Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1353
Peer-review started: October 1, 2016
First decision: October 20, 2016
Revised: November 8, 2016
Accepted: December 8, 2016
Article in press: December 8, 2016
Published online: February 28, 2017
Processing time: 149 Days and 19.9 Hours
To evaluate the anti-inflammatory intestinal effect of the ethanolic extract (EtOHE) and hexane phase (HexP) obtained from the leaves of Combretum duarteanum (Cd).
Inflammatory bowel disease was induced using trinitrobenzenesulfonic acid in acute and relapsed ulcerative colitis in rat models. Damage scores, and biochemical, histological and immunohistochemical parameters were evaluated.
Both Cd-EtOHE and Cd-HexP caused significant reductions in macroscopic lesion scores and ulcerative lesion areas. The vegetable samples inhibited myeloperoxidase increase, as well as pro-inflammatory cytokines TNF-α and IL-1β. Anti-inflammatory cytokine IL-10 also increased in animals treated with the tested plant samples. The anti-inflammatory intestinal effect is related to decreased expression of cyclooxygenase-2, proliferating cell nuclear antigen, and an increase in superoxide dismutase.
The data indicate anti-inflammatory intestinal activity. The effects may also involve participation of the antioxidant system and principal cytokines relating to inflammatory bowel disease.
Core tip: Inflammatory bowel diseases are chronic inflammatory disorders that include Crohn’s disease and ulcerative colitis (UC). Genetic, immunologic and environmental factors are postulated as possible etiologic agents. Their conventional treatment is centered in reducing inflammation and abnormal symptom relief. A variety of herbal medicines have been demonstrated to produce promising results in the treatment of those diseases. Combretum duarteanum is a species popularly used in folk medicine to treat inflammation. Thus, the present study was designed to evaluate the intestinal anti-inflammatory effect in an UC rat model, contributing to the safe use and collaborating with the scientific knowledge of natural products.
- Citation: de Morais Lima GR, Machado FDF, Périco LL, de Faria FM, Luiz-Ferreira A, Souza Brito ARM, Pellizzon CH, Hiruma-Lima CA, Tavares JF, Barbosa Filho JM, Batista LM. Anti-inflammatory intestinal activity of Combretum duarteanum Cambess. in trinitrobenzene sulfonic acid colitis model. World J Gastroenterol 2017; 23(8): 1353-1366
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1353
The inflammatory bowel diseases (IBDs) are chronic disorders of the gastrointestinal (GI) tract characterized by alternating periods of remission and relapse[1]. These diseases represent a large group of inflammatory disorders, the most common being Crohn’s disease (CD) and ulcerative colitis (UC)[2,3].
CD can affect any part of the GI tract and has the classic symptoms of fatigue, prolonged diarrhea (with or without bleeding), abdominal pain, weight loss, and fever[4,5]. UC is a type of chronic inflammation restricted to the colon; however, the entire large intestine may be affected[6]. Affected patients show symptoms such as rectal bleeding, frequent bowel movements, tenesmus, rectal mucus discharge, and abdominal pain[5].
The etiology of IBDs is still not fully understood; however, it is believed that environmental, genetic and immunologic factors have important roles in their occurrence and progression[4,7].
Emerging models in the study of IBD pathogenesis suggest three key disease development factors: (1) breaking the intestinal barrier function; (2) lamina immune cell exposure to luminal contents; and (3) exacerbation of immune response. However, the factors responsible for initiation and perpetuation of the cycle leading to exacerbation of the disease are still unclear[8,9].
A combination of genetic and environmental factors may foment changes in the intestinal mucosal barrier function; this allows translocation of luminal antigens (commensal bacteria or microbial products) into the intestinal wall, and consequent immune cell activation and excessive production of cytokines, causing the acute phase of inflammation. If the acute inflammatory process is not resolved by anti-inflammatory mechanisms and suppression of pro-inflammatory cytokines, chronic intestinal inflammation develops. This can lead to tissue destruction and complications of the disease[10].
Conventional treatments are aimed at reducing inflammation and consequent abnormal symptom relief. Patients with UC are treated with amino-salicylates, corticosteroids, and immunomodulatory drugs[11].
Natural products have become the most attractive source of new drugs for the treatment and prevention of diseases and their use is constantly expanding worldwide. A variety of herbal medicines have been shown to produce promising results in the treatment of peptic ulcer and IBD[11-14].
Combretum duarteanum (C. duarteanum, Cd) Cambess., the species selected for this study, is popularly known as “mufumbo, cipiúba, cipaúba”. This shrubby species is exclusive to South America with registrations in Bolivia, Paraguay, and Brazil. It occurs in the northern and northeastern regions of Brazil, being associated with the “caatinga” biome[15,16].
In folk medicine, C. duarteanum is used to treat pain, inflammation and GI tract disorders, which justifies its selection, using ethno-pharmacological indication as the criterion of choice. Phytochemical studies suggest the presence of flavonoids and triterpenes, whose pharmacological effects have been attributed[16-18].
C. duarteanum has presented in vitro and in vivo anti-inflammatory, anti-nociceptive, and antioxidant capacities[15]. Quintans et al[17] demonstrated orofacial nociceptive activity as promoted by the hexane phase and Fridelin terpenes, isolated from the species studied.
de Morais Lima et al[16,19] demonstrated gastro-protective and antiulcer activity in C. duarteanum in different models of acute ulcer induction (acidified ethanol, ethanol, nonsteroidal anti-inflammatory drugs (commonly known as NSAIDs), stress, pylorus ligature, acetic acid) in animals. Previous studies demonstrated low toxicity and no change of liver enzymes in animals treated with the tested plant sample for 15 d in acid acetic-induced gastric ulcer model[19].
Given the need for new IBD therapies, this study aimed to evaluate the intestinal anti-inflammatory activity promoted by the species C. duarteanum, validating its popular use and contributing to the search for new therapies for diseases that affect the GI tract.
The drugs and reagents were prepared immediately before use. The following drugs were used: trinitrobenzenesulfonic acid (TNBS) (Sigma-Aldrich), ketamine 5% (Vetanarcol), xilazine 2% (Dorcipec), ethanol (Merck®), Tween 80 (Merck®), sodium chloride (Sigma-Aldrich). TNF-α, IL-1β and IL-10 in enzyme-linked immunosorbent assay (ELISA) kits were provided by R&D Systems Inc.
Plant samples used in intestinal anti-inflammatory activity research experiments in rats were obtained from the leaves of C. duarteanum Cambess., collected at Serra Branca City, Paraíba State, Brazil, in 2010. The species was identified by Dr. Maria Fatima Agra and a voucher specimen (No. 6767) was deposited in the Herbarium Prof. Lauro Pires Xavier (JPB) at the Universidade Federal da Paraíba.
The ethanolic extract (Cd-EtOHE) and the hexane phase (Cd-HexP) obtained from the leaves of C. duarteanum Cambess. were provided by Dr. Josean Fechine Tavares and his group, all of PgPNSB/UFPB.
The dried leaves (5 kg) were powdered, extracted with ethanol, stirred, and macerated at room temperature for approximately 48 h, with the procedure being repeated three times. The solvent was fully evaporated under reduced pressure, and the extract (yield of 200 g) was concentrated. The Cd-EtOHE was subjected to liquid-liquid partition with the following solvents: hexane, chloroform (CHCl3), and ethyl acetate (EtOAc), obtaining their respective phases. This step was repeated to secure the required quantity for the study.
Investigation of Cd-EtOHE and Cd-HexP effects on acute phase intestinal inflammation (TNBS)-induced in rats: The intestinal anti-inflammatory activity of Cd-EtOHE and Cd-HexP was assessed in rats using the Morris et al[20] method. Male Wistar rats (n = 5-8) fasted for 24 h were divided into four groups: non-colitic, colitic, Cd-EtOHE and Cd-HexP. The animals were anesthetized for rectal administration of TNBS (2,4,6-trinitro-benzene sulfonic acid) - 10 mg solubilized in 0.25 mL of 50% v/v ethanol. The induction of inflammation was performed with the aid of a probe (2-mm diameter), which was inserted about 8 cm into the rectum of the animal. After TNBS administration, animals were maintained upside down for 15 min to enable total absorption of the administered inducing agent. The non-colitic group underwent the same procedures but they did not receive TNBS.
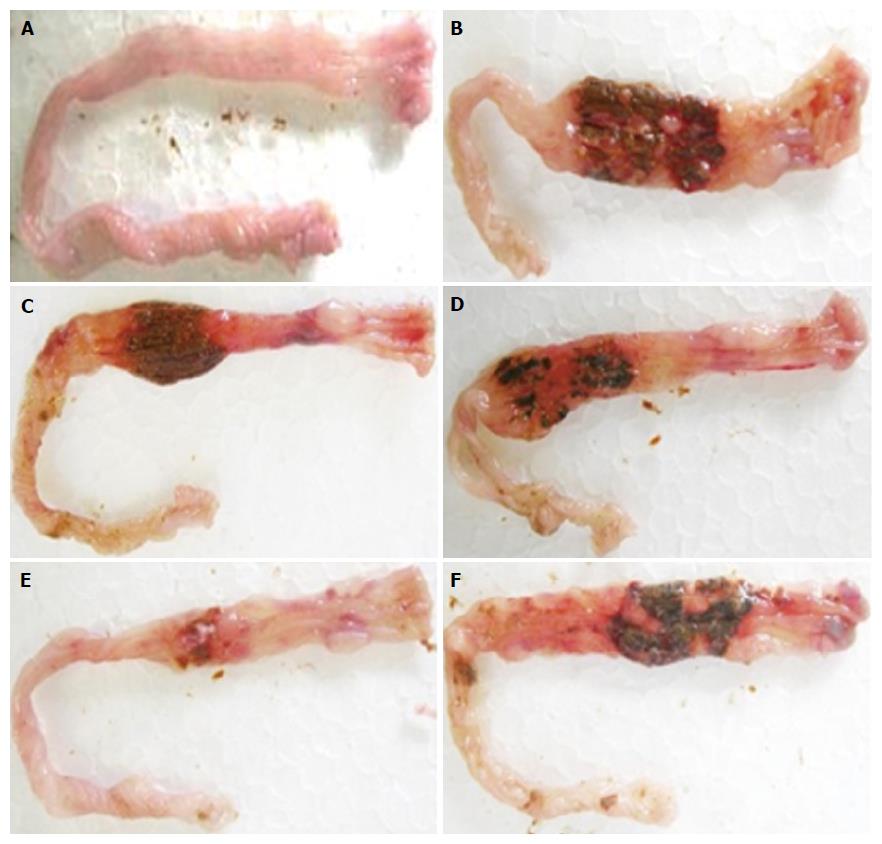
Each group of rats was pretreated with vehicle (12% Tween 80), Cd-EtOHE (31.25, 62.5, 125, 250 mg/kg) or Cd-HexP (31.25, 62.5, 125, 250 mg/kg), at 48, 24 and 1 h prior to administration of TNBS/50% ethanol, and at 24 h after colitis induction. At 48 h after inducing inflammation, the animals were euthanized and colonic segments were removed, opened, washed, and photographed for quantification of ulcerative lesion area (ULA) and macroscopic score evaluation of the intestinal inflammatory process. Analysis of the extent of intestinal injury was performed according to the scale described previously by Bell et al[21]. General parameters such as diarrhea and the colon weight/length ratio were also evaluated. The most effective doses obtained in this model were used in the chronic model with relapse of UC in rats.
Investigation of Cd-EtOHE and Cd-HexP effects in the chronic phase with intestinal inflammation relapse induced by TNBS in rats: Male Wistar rats (n = 7-9) were divided into non-colitic, colitic, Cd-EtOHE and Cd-Hexp groups. After 24 h of fasting, induction of intestinal inflammation was performed with TNBS (10 mg/0.25 mL ethanol 50% v/v, rectally)[20]. At 24 h after initial induction, the animal groups were treated orally with 12% Tween 80 solution (non-colitic and colitic), Cd-EtOHE (125 mg/kg) or Cd-HexP (62.5 mg/kg). On day 14 after the first induction, the second administration (relapse) was performed with TNBS (10 mg/0.25 mL ethanol 50% v/v, rectally) to mimic recurrent relapses in IBDs in humans.
General parameters such as diarrhea, water and food intake, and body weight were recorded daily throughout the treatment period. At day 21 all animals were euthanized, the colon removed, opened, and washed for macroscopic lesion analysis and evaluation of the intestinal inflammatory process[21]. Collection of material for biochemical and histological analysis was also performed. The samples were stored at -80 °C for evaluation of myeloperoxidase (MPO) and cytokines involved in intestinal inflammation.
Colonic segments intended for light microscopy were collected. For this, the tissues were fixed in Alfac solution for 24 h at room temperature. Afterwards, the pieces were kept in 80% alcohol until the block assembly time. The pieces were dehydrated and embedded in paraplast forming blocks, and then cut to 10-mm thickness for mounting on slides. These were stained with hematoxylin and eosin for morphological analysis[22].
Colon segments, stored at -80 °C were used with dosages of MPO and pro-inflammatory and anti-inflammatory cytokines. The samples were homogenized in hexadecyltrimethylammonium bromide buffer (HTAB) (0.5% in 50 mmol/L sodium phosphate buffer, pH 6.0) that acts as a detergent, lysing granules of neutrophils containing MPO, which is released. The sample was centrifuged for 10 min at 4 °C. The homogenate was subjected to a three-fold freezing and thawing process to facilitate the rupturing of cell structures and the consequent release of the enzyme. On ELISA plates were placed 50 μL of supernatant from each sample and 150 μL of reaction buffer[23].
The results were expressed as MPO units per gram of tissue, where 1 U of MPO activity is defined as that degrading 1 μmol of hydrogen peroxide per min at 25 °C.
TNF-α, IL-1β and IL-10 levels were determined from colonic specimens, frozen in -80 °C, and collected in the UC relapse model. For this, we used PBS buffer pH 7.4 (1:5) to homogenize the samples. The homogenate tubes were centrifuged at 12000 rpm for 10 min. The supernatants were frozen at -80 °C until assay. Subsequently, the samples were shaken in water bath at 37 °C for 20 min and then centrifuged at 10000 rpm for 5 min at 4 °C. The supernatant was collected and the cytokines TNF-α, IL-1β and IL-10 were quantitated using ELISA assay kits (DuoSet®; R & D Systems Inc.). The concentrations of the cytokines in relation to the amount of total protein was quantified by bicinchoninic acid method[24].
Histological samples were incubated with anti-cyclooxygenase-2 (COX-2) secondary antibody (marker for assessing anti-inflammatory effect), anti-proliferating cell nuclear antigen (PCNA) (cell division marker to assess potential for regeneration), and anti-superoxide dismutase (SOD) (marker to evaluate the antioxidant effect). The positively stained cells were counted for the various immunohistochemical reactions in a fixed number of fields by means of an image analyzer (Q-Win Standard Version 3.1.0; Leica) coupled to the Leica DM microscope. They were photographed and analyzed by AVSoft program Bioview Spectra and Seeker 4.0.
The experimental protocols were approved by the Committee for Ethics in Animal Experimentation (CEPA/LTF/UFPB) under number 1112/10. Male Wistar albino rats (180-250 g) from the “Prof. Thomas George Vivarium” of LTF/UFPB were fed a certified Presence® diet, with free access to water under fixed conditions of illumination (12/12 h light/dark cycle), humidity (60% ± 1.0%), and temperature (21.5 ± 1.0 °C). Fasting was used prior to all assays since standard drugs were administered orally (by gavage) or by intra-rectal route, using a 12% solution of Tween 80 (10 mL/kg) as the vehicle. The animals were kept in cages with raised, wide-mesh floors to prevent coprophagy.
Results with parameter values (inflammatory bowel lesion area and weight/length ratio) were subjected to analysis of variance (ANOVA) followed by Dunnett’s or Tukey test, and expressed as mean ± SD of the average. In quantitation assays of antioxidant enzymes, cytokines and MPO values obtained were presented as mean ± standard error of mean (SEM).
For nonparametric values (score of intestinal inflammation), the Kruskal-Wallis test (ANOVA, Dunn's post-test) was used. The results were expressed as median (minimum-maximum). Data were analyzed using the software GraphPad Prism 6.0, and the significance level was set at P < 0.05.
A significant reduction in the intestinal ULA for rats treated with Cd-EtOHE at doses of 62.5 and 125 mg/kg (46 ± 12, P < 0.01 and 19 ± 8, P < 0.001 respectively) was observed compared to the colitic group (107 ± 38). In the experimental evaluation of the effect of Cd-HexP, a significant reduction was observed at doses of 31.25 and 62.5 mg/kg (52 ± 18, P < 0.01 and 21 ± 7, P < 0.001 respectively), when compared to the colitic animals (101 ± 45) (Table 1).
| Group | Dose (mg/kg) | ULA (mm2) | Inhibition (%) | Lesion score | Weight/length (mg/cm) | Diarrhea (%) |
| Non-colitics | - | - | 100 | 110 ± 7.7 | 0 | |
| Colitics | - | 107 ± 38 | - | 6.0 (5-7) | 148 ± 17f | 100 |
| Cd-EtOHE | 31.25 | 79 ± 31 | 26 | 5.0 (3-7) | 152 ± 19f | 100 |
| 62.5 | 46 ± 12b | 57 | 4.0 (1-5)a | 146 ± 19f | 57 | |
| 125 | 19 ± 8c | 82 | 3.0 (2-5)b | 134 ± 5d | 14b | |
| 250 | 76 ± 23 | 29 | 6.0 (4-7) | 152 ± 14f | 86 | |
| Non-colitics | - | - | - | - | 102 ± 14 | 0 |
| Colitics | - | 101 ± 45 | - | 7.0 (5-8) | 154 ± 27f | 87 |
| Cd-HexP | 31.25 | 52 ± 18b | 49 | 5.0 (1-6)a | 142 ± 20e | 87 |
| 62.5 | 21 ± 7c | 79 | 5.0 (4-5)b | 129 ± 20a | 29a | |
| 125 | 95 ± 33 | 7 | 6.0 (5-6) | 154 ± 10f | 71 | |
| 250 | 80 ± 12 | 22 | 5.0 (4-7) | 155 ± 16f | 86 |
For the lesion score, Cd-EtOHE at doses of 62.5 and 125 mg/kg significantly reduced the amounts of lesion to 4.0 (1-5) (P < 0.05) and 3.0 (2-5) (P < 0.01) respectively, compared to the colitic control of 6 (5-7). Cd-HexP 31.25 and 62.5 mg/kg significantly reduced lesion to 5.0 (1-6) (P < 0.05) and 5.0 (4-5) (P < 0.01) respectively, compared to the colitic group of 7 (5-8) (Table 1).
A significant increase in weight/length for the colitic group (148 ± 17, P < 0.001, 154 ± 27, P < 0.001) was also observed when compared to the non-colitic group (110 ± 8, 102 ± 14 respectively). Treatment with different doses of Cd-EtOHE (31.25, 62.5, 125 and 250 mg/kg) did not reduce the weight/length ratio (152 ± 19, 146 ± 19, 134 ± 5 and 152 ± 14 respectively) for the parameter compared to the colitic group (148 ± 17). However, treatment with Cd-HexP at a dose of 62.5 mg/kg significantly reduced the ratio to 129 ± 20 (P < 0.05), compared to their respective colitic group (154 ± 27) (Table 1).
The administration of TNBS resulted in a diarrhea rate of 100% in the colitic animals. Cd-EtOHE at a dose of 125 mg/kg significantly reduced the diarrhea involvement to 14%. For Cd-HexP, treatment at dose of 62.5 mg/kg significantly reduced diarrhea to 29% (P < 0.05) when compared to their respective colitic control (87%) (Table 1).
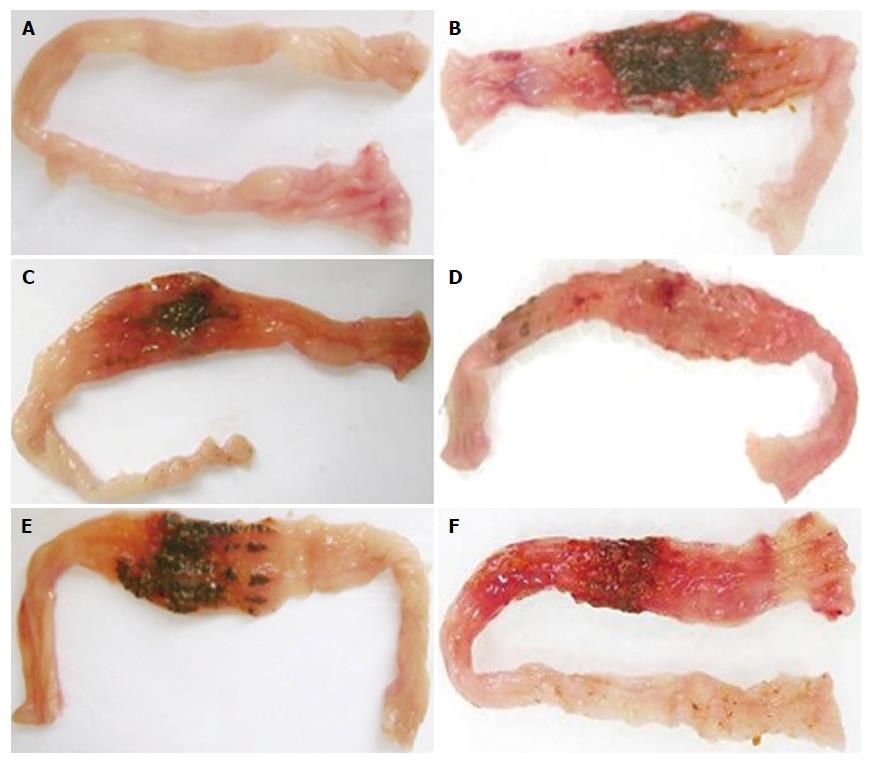
Intestines of colitic, non-colitic and treated rats with different tested doses of Cd-EtOHE or Cd-HexP in the model can be seen in Figures 1 and 2 respectively.
A significant reduction in macroscopic damage scores was observed for both Cd-EtOHE (125 mg/kg) and Cd-HexP (62.5 mg/kg), to 1.0 (1-4) (P < 0.05) and 1.0 (1-4) (P < 0.01) respectively, compared to the colitic control 4 (3-6). Moreover, the tested plant sample reduced the onset of diarrhea by 56% when compared to colitic animals (94%) (see Table 2).
The weight/length ratio significantly increased in the colitic, Cd-EtOHE and Cd-HexP groups (143 ± 14, P < 0.001; 132 ± 11, P < 0.001; 122 ± 12, P < 0.01, respectively) compared to the non-colitic group (97 ± 9). However, Cd-HexP 62.5 mg/kg caused a significant reduction (122 ± 12, P < 0.01), compared to the colitic group (143 ± 14). These results are shown in Table 2, and can be seen best in Figure 3.
A significant decrease in water (28 ± 3, P < 0.01) and food (19 ± 2, P < 0.01) intake was observed in the colitic group, compared to the non-colitic animals (31 ± 4 and 22 ± 2 respectively). Only treatment with Cd-HexP increased water (31 ± 2, P < 0.05) and food (22 ± 3, P < 0.01) intake, compared to colitic animals (28 ± 3, P < 0.01 and 19 ± 2, P < 0.01 respectively) (Table 3).
As an additional parameter to the data described above, we evaluated the effect of repeated administrations of Cd-EtOHE (125 mg/kg) and Cd-HexP (62.5 mg/kg) on the body weights of animals affected with intestinal inflammation. At the end of the experiment, there was a significant reduction in mean body weight for the colitic group (215 ± 21, P < 0.001) when compared to the non-colitic group (261 ± 35). However, when the treatments were performed with Cd-HexP (62.5 mg/kg), a significant increase in mean body weight (239 ± 17, P < 0 05) was observed compared to the colitic animals (215 ± 21) (Table 4).
A significant increase was observed in spleen weight in the colitic group (2.8 ± 0.5, P < 0.05) compared to the non-colitic group (2.1 ± 0.2). Analyzing the organs of animals treated with Cd-EtOHE or Cd-HexP, a significant increase in spleen weight for animals treated with Cd-EtOHE as compared to the non-colitic group was demonstrated (Table 5). In other evaluations (heart, liver and kidneys) no significant changes, compared to the non-colitic group, were observed.
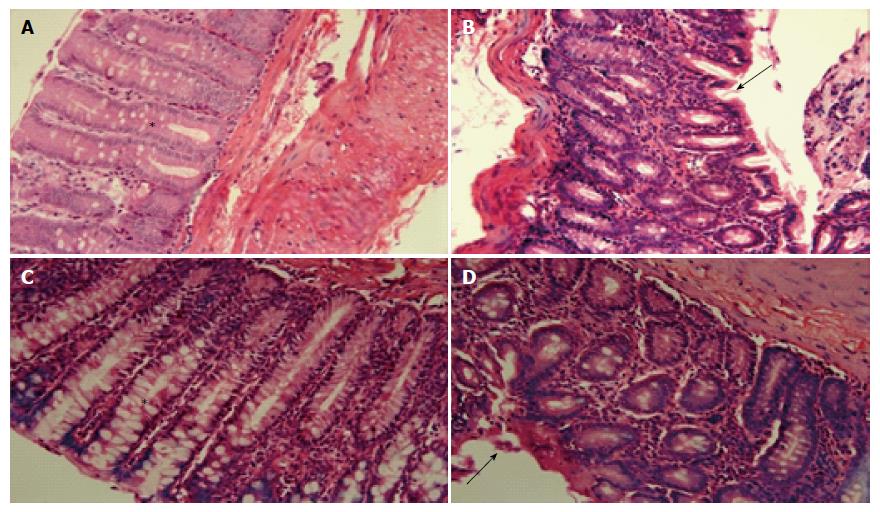
Histological examination of the colon of non-colitic rats showed normal histological structure, highlighting the structure of the mucosal straight intestinal glands with large numbers of goblet cells and lamina propria classical (or normal). The animals belonging to the colitic group had transmural inflammation, necrosis of the mucosa with disruption of glands, and loss of goblet and epithelial cells. The presence of granulation, highlighting neutrophilic and lymphocytic infiltration, was also observed.
Treatment with Cd-EtOHE (125 mg/kg) or Cd-HexP (62.5 mg/kg) maintained some areas of the mucosal structure and epithelium intact, reducing the reducing the Inflammatories cells in lamina propria as compared to the colitic group, suggesting the re-epithelialization of the animals treated with the vegetable samples (Figure 4).
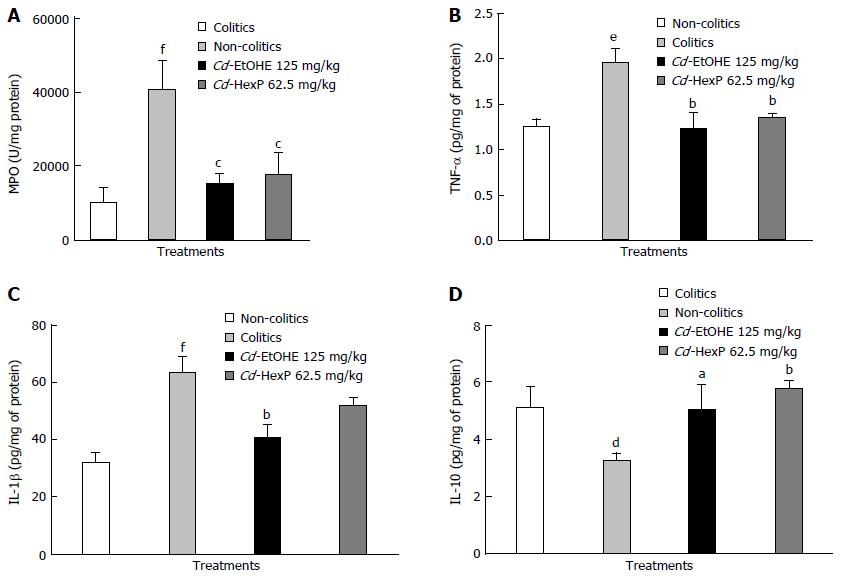
According to the results obtained, there was a significant increase in MPO to 40.270 ± 3.077 (P < 0.001) in the colitic group when compared to non-colitics (10.120 ± 1.672). When compared to the colitic controls (40.270 ± 3.077), treatments with Cd-EtOHE (15.187 ± 1.158, P < 0.001) or Cd-HexP (17.620 ± 2.395, P < 0.001) significantly reduced MPO (Figure 5).
The results showed a significant increase in TNF-α levels in colitic animals (2.0 ± 0.2, P < 0.01) compared to non-colitics (1.3 ± 0.1). However, the treatment of Cd-EtOHE (125 mg/kg) or Cd-HexP (62.5 mg/kg) resulted in TNF-α level reduction (1.2 ± 0.2 and 1.4 ± 0.1, P < 0.01) respectively, compared to the colitic group (2.0 ± 0.2) (Figure 5).
IL-1β levels increased in colitic animals (63 ± 6, P < 0.001) when compared to the non-colitic group (32 ± 4.0). Treatment with Cd-EtOHE significantly reduced to 41 ± 5 (P < 0.01) IL-1-β, compared to the colitic group (63 ± 6.2). Cd-HexP did not cause significant change when compared to the negative control (Figure 5).
The results showed significant reduction of IL-10 in intestinal tissues, comparing the colitic group 3.2 ± 0.3 (P < 0.05) to the non-colitic group (5.1 ± 0.8). Treatment with Cd-EtOHE or Cd-HexP caused significant increases (5.0 ± 0.4, P < 0.05, 5.8 ± 0.3, P < 0.01, respectively) when compared to the colitic group (3 2 ± 0.3) (Figure 5).
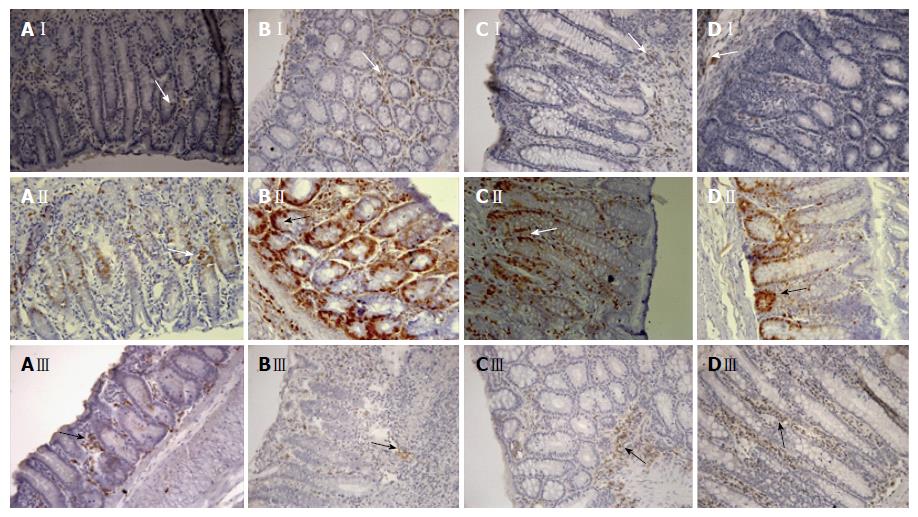
In analyzing the results of COX-2 expression shown in Table 6 and Figure 6, we observed a significant increase in COX-2 expression in the colitic group animals to 505 (100-1450) (P < 0.05) when compared the non-colitic group of 230 (110-700). However, treatment with Cd-EtOHE (125 mg/kg) or Cd-HexP (62.5 mg/kg) significantly reduced the expression of COX-2 to 90 (10-2590) (P < 0.001) and 205 (20-790) (P < 0.001) respectively, compared to the colitic group of 505 (100-1450).
A significant increase in PCNA expression was observed in the animals of the colitic group of 5380 (850-15960) (P < 0.001) when compared to the non-colitic group of 1425 (60-7890). However, treatment with Cd-EtOHE (125 mg/kg) or Cd-HexP (62.5 mg/kg) significantly reduced PCNA expression to 1.630 (90-14790) (P < 0.001) and 1.570 (250-9500) (P < 0.001) respectively, compared to 5.380 of the colitic group (850-15960) (P < 0.001) (Table 6 and Figure 6).
The results of this analysis demonstrated a significant decrease in the expression of SOD in colitic controls to 165 (10-1000) (P < 0.001) when compared to the non-colitic group of 400 (80-1115). The treatment of the animals with Cd-EtOHE (125 mg/kg) or Cd-HexP (62.5 mg/kg) increased the expression of SOD to 400 (50-1480) (P < 0.05) and 435 (20-1650) (P < 0.001) respectively, compared to the colitic control (Table 6 and Figure 6).
A promising area for research, many plant species and their chemical constituents exert therapeutic actions. This has led to the development of new, effective and safe drugs for the treatment of various pathological processes. There is also an interest in targeted therapy for diseases derived from oxidative stress, such as IBD[11,25].
IBDs are progressive and destructive chronic disorders of the GI tract, the most common being CD or UC. There is evidence that the pathogenesis of IBD is related to a dysfunctional interaction between the bacteria of the intestinal microflora and mucosal immune system[26,27].
CD and UC are immunologically different diseases. CD is characterized by an exaggerated cellular Th1 response (CD4+) and Th17, characterized by high levels of INF-γ/IL-17 and IL-12/IL-23. UC is characterized by a heightened Th2 response, and excessive IL-5 and IL-13[28-30].
TNBS is a hapten, administered by enema in rats in combination with 50% ethanol to break the mucus barrier and facilitate penetration of the hapten into the intestinal epithelium. TNBS reacts with autologous proteins and stimulates the development of hypersensitivity, leading to the activation of antigen-specific T cells. The immune response induced by the hapten causes severe ulceration of the mucosal and epithelial barrier, characterized by trans-mural infiltration of mononuclear cells[20].
Preventive treatment with Cd-EtOHE (62.5 to 125 mg/kg) or Cd-HexP (31.25 and 62.5 mg/kg) caused a significant reduction in the severity and extent of injury as reflected in the macroscopic lesion score. The macroscopic/microscopic damage scores and colon weight/length ratio can be considered as sensitive and reliable markers to estimate the severity of the disease, and thus the anti-inflammatory effect promoted by the test drug[31].
A low incidence of diarrhea in animals treated with Cd-EtOHE and Cd-HexP was also observed. Diarrhea is a major symptom of disease in both animals and humans and indicates loss of the absorptive capacity of the colon, which is impaired in intestinal inflammation[32]. The results suggest that treatment with the plant samples restored the intestinal absorptive capacity.
Cd-HexP at 62.5 mg/kg significantly reduced the weight/length ratio. This effect is possibly related to the scavenging capacity of free radicals caused by treatment at this dose[33]. These results demonstrate, for the first time, anti-inflammatory intestinal activity for a species belonging to the genus Combretum.
The more effective doses of Cd-EtOHE (125 mg/kg) or Cd-HexP (62.5 mg/kg) were selected to investigate their effects in the chronic phase with relapse in TNBS-induced UC in rats. This model mimics the disease in humans and can be used to evaluate new treatments potentially applicable in IBD[31].
Cd-EtOHE at 125 mg/kg or Cd-HexP at 62.5 mg/kg significantly decreased signs of disease, such as macroscopic lesion (lesion area and score), weight/length ratio and diarrhea, showing the anti-inflammatory effect at 21 d of treatment.
Intestinal inflammation in the TNBS-induced model promoted the loss of 8% to 10% of body weight notably at 1 wk after induction, this is related to reduction in food intake due to abdominal pain and diarrhea during the active phase of the disease[34]. Treatment with Cd-HexP at 62.5 mg/kg reversed low water and food intake (and body weight loss) caused by disease.
The spleen recycles and acts as a reserve of red blood cells. The organ is the center of reticuloendothelial system activity, and is an essential part of the immune system[35]. A significant increase in spleen weight of colitic animals when compared to non-colitic animals was demonstrated. UC and CD are mentioned in lists of factors that can cause increased spleen size, which can occur through lymphoid cell accumulation in immune functions[35,36].
MPO is an enzyme found in neutrophils and has been used as a quantitative index of neutrophil influx into inflamed intestines. The recruitment and activation of neutrophils results in a significant increase in free radical production, capable of overcoming antioxidant protections, and resulting in oxidative stress and inflammation[37,38]. Cd-EtOHE at 125 mg/kg and Cd-HexP at 62.5 mg/kg significantly decreased MPO activity when compared to colitic animals. This may be interpreted as a manifestation of anti-inflammatory effect for C. duarteanum species.
The inflammation in the TNBS-induced colitis model is characterized by a Th1 pathway immune response, in which there is an increase in TNF-α, IL-1β, IL-12, IL-17, IL-18 and IL-6[39-41].
Up-regulation of the inflammatory state with increased TNF-α and IL-1β levels in colitic rats was observed, which corroborates the literature findings[42]. The treatment with Cd-EtOHE at 125 mg/kg or Cd-HexP at 62.5 mg/kg was able to significantly reduce TNF-α levels when compared to the colitic controls, suggesting that TNF-α suppression is related to the anti-inflammatory effect promoted by the vegetable samples studied. Cd-EtOHE at 125 mg/kg was able to decrease IL-1β levels, suggesting that compounds of this plant sample may interfere with the synthesis machinery of IL-1β activation by inhibiting production[10,43,44].
IL-10 suppresses production of pro-inflammatory cytokines, such as IL-12, IL-6, IL-1 and TNF-α, in activated macrophages in vitro, and blocks the ability of macrophages stimulating the production of interferon by Th1 cells. IL-10 is produced in large amounts by TCD4+ regulatory cells subtype (Tregs). Tregs maintain homeostasis by suppressing the adaptive response of T cells and preventing autoimmunity[45].
A significant elevation in IL-10 levels in animals treated with Cd-EtOHE at 125 mg/kg or Cd-HexP at 62.5 mg/kg, compared to the colitic group, was demonstrated. This increase is attributable to compensatory mechanisms against colonic injury, possibly playing a role in reducing mucosal inflammation and preventing it from becoming uncontrolled. IL-10 down-regulates antigen presentation, and thereafter the release of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6[46,47].
Studies have shown that COX-2 is expressed predominantly in experimental colitis. Colotic humans and animals show considerable improvement in the inflammatory process when COX-2 inhibitors are used[48]. The vegetable samples tested prevented the increase in expression of this enzyme, and suggest that the intestinal anti-inflammatory effect promoted by Cd-EtOHE at 125 mg/kg or Cd-HexP at 62.5 mg/kg is mediated by a reduction in COX-2 expression.
PCNA is an intra-nuclear protein, whose expression is related to cell proliferation and DNA repair. It is highly expressed during the S phase of the cell cycle[49]. Studies show that PCNA expression is up-regulated during chronic inflammation, inducing the proliferation of epithelial cells to repair the mucous tissue[50,51].
The positive expression of PCNA was increased in colitic animals in this study, while Cd-EtOHE at 125 mg/kg or Cd-HexP at 62.5 mg/kg treatment significantly decreased expression of this protein. Treatment with the plant samples studied protected against intestinal epithelial cell damage induced by TNBS and the effect was mediated via regulation of PCNA.
SOD is a key enzyme that converts superoxide to H2O2, a more stable metabolite. During oxidative stress and inflammation, SOD activity is decreased in inflamed tissues as compared to non-inflamed tissues. The decreased SOD activity allows superoxide accumulation and subsequent oxidative effects in the intestinal tissue, as well as increased expression of adhesion molecules[52].
Treatment with Cd-EtOHE at 125 mg/kg or Cd-HexP at 62.5 mg/kg significantly increased the expression of this enzyme when compared to colitic animals, suggesting involvement of an antioxidant effect in the intestinal anti-inflammatory activity promoted by the vegetable samples.
In conclusion, C. duarteanum presents promising anti-inflammatory intestinal effects that are related to reduced levels of the pro-inflammatory cytokines (TNF-α and IL-1β) and increased anti-inflammatory cytokine (IL-10), which feature regulatory effects on the immune response, with reduction in the expression of COX-2, PCNA, and an increase in the antioxidant enzyme SOD.
We are grateful to José Crispim Duarte, Rodrigo de Oliveira Formiga and the members of the Laboratório de Farmacologia do Trato Gastrintestinal of the Programa de Pós-Graduação em Produtos Naturais e Sintéticos Bioativos for technical support. The authors also thank David Peter Harding for the careful language assistance.
A variety of herbal medicines have been shown to produce promising results in the treatment of peptic ulcer and inflammatory bowel disease. Combretum duarteanum (C. duarteanum, Cd), the species selected for this study, is popularly known as “mufumbo, cipiúba, cipaúba”. In folk medicine, it is used to treat pain, inflammation and gastrointestinal (GI) tract disorders. Given the need for new inflammatory bowel disease therapies, this study aimed to evaluate, for the first time, the intestinal anti-inflammatory activity promoted by the species C. duarteanum, validating its popular use and contributing to the search for new therapies for diseases that affect the GI tract.
C. duarteanum has presented in vitro and in vivo anti-inflammatory, antinociceptive and antioxidant capacities. Furthermore, it has demonstrated low toxicity, gastroprotective and antiulcer activity in different models of acute ulcer induction (acidified ethanol, ethanol, nonsteroidal anti-inflammatory drugs, stress, pylorus ligature and acetic acid) in animals.
This study evaluated, for the first time, the intestinal anti-inflammatory activity promoted by the species C. duarteanum Cambess.
This study validated the popular use of C. duarteanum and contributes to the search for new therapies for diseases that affect the GI tract.
The authors demonstrated that Cd-EtOHE and Cd-HexP obtained from leaves of C. duarteanum displays anti-inflammatory effect in trinitrobenzenesulfonic acid colitis model in rats. These results are promising. The vegetal samples may have a role in antioxidant activity and mucosal healing in ulcerative colitis.
| 1. | Loftus EV, Sandborn WJ. Epidemiology of inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 412] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 3. | Yen EF, Pardi DS. Non-IBD colitides (eosinophilic, microscopic). Best Pract Res Clin Gastroenterol. 2012;26:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Kondamudi PK, Malayandi R, Eaga C, Aggarwal D. Drugs as causative agents and therapeutic agents in inflammatory bowel disease. Acta Pharm Sin B. 2013;3:289-296. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 356] [Cited by in RCA: 441] [Article Influence: 36.8] [Reference Citation Analysis (12)] |
| 6. | Langholz E. Current trends in inflammatory bowel disease: the natural history. Therap Adv Gastroenterol. 2010;3:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Dharmani P, Chadee K. Biologic therapies against inflammatory bowel disease: a dysregulated immune system and the cross talk with gastrointestinal mucosa hold the key. Curr Mol Pharmacol. 2008;1:195-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 9. | Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124:3-20; quiz 21-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1311] [Article Influence: 77.1] [Reference Citation Analysis (2)] |
| 10. | Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1545] [Cited by in RCA: 2087] [Article Influence: 173.9] [Reference Citation Analysis (1)] |
| 11. | Awaad AS, El-Meligy RM, Soliman GA. Natural products in treatment of ulcerative colitis and peptic ulcer. J Saudi Chem Soc. 2013;17:101-124. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Pagano E, Capasso R, Piscitelli F, Romano B, Parisi OA, Finizio S, Lauritano A, Marzo VD, Izzo AA, Borrelli F. An Orally Active Cannabis Extract with High Content in Cannabidiol attenuates Chemically-induced Intestinal Inflammation and Hypermotility in the Mouse. Front Pharmacol. 2016;7:341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Borrelli F, Romano B, Petrosino S, Pagano E, Capasso R, Coppola D, Battista G, Orlando P, Di Marzo V, Izzo AA. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br J Pharmacol. 2015;172:142-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 14. | Capasso R, Orlando P, Pagano E, Aveta T, Buono L, Borrelli F, Di Marzo V, Izzo AA. Palmitoylethanolamide normalizes intestinal motility in a model of post-inflammatory accelerated transit: involvement of CB1 receptors and TRPV1 channels. Br J Pharmacol. 2014;171:4026-4037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Gouveia MG, Xavier MA, Barreto AS, Gelain DP, Santos JP, Araújo AA, Silva FA, Quintans JS, Agra MF, Cabral AG. Antioxidant, antinociceptive, and anti-inflammatory properties of the ethanolic extract of Combretum duarteanum in rodents. J Med Food. 2011;14:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | de Morais Lima GR, de Albuquerque Montenegro C, de Sousa Falcão H, de Jesus NZ, Cabral AG, Gomes IF, Agra Mde F, Tavares JF, Batista LM. Gastroprotective activity of the ethanolic extract and hexane phase of Combretum duarteanum Cambess. (Combretaceae). J Nat Med. 2013;67:492-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Quintans JS, Costa E V, Tavares JF, Souza TT, Araújo SS, Estevam CS, Barison A, Cabral AG, Silva MS, Serafini MR. Phytochemical study and antinociceptive effect of the hexanic extract of leaves from Combretum duarteanum and friedelin, a triterpene isolated from the hexanic extract, in orofacial nociceptive protocols. Rev Bras Farmacogn. 2014;24:60-66. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Guimarães AG, Oliveira GF, Melo MS, Cavalcanti SC, Antoniolli AR, Bonjardim LR, Silva FA, Santos JP, Rocha RF, Moreira JC. Bioassay-guided evaluation of antioxidant and antinociceptive activities of carvacrol. Basic Clin Pharmacol Toxicol. 2010;107:949-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | de Morais Lima GR. Atividade Gastroprotetora de Combretum duarteanum Cambess (Combretaceae) em modelos animais [Dissertação]: Universidade Federal da Paraíba, 2011; João Pessoa, Paraiba, Brazil. 133 p. Available from: http://tede.biblioteca.ufpb.br/bitstream/tede/6735/1/arquivototal.pdf. |
| 20. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 637] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 21. | Bell CJ, Gall DG, Wallace JL. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol. 1995;268:G622-G630. [PubMed] |
| 22. | Behmer OA, Tolosa EMC, Freitas-Neto AG. Manual de técnicas para histologia normal e patológica. São Paulo/SP: Editora da Universidade de São Paulo; 1976. . |
| 23. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. [PubMed] |
| 24. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189576] [Cited by in RCA: 159181] [Article Influence: 3183.6] [Reference Citation Analysis (1)] |
| 25. | Calixto JB, Campos MM, Otuki MF, Santos AR. Anti-inflammatory compounds of plant origin. Part II. modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004;70:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 270] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 26. | Podolsky DK. The current future understanding of inflammatory bowel disease. Best Pract Res Clin Gastroenterol. 2002;16:933-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Swidsinski A, Loening-Baucke V, Vaneechoutte M, Doerffel Y. Active Crohn’s disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm Bowel Dis. 2008;14:147-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 28. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1021] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 29. | Sheikh SZ, Plevy SE. The role of the macrophage in sentinel responses in intestinal immunity. Curr Opin Gastroenterol. 2010;26:578-582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1358] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 31. | Yang XL, Guo TK, Wang YH, Gao MT, Qin H, Wu YJ. Therapeutic effect of ginsenoside Rd in rats with TNBS-induced recurrent ulcerative colitis. Arch Pharm Res. 2012;35:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Sánchez de Medina F, Vera B, Gálvez J, Zarzuelo A. Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci. 2002;70:3097-3108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Luchini AC, Rodrigues-Orsi P, Cestari SH, Seito LN, Witaicenis A, Pellizzon CH, Di Stasi LC. Intestinal anti-inflammatory activity of coumarin and 4-hydroxycoumarin in the trinitrobenzenesulphonic acid model of rat colitis. Biol Pharm Bull. 2008;31:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Campos FG, Waitzberg DL, Teixeira MG, Mucerino DR, Habr-Gama A, Kiss DR. Inflammatory bowel diseases: principles of nutritional therapy. Rev Hosp Clin Fac Med Sao Paulo. 2014;57:187-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Cho EJ, Shin JS, Noh YS, Cho YW, Hong SJ, Park JH, Lee JY, Lee JY, Lee KT. Anti-inflammatory effects of methanol extract of Patrinia scabiosaefolia in mice with ulcerative colitis. J Ethnopharmacol. 2011;136:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 36. | Pereira JL, Hughes LE, Young HL. Spleen size in patients with inflammatory bowel disease. Does it have any clinical significance? Dis Colon Rectum. 1987;30:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Lima de Albuquerque C, Comalada M, Camuesco D, Rodríguez-Cabezas ME, Luiz-Ferreira A, Nieto A, Monteiro de Souza Brito AR, Zarzuelo A, Gálvez J. Effect of kale and papaya supplementation in colitis induced by trinitrobenzenesulfonic acid in the rat. E Spen Eur E J Clin Nutr Metab. 2010;5:e111-e116. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Veljaca M, Lesch CA, Pllana R, Sanchez B, Chan K, Guglietta A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J Pharmacol Exp Ther. 1995;272:417-422. [PubMed] |
| 39. | Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 636] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 40. | Shi XZ, Winston JH, Sarna SK. Differential immune and genetic responses in rat models of Crohn’s colitis and ulcerative colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G41-G51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Zhu MY, Lu YM, Ou YX, Zhang HZ, Chen WX. Dynamic progress of 2,4,6-trinitrobenzene sulfonic acid induced chronic colitis and fibrosis in rat model. J Dig Dis. 2012;13:421-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Witaicenis A, Luchini AC, Hiruma-Lima CA, Felisbino SL, Garrido-Mesa N, Utrilla P, Gálvez J, Di Stasi LC. Suppression of TNBS-induced colitis in rats by 4-methylesculetin, a natural coumarin: comparison with prednisolone and sulphasalazine. Chem Biol Interact. 2012;195:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 43. | Ogryzko NV, Renshaw SA, Wilson HL. The IL-1 family in fish: swimming through the muddy waters of inflammasome evolution. Dev Comp Immunol. 2014;46:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Li P, Allen H, Banerjee S, Franklin S, Herzog L, Johnston C, McDowell J, Paskind M, Rodman L, Salfeld J. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80:401-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1120] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 45. | Jones-Hall YL, Grisham MB. Immunopathological characterization of selected mouse models of inflammatory bowel disease: Comparison to human disease. Pathophysiology. 2014;21:267-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Schreiber S, Heinig T, Thiele HG, Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995;108:1434-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 263] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Arab HH, Salama SA, Eid AH, Omar HA, Arafa el-SA, Maghrabi IA. Camel’s milk ameliorates TNBS-induced colitis in rats via downregulation of inflammatory cytokines and oxidative stress. Food Chem Toxicol. 2014;69:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Mahadevan U, Loftus EV, Tremaine WJ, Sandborn WJ. Safety of selective cyclooxygenase-2 inhibitors in inflammatory bowel disease. Am J Gastroenterol. 2002;97:910-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Prelich G, Stillman B. Coordinated leading and lagging strand synthesis during SV40 DNA replication in vitro requires PCNA. Cell. 1988;53:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 315] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 50. | Fukui T, Takeda H, Shu HJ, Ishihama K, Otake S, Suzuki Y, Nishise S, Ito N, Sato T, Togashi H. Investigation of Musashi-1 expressing cells in the murine model of dextran sodium sulfate-induced colitis. Dig Dis Sci. 2006;51:1260-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Sánchez-Fidalgo S, Cárdeno A, Sánchez-Hidalgo M, Aparicio-Soto M, de la Lastra CA. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J Nutr Biochem. 2013;24:1401-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 52. | Kannan N, Guruvayoorappan C. Protective effect of Bauhinia tomentosa on acetic acid induced ulcerative colitis by regulating antioxidant and inflammatory mediators. Int Immunopharmacol. 2013;16:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Capasso R, Gassler N, Lakatos PL S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF