] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 9. | Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 335] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 10. | Gomez EV, Rodriguez YS, Bertot LC, Gonzalez AT, Perez YM, Soler EA, Garcia AY, Blanco LP. The natural history of compensated HCV-related cirrhosis: a prospective long-term study. J Hepatol. 2013;58:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1892] [Cited by in RCA: 2216] [Article Influence: 110.8] [Reference Citation Analysis (3)] |
| 12. | Lonardo A, Sookoian S, Chonchol M, Loria P, Targher G. Cardiovascular and systemic risk in nonalcoholic fatty liver disease - atherosclerosis as a major player in the natural course of NAFLD. Curr Pharm Des. 2013;19:5177-5192. [PubMed] |
| 13. | Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, Lonardo A. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (4)] |
| 14. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] |
| 15. | Morisco F, Pagliaro L, Caporaso N, Bianco E, Sagliocca L, Fargion S, Smedile A, Salvagnini M, Mele A. Consensus recommendations for managing asymptomatic persistent non-virus non-alcohol related elevation of aminotransferase levels: suggestions for diagnostic procedures and monitoring. Dig Liver Dis. 2008;40:585-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1127] [Article Influence: 93.9] [Reference Citation Analysis (1)] |
| 17. | Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8720] [Cited by in RCA: 10967] [Article Influence: 645.1] [Reference Citation Analysis (0)] |
| 18. | Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1021] [Article Influence: 42.5] [Reference Citation Analysis (1)] |
| 19. | Tellez-Avila FI, Sanchez-Avila F, García-Saenz-de-Sicilia M, Chavez-Tapia NC, Franco-Guzman AM, Lopez-Arce G, Cerda-Contreras E, Uribe M. Prevalence of metabolic syndrome, obesity and diabetes type 2 in cryptogenic cirrhosis. World J Gastroenterol. 2008;14:4771-4775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Carulli L, Dei Cas A, Nascimbeni F. Synchronous cryptogenic liver cirrhosis and idiopathic pulmonary fibrosis: a clue to telomere involvement. Hepatology. 2012;56:2001-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Ratziu V, Bonyhay L, Di Martino V, Charlotte F, Cavallaro L, Sayegh-Tainturier MH, Giral P, Grimaldi A, Opolon P, Poynard T. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 317] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 22. | Cacciatore L, Cozzolino G, Giardina MG, De Marco F, Sacca L, Esposito P, Francica G, Lonardo A, Matarazzo M, Varriale A. Abnormalities of glucose metabolism induced by liver cirrhosis and glycosylated hemoglobin levels in chronic liver disease. Diabetes Res. 1988;7:185-188. [PubMed] |
| 23. | Afzali A, Weiss NS, Boyko EJ, Ioannou GN. Association between serum uric acid level and chronic liver disease in the United States. Hepatology. 2010;52:578-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Lonardo A, Loria P, Leonardi F, Borsatti A, Neri P, Pulvirenti M, Verrone AM, Bagni A, Bertolotti M, Ganazzi D. Fasting insulin and uric acid levels but not indices of iron metabolism are independent predictors of non-alcoholic fatty liver disease. A case-control study. Dig Liver Dis. 2002;34:204-211. [PubMed] |
| 25. | Petta S, Cammà C, Cabibi D, Di Marco V, Craxì A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2011;34:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Carulli L, Ballestri S, Lonardo A, Lami F, Violi E, Losi L, Bonilauri L, Verrone AM, Odoardi MR, Scaglioni F. Is nonalcoholic steatohepatitis associated with a high-though-normal thyroid stimulating hormone level and lower cholesterol levels? Intern Emerg Med. 2013;8:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Chung GE, Kim D, Kim W, Yim JY, Park MJ, Kim YJ, Yoon JH, Lee HS. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 28. | Loria P, Lonardo A, Lombardini S, Carulli L, Verrone A, Ganazzi D, Rudilosso A, D’Amico R, Bertolotti M, Carulli N. Gallstone disease in non-alcoholic fatty liver: prevalence and associated factors. J Gastroenterol Hepatol. 2005;20:1176-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Ruhl CE, Everhart JE. Relationship of non-alcoholic fatty liver disease with cholecystectomy in the US population. Am J Gastroenterol. 2013;108:952-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 30. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. [PubMed] |
| 31. | Kojima H, Sakurai S, Matsumura M, Umemoto N, Uemura M, Morimoto H, Tamagawa Y, Fukui H. Cryptogenic cirrhosis in the region where obesity is not prevalent. World J Gastroenterol. 2006;12:2080-2085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | O’Leary JG, Landaverde C, Jennings L, Goldstein RM, Davis GL. Patients with NASH and cryptogenic cirrhosis are less likely than those with hepatitis C to receive liver transplants. Clin Gastroenterol Hepatol. 2011;9:700-704.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 33. | Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, Adams LA, Charatcharoenwitthaya P, Topping JH, Bugianesi E. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208-1216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 34. | Hui JM, Kench JG, Chitturi S, Sud A, Farrell GC, Byth K, Hall P, Khan M, George J. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 294] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 35. | Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Heuman D, Coterrell A, Fisher RA. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 367] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 36. | Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 982] [Article Influence: 61.4] [Reference Citation Analysis (1)] |
| 37. | Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 428] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 38. | Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology. 2016;63:827-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 480] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 39. | Senanayake SM, Niriella MA, Weerasinghe SK, Kasturiratne A, de Alwis JP, de Silva AP, Dassanayake AS, de Silva HJ. Survival of patients with alcoholic and cryptogenic cirrhosis without liver transplantation: a single center retrospective study. BMC Res Notes. 2012;5:663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Adinolfi LE, Zampino R, Restivo L, Lonardo A, Guerrera B, Marrone A, Nascimbeni F, Florio A, Loria P. Chronic hepatitis C virus infection and atherosclerosis: clinical impact and mechanisms. World J Gastroenterol. 2014;20:3410-3417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Contos MJ, Cales W, Sterling RK, Luketic VA, Shiffman ML, Mills AS, Fisher RA, Ham J, Sanyal AJ. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363-373. [PubMed] |
| 42. | Álamo JM, Bernal C, Barrera L, Marín LM, Suárez G, Serrano J, Gómez MA, Padillo FJ. Liver transplantation in patients with cryptogenic cirrhosis: long-term follow-up. Transplant Proc. 2011;43:2230-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Giannini EG, Marabotto E, Savarino V, Trevisani F, di Nolfo MA, Del Poggio P, Benvegnù L, Farinati F, Zoli M, Borzio F. Hepatocellular carcinoma in patients with cryptogenic cirrhosis. Clin Gastroenterol Hepatol. 2009;7:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 44. | Berretta M, Stanzione B, Di Francia R, Tirelli U. The expression of PD-L1 APE1 and P53 in hepatocellular carcinoma and its relationship to clinical pathology. Eur Rev Med Pharmacol Sci. 2015;19:4207-4209. [PubMed] |
| 45. | Perumpail RB, Liu A, Wong RJ, Ahmed A, Harrison SA. Pathogenesis of hepatocarcinogenesis in non-cirrhotic nonalcoholic fatty liver disease: Potential mechanistic pathways. World J Hepatol. 2015;7:2384-2388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Nakajima H, Prieto J, Ruiz-Margain A S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF