Published online Feb 28, 2017. doi: 10.3748/wjg.v23.i8.1338
Peer-review started: July 16, 2016
First decision: August 8, 2016
Revised: September 3, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: February 28, 2017
Processing time: 228 Days and 9.9 Hours
To examined the bile acid receptor TGR5 expression in squamous mucosa, Barrett’s mucosa, dysplasia and esophageal adenocarcinoma (EA).
Slides were stained with TGR5 antibody. The staining intensity was scored as 1+, 2+ and 3+. The extent of staining (percentage of cells staining) was scored as follows: 1+, 1%-10%, 2+, 11%-50%, 3+, 51%-100%. A combined score of intensity and extent was calculated and categorized as negative, weak, moderate and strong staining. TGR5 mRNA was measured by real time PCR.
We found that levels of TGR5 mRNA were significantly increased in Barrett’s dysplastic cell line CP-D and EA cell line SK-GT-4, when compared with Barrett’s cell line CP-A. Moderate to strong TGR5 staining was significantly higher in high-grade dysplasia and EA cases than in Barrett’s esophagus (BE) or in low-grade dysplasia. Moderate to strong staining was slightly higher in low-grade dysplasia than in BE mucosa, but there is no statistical significance. TGR5 staining had no significant difference between high-grade dysplasia and EA. In addition, TGR5 staining intensity was not associated with the clinical stage, the pathological stage and the status of lymph node metastasis.
We conclude that TGR5 immunostaining was much stronger in high-grade dysplasia and EA than in BE mucosa or low-grade dysplasia and that its staining intensity was not associated with the clinical stage, the pathological stage and the status of lymph node metastasis. TGR5 might be a potential marker for the progression from BE to high-grade dysplasia and EA.
Core tip: The expression of a bile acid receptor TGR5 at moderate to strong intensity was significantly higher in high-grade dysplasia and esophageal adenocarcinoma (EA) cases than in BE or in low-grade dysplasia, suggesting that TGR5 may play an important role in the progression from Barrett’s esophagus to high-grade dysplasia and EA. TGR5 might be a potential marker for this progression. TGR5 staining intensity was not associated with the clinical stage, the pathological stage and the status of lymph node metastasis, indicating that TGR5 may not be a marker for the prognosis of EA.
- Citation: Marketkar S, Li D, Yang D, Cao W. TGR5 expression in benign, preneoplastic and neoplastic lesions of Barrett’s esophagus: Case series and findings. World J Gastroenterol 2017; 23(8): 1338-1344
- URL: https://www.wjgnet.com/1007-9327/full/v23/i8/1338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i8.1338
Esophageal adenocarcinoma (EA) is a deadly cancer with an increasing incidence[1-3]. It has a poor prognosis with a median survival of less than one year[4,5] and a five-year survival rate of 12.5%-20%[6,7]. Gastroesophageal reflux disease (GERD) complicated by Barrett’s esophagus (BE)[8-10] is a major risk factor for EA. BE carries nearly a 30-125-fold increased risk for the development of EA, with best estimates of cancer incidence of 0.12%-0.8% per year[2,10-14]. However, mechanisms of the progression from BE (intestinal metaplasia) to EA are not fully understood.
Bile acids may play an important role in the progression from BE to EA[15,16] since (1) exposure of the lower esophagus to duodenal juice in animal models leads to EA[17-19]; (2) bile acid causes the production of reactive oxygen species and DNA damage in a non-neoplastic Barrett’s cell line BAR-T[20]; (3) bile salts may activate the mitogen-activated protein kinase and NF-κB pathways[21,22] thereby enhancing cell proliferation and preventing cell apoptosis; and (4) Barrett’s cells become tumorigenic after long-term exposure to acid and bile acid in vitro[23].
A G protein-coupled receptor TGR5 has been shown to mediate bile acids’ effects as a cell-surface receptor[24]. The TGR5 receptor is abundantly expressed in human monocytes and macrophages, and participates in the regulation of cell metabolism[25,26]. Primary bile acids (cholic acid, taurocholic acid and glycocholic acid) and secondary bile acids (deoxycholic acid, taurodeoxycholic acid, glycodeoxycholic acid and taurolithocholic acid) have been shown to bind to TGR5 receptors[24]. Primary bile acids are much weaker at inducing cyclic AMP production via activation of TGR5 than secondary bile acids. Deoxycholic acid, taurodeoxycholic acid, and glycodeoxycholic acid have similar strengths at inducing cyclic AMP production[24]. TGR5 has been reported to be expressed in human gastric cancers, to promote epithelial-mesenchymal transition in gastric cancer cell lines[27], and to mediate bile acid-induced cholangiocyte proliferation in vivo and in vitro[28].
We have previously shown that TGR5 receptors are present in EA cells and that TGR5 receptors mediate bile acid-induced increase in cell proliferation[29]. The expression of TGR5 in EA tissues is not well understood. In this study, we examined the bile acid receptor TGR5 expression in squamous mucosa, Barrett’s mucosa, dysplasia and EA by immunohistochemistry. We found that TGR5 immunostaining was much stronger in high grade and EA than in BE mucosa or low-grade dysplasia.
Archival cases of BE, low grade dysplasia, high grade dysplasia and EA from 34 patients (18 cases with squamous mucosa, 15 cases with BE, 8 cases with low grade dysplasia, 9 cases with high grade dysplasia and 16 cases with adenocarcinoma) were collected between the years of 2005 and 2013 from the archives of the Department of Pathology at the Rhode Island Hospital (RIH). BE was made based on the histological finding of intestinal metaplasia and the endoscopic finding of column-type mucosa. Patients with previous history of chemoradiation therapy were excluded from the study. Stage was defined according to American Joint Committee on Cancer criteria[30]. This study was approved by the Institutional Review Board at the RIH. All tissue samples were formalin-fixed and paraffin-embedded. The corresponding hematoxylin-eosin slides were reviewed for confirmation of diagnosis and adequacy of material by Dr. Cao W. The detailed clinicopathological features of the study population are given in Table 1.
| Number | 34 |
| Age (yr) | |
| Range | 48-91 |
| Median | 65.5 |
| Sex, n (%) | |
| Male | 28 (82.4) |
| Female | 6 (17.6) |
Immunohistochemistry for TGR5 was performed on 4-μm paraffin sections. Slides were stained with TGR5 antibody (1:1000, Sigma-Aldrich Co., St. Louis, MO) using the DAKO Envision + Dual Link System and the DAKO Liquid 3,3’-diaminobenzidine (DAB+) Substrate Chromagen System (DAKO North America, Inc., Carpinteria, CA). Bile ducts from liver tissue were used as positive controls. Negative controls included replacement of the primary antibody with non-reacting antibodies of the same species. The specificity of TGR5 antibody has been confirmed by Western Blot analysis in our lab[31].
Cancers and non-neoplastic mucosa that displayed a strong, well-localized, strong staining pattern for TGR5 were scored as +3, moderately intense staining as +2, and weak staining as +1. The extent of staining (percentage of cells staining) was scored as follows: 1+ 1%-10%, 2+ 11%-50%, 3+ 51%-100%. A combined score of intensity and extent was calculated and categorized as follows: weak staining 1-2, moderate staining 3-4, strong staining 5-6. All sections were scored independently by WC and SM without knowledge of the clinicopathologic features or clinical outcome.
Cell culture was similar to those we described previously[29,32,33]. Briefly, human esophageal squamous HET-1A cells were purchased from ATCC, Manassas, VA in 2011 and cultured in the bronchial epithelial cell medium (BEGM BulletKit, Cambrex, East Rutherford, NJ). Human Barrett’s cell line CP-A and Barrett’s dysplastic cell line CP-D were bought from ATCC (Manassas, VA) and cultured in Barrett’s medium containing keratinocyte medium-2 (Cambrex, Rockland, ME), 1.8 mmol/L CaCl2, 5% fetal bovine serum, 400 ng/mL hydrocortisone, 20 ng/ml epidermal growth factor, 0.1 nmol/L cholera toxin, 20 μg/mL adenine, 5 μg/mL insulin, 70 μg/mL bovine pituitary extract, and antibiotics. EA cell line SK-GT-4 was purchased from Sigma and cultured in the Barrett’s medium.
For immunohistochemical data, statistical differences were determined by χ2 test. For TGR5 mRNA data, data was expressed as mean ± SE. Statistical differences between two groups were determined by Student’s t test. Differences among multiple groups were tested using analysis of variance and checked for significance using Fisher’s protected least significant difference test. P values of 0.05 or less were considered statistically significant.
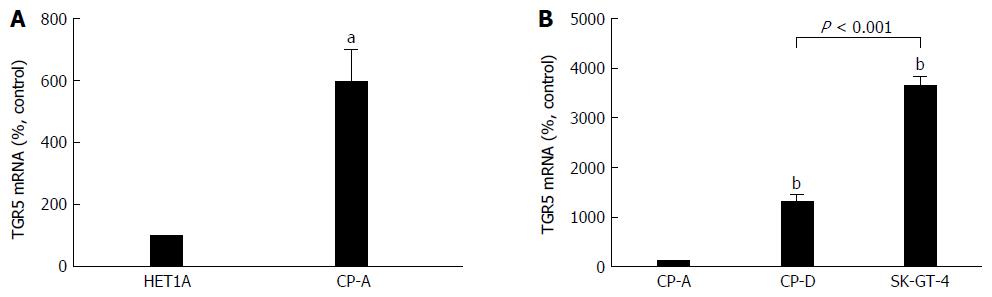
We have previously shown that the levels of TGR5 mRNA and protein expression are significantly increased in Barrett’s mucosal tissue, when compared with normal esophageal mucosa. TGR5 mRNA and protein levels are significantly higher in EA tissue than in normal esophageal mucosa or in Barrett’s mucosa[29]. Consistent with our previous findings, TGR5 mRNA level was significantly higher in Barrett’s cell line CP-A than in squamous cell line HET-1A (Figure 1A). Levels of TGR5 mRNA were significantly increased in Barrett’s dysplastic cell line CP-D and EA cell line SK-GT-4, when compared with CP-A cells. In addition, TGR5 mRNA was significantly higher in SK-GT-4 cells than in CP-D cells (Figure 1B). These data suggest that TGR5 may play an important role in the progression from BE to EA.
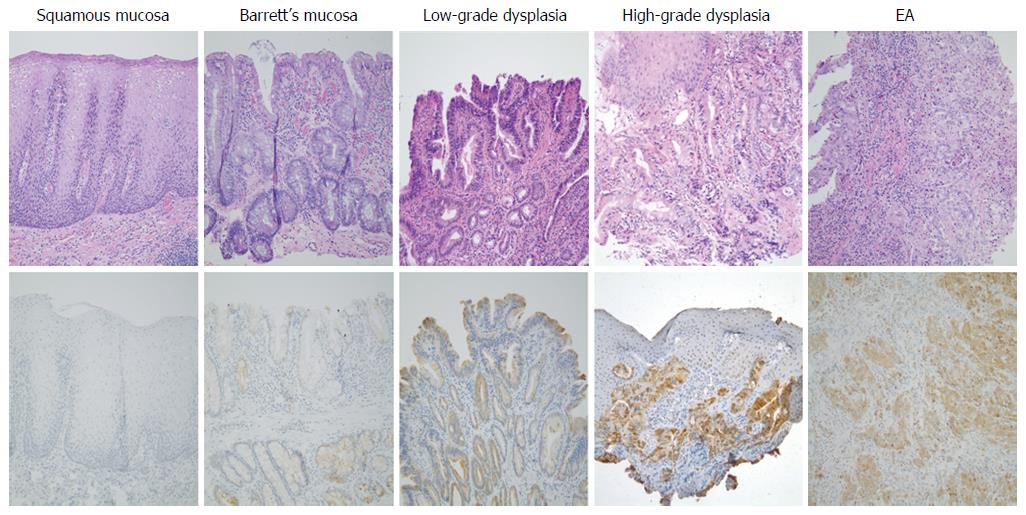
We have previously shown that TGR5 antibody is relatively specific since only one band was detectable by using TGR5 antibody[31]. The expression of TGR5 in squamous mucosa, BE mucosa, low-grade dysplasia and EA was further examined by immunohistochemistry. We found that 93.3% Barrett’s mucosa showed weak to moderate TGR5 staining, which was significantly higher than squamous mucosa (27.8%) (Figure 2 and Table 2). Moderate to strong TGR5 staining was significantly higher in EA cases (100%) than in BE (13.3%, P < 0.001) or in low-grade dysplasia (37.5%, P < 0.01) (Figure 2 and Table 2). Similarly, moderate to strong TGR5 staining was significantly higher in high-grade dysplasia cases (88.9%) than in BE (13.3%, P < 0.001) or in low-grade dysplasia (37.5%, P < 0.05) (Figure 2 and Table 2). Moderate to strong staining was slightly higher in low-grade dysplasia (37.5%) than in BE mucosa (13.3%), but there is no statistical significance. TGR5 staining had no significant difference between high-grade dysplasia and EA.
| Negative | Weak | Moderate | Strong | ||
| Squamous mucosa, n = 18 | 13 (72) | 5 (28) | 0 | 0 | |
| Barrett’s esophagus, n = 15 | 1 (6.7) | 12 (80) | 2 (13.3) | 0 | P < 0.001, compared with squamous mucosa |
| Low grade dysplasia, n = 8 | 0 | 5 (62.5) | 2 (25) | 1 (12.5) | P > 0.05, compared with BE |
| High grade dysplasia, n = 9 | 1 (11.1) | 0 | 2 (22.2) | 6 (66.7) | P < 0.001, compared with BE |
| P < 0.05, compared with low grade dysplasia | |||||
| EA, n = 16 | 0 | 0 | 7 (43.7) | 9 (56.3) | P < 0.001, compared with BE |
| P < 0.01, compared with low grade dysplasia |
Next, we compared the expression of TGR5 in different clinical and pathological stages of EA tissues. We found that 100% stage III and IV cases showed moderate to strong staining, which was the same as stage I and II cases (100%; Table 3), indicating that the degree of TGR5 expression might not be associated with clinical stages. T3 and T4 cancers had 63.6% strong TGR5 staining, which was not different from T1 and T2 cancers (P > 0.05; Table 4). In addition, TGR5 expression had no significant difference between tumors with and without lymph node metastasis (Table 5).
| Clinical stage | Negative | Weak | Moderate | Strong | |
| I and II, n = 9 | 0 | 0 | 4 (44.4) | 5 (55.6) | |
| III and IV, n = 7 | 0 | 0 | 3 (42.9) | 4 (57.1) | P > 0.05 |
| Pathological stage | Negative | Weak | Moderate | Strong | |
| T1 and T2 | 0 | 0 | 2 (40) | 3 (60) | |
| T3 and T4 | 0 | 0 | 4 (36.4) | 7 (63.6) | P > 0.05 |
| Lymph node metastasis | Negative | Weak | Moderate | Strong | |
| Positive | 0 | 0 | 2 (33.3) | 4 (66.7) | P > 0.05 |
| Negative | 0 | 0 | 5 (50) | 5 (50) |
GERD complicated by BE[8-10] is a major risk factor for EA. There is a progression from BE, to dysplasia and to EA. The mechanisms of progression from BE to EA are not fully understood. Many genetic and epigenetic alterations, chromosomal gains and losses, and hypermethylation of gene promoters may be involved in this progression[13,34]. Bile acids have also been indicated to be involved in this progression[15,16].
We have previously shown that TGR5 receptors are present in EA cells and that TGR5 mediates bile-acid-induced increase in cell proliferation, suggesting that TGR5 may be important in the development of EA[29]. We have also reported that moderate to strong TGR5 staining is associated with decreased patient survival in all gastric adenocarcinomas, suggesting that TGR5 may be a negative prognostic marker in gastric cancer[31]. The histological expression of TGR5 in EA has not been reported.
In this study, we examined TGR5 mRNA expression in different cell lines and found that Barrett’s cells CP-A had significantly higher levels of TGR5 mRNA than squamous cells HET-1A. Moreover, Barrett’s dysplastic cells CP-D had significantly higher levels of TGR5 mRNA than CP-A cells. EA cells SK-GT-4 had much higher levels of TGR5 mRNA than CP-A or CP-D. These data suggest that TGR5 may be involved in the progression from BE to EA.
Next, we examined the TGR5 expression in squamous mucosa, Barrett’s mucosa, dysplasia and EA. We found that moderate to strong TGR5 staining was significantly higher in high-grade dysplasia and EA cases than in BE or in low-grade dysplasia. Moderate to strong staining was slightly higher in low-grade dysplasia than in BE mucosa, but there is no statistical significance. TGR5 staining had no significant difference between high-grade dysplasia and EA. These data further support our above results that TGR5 may play an important role in the progression from BE to EA. How TGR5 is involved in this progression is not clear. Recently we found that TGR5 mediates bile acid-induced activation of cyclic AMP response element binding protein (CREB) and NADPH oxidase NOX5-S, which produces reactive oxygen species and causes DNA damage[35]. TGR5 is present in human gastric cancers and promotes epithelial-mesenchymal transition in gastric cancer cell lines[27]. It also mediates bile acid-induced cholangiocyte proliferation in vivo and in vitro[28]. Therefore, we speculate that in Barrett’s patients bile acids may activate TGR5 receptors, which activate CREB and NOX5-S. NOX5-S-derived ROS may increase cell proliferation and cause DNA damage, thereby contributing to the progression from BE to EA. TGR5 might be a potential marker for the progression from BE to high-grade dysplasia and EA. In addition, we compared the expression of TGR5 in different clinical stages of EA tissues. We found that 100% stage III and IV cases showed moderate to strong staining, which was the same as stage I and II cases, indicating that the degree of TGR5 expression might not be associated with clinical stages. Moreover, TGR5 expression had no significant difference between tumors with and without lymph node metastasis, indicating that the degree of TGR5 expression may not be related to the status of lymph node metastasis. These data suggest that TGR5 may not be a marker for the prognosis of EA.
In conclusion, TGR5 immunostaining was much stronger in high-grade dysplasia and EA than in BE mucosa or low-grade dysplasia. Its staining intensity was not associated with the clinical stage, pathological stage and the status of lymph node metastasis.
This work was presented in part at CAP 2015.
Gastroesophageal reflux disease complicated by Barrett’s esophagus (BE) is a major risk factor for esophageal adenocarcinoma (EA). However, mechanisms of the progression from BE (intestinal metaplasia) to EA are not fully understood. Recent data suggest that bile acids may play an important role in the progression from BE to EA. We have previously shown that TGR5 receptors are present in EA cells and that TGR5 receptors mediate bile acid-induced increase in cell proliferation. The expression of TGR5 in EA tissues is not well understood. In this study, we examined the bile acid receptor TGR5 expression in squamous mucosa, Barrett’s mucosa, dysplasia and EA by immunohistochemistry.
Bile acids may contribute to the progression from BE (intestinal metaplasia) to EA. The role of a bile acid receptor TGR5 in this progression is not clear.
The expression of a bile acid receptor TGR5 at moderate to strong intensity was significantly higher in high-grade dysplasia and EA cases than in BE or in low-grade dysplasia, suggesting that TGR5 may play an important role in the progression from BE to high-grade dysplasia and EA. TGR5 might be a potential marker for this progression. However, TGR5 may not be a marker for the prognosis of EA.
TGR5 might be a potential marker for the progression from BE to EA.
TGR5 is a G protein-coupled bile acid receptor.
This article is of tremendous importance in highlighting the different expression of TGR5 among different stages of EA.
| 1. | Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1155] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 2. | Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400-412. [PubMed] |
| 3. | Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155-3162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 4. | Falk GW. Barrett’s esophagus. Gastroenterology. 2002;122:1569-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Crane SJ, Locke GR, Harmsen WS, Zinsmeister AR, Romero Y, Talley NJ. Survival trends in patients with gastric and esophageal adenocarcinomas: a population-based study. Mayo Clin Proc. 2008;83:1087-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Sihvo EI, Luostarinen ME, Salo JA. Fate of patients with adenocarcinoma of the esophagus and the esophagogastric junction: a population-based analysis. Am J Gastroenterol. 2004;99:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, Feuer EJ. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 400] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 8. | Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2043] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 9. | Pohl H, Wrobel K, Bojarski C, Voderholzer W, Sonnenberg A, Rösch T, Baumgart DC. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol. 2013;108:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Kahrilas PJ. The problems with surveillance of Barrett’s esophagus. N Engl J Med. 2011;365:1437-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Kim R, Weissfeld JL, Reynolds JC, Kuller LH. Etiology of Barrett’s metaplasia and esophageal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 1997;6:369-377. [PubMed] |
| 12. | Haggitt RC. Barrett’s esophagus, dysplasia, and adenocarcinoma. Hum Pathol. 1994;25:982-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 441] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Wild CP, Hardie LJ. Reflux, Barrett’s oesophagus and adenocarcinoma: burning questions. Nat Rev Cancer. 2003;3:676-684. [PubMed] |
| 14. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 1003] [Article Influence: 66.9] [Reference Citation Analysis (1)] |
| 15. | Kauer WK, Stein HJ. Emerging concepts of bile reflux in the constellation of gastroesophageal reflux disease. J Gastrointest Surg. 2010;14 Suppl 1:S9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Jankowski JA, Anderson M. Review article: management of oesophageal adenocarcinoma -- control of acid, bile and inflammation in intervention strategies for Barrett’s oesophagus. Aliment Pharmacol Ther. 2004;20 Suppl 5:71-80; discussion 95-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Attwood SE, Smyrk TC, DeMeester TR, Mirvish SS, Stein HJ, Hinder RA. Duodenoesophageal reflux and the development of esophageal adenocarcinoma in rats. Surgery. 1992;111:503-510. [PubMed] |
| 18. | Clark GW, Smyrk TC, Mirvish SS, Anselmino M, Yamashita Y, Hinder RA, DeMeester TR, Birt DF. Effect of gastroduodenal juice and dietary fat on the development of Barrett’s esophagus and esophageal neoplasia: an experimental rat model. Ann Surg Oncol. 1994;1:252-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Fein M, Peters JH, Chandrasoma P, Ireland AP, Oberg S, Ritter MP, Bremner CG, Hagen JA, DeMeester TR. Duodenoesophageal reflux induces esophageal adenocarcinoma without exogenous carcinogen. J Gastrointest Surg. 1998;2:260-268. [PubMed] |
| 20. | Peng S, Huo X, Rezaei D, Zhang Q, Zhang X, Yu C, Asanuma K, Cheng E, Pham TH, Wang DH. In Barrett’s esophagus patients and Barrett’s cell lines, ursodeoxycholic acid increases antioxidant expression and prevents DNA damage by bile acids. Am J Physiol Gastrointest Liver Physiol. 2014;307:G129-G139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Looby E, Abdel-Latif MM, Athié-Morales V, Duggan S, Long A, Kelleher D. Deoxycholate induces COX-2 expression via Erk1/2-, p38-MAPK and AP-1-dependent mechanisms in esophageal cancer cells. BMC Cancer. 2009;9:190. [PubMed] |
| 22. | Wu J, Gong J, Geng J, Song Y. Deoxycholic acid induces the overexpression of intestinal mucin, MUC2, via NF-kB signaling pathway in human esophageal adenocarcinoma cells. BMC Cancer. 2008;8:333. [PubMed] |
| 23. | Das KM, Kong Y, Bajpai M, Kulkarni D, Geng X, Mishra P, Banerjee D, Hirshfield K. Transformation of benign Barrett’s epithelium by repeated acid and bile exposure over 65 weeks: a novel in vitro model. Int J Cancer. 2011;128:274-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435-9440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1272] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 25. | Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 328] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 26. | Yang JI, Yoon JH, Myung SJ, Gwak GY, Kim W, Chung GE, Lee SH, Lee SM, Kim CY, Lee HS. Bile acid-induced TGR5-dependent c-Jun-N terminal kinase activation leads to enhanced caspase 8 activation in hepatocytes. Biochem Biophys Res Commun. 2007;361:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Carino A, Graziosi L, D’Amore C, Cipriani S, Marchianò S, Marino E, Zampella A, Rende M, Mosci P, Distrutti E. The bile acid receptor GPBAR1 (TGR5) is expressed in human gastric cancers and promotes epithelial-mesenchymal transition in gastric cancer cell lines. Oncotarget. 2016;7:61021-61035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Reich M, Deutschmann K, Sommerfeld A, Klindt C, Kluge S, Kubitz R, Ullmer C, Knoefel WT, Herebian D, Mayatepek E. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut. 2016;65:487-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 29. | Hong J, Behar J, Wands J, Resnick M, Wang LJ, DeLellis RA, Lambeth D, Souza RF, Spechler SJ, Cao W. Role of a novel bile acid receptor TGR5 in the development of oesophageal adenocarcinoma. Gut. 2010;59:170-180. [PubMed] |
| 30. | American-Joint-Committee-on-Cancer. AJCC Cancer Stagng Manual. 7th ed. New York: Springer 2010: 117-121. . |
| 31. | Cao W, Tian W, Hong J, Li D, Tavares R, Noble L, Moss SF, Resnick MB. Expression of bile acid receptor TGR5 in gastric adenocarcinoma. Am J Physiol Gastrointest Liver Physiol. 2013;304:G322-G327. [PubMed] |
| 32. | Hong J, Li D, Wands J, Souza R, Cao W. Role of NADPH oxidase NOX5-S, NF-κB, and DNMT1 in acid-induced p16 hypermethylation in Barrett’s cells. Am J Physiol Cell Physiol. 2013;305:C1069-C1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Hong J, Li D, Cao W. Rho Kinase ROCK2 Mediates Acid-Induced NADPH Oxidase NOX5-S Expression in Human Esophageal Adenocarcinoma Cells. PLoS One. 2016;11:e0149735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Souza RF, Morales CP, Spechler SJ. Review article: a conceptual approach to understanding the molecular mechanisms of cancer development in Barrett’s oesophagus. Aliment Pharmacol Ther. 2001;15:1087-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Li D, Cao W. Bile acid receptor TGR5, NADPH Oxidase NOX5-S and CREB Mediate Bile Acid-Induced DNA Damage In Barrett’s Esophageal Adenocarcinoma Cells. Sci Rep. 2016;6:31538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Dar NA S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF