Published online Feb 14, 2017. doi: 10.3748/wjg.v23.i6.1044
Peer-review started: October 10, 2016
First decision: October 20, 2016
Revised: November 17, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: February 14, 2017
Processing time: 129 Days and 2.5 Hours
AIM
To investigate presence and extent of eosinophilic cholangitis (EC) as well as IgG4-related disease in patients with indeterminate biliary stricture (IBS).
METHODS
All patients with diagnosis of sclerosing cholangitis (SC) and histopathological samples such as biopsies or surgical specimens at University Hospital Frankfurt from 2005-2015 were included. Histopathological diagnoses as well as further clinical course were reviewed. Tissue samples of patients without definite diagnosis after complete diagnostic work-up were reviewed regarding presence of eosinophilic infiltration and IgG4 positive plasma cells. Eosinophilic infiltration was as well assessed in a control group of liver transplant donors and patients with primary sclerosing cholangitis.
RESULTS
one hundred and thirty-five patients with SC were included. In 10/135 (13.5%) patients, no potential cause of IBS could be identified after complete diagnostic work-up and further clinical course. After histopathological review, a post-hoc diagnosis of EC was established in three patients resulting in a prevalence of 2.2% (3/135) of all patients with SC as well as 30% (3/10) of patients, where no cause of IBS was identified. 2/3 patients with post-hoc diagnosis of EC underwent surgical resection with suspicion for malignancy. Diagnosis of IgG4-related cholangitis was observed in 7/135 patients (5.1%), whereas 3 cases were discovered in post-hoc analysis. 6/7 cases with IgG4-related cholangitis (85.7%) presented with eosinophilic infiltration in addition to IgG4 positive plasma cells. There was no patient with eosinophilic infiltration in the control group of liver transplant donors (n = 27) and patients with primary sclerosing cholangitis (n = 14).
CONCLUSION
EC is an underdiagnosed benign etiology of SC and IBS, which has to be considered in differential diagnosis of IBS.
Core tip: To differentiate benign from malignant disease in indeterminate biliary strictures (IBS) is crucial for clinical management. To date, data on eosinophilic cholangitis (EC) as a potential cause of IBS are lacking. In this retrospective study, we demonstrate that EC occurs in up to 30% of patients presenting with IBS and unclear clinical and histopathological findings at the end of diagnostic work-up. We thereby demonstrate that EC is a potentially underdiagnosed benign disease, which has to be considered in differential diagnoses of IBS to prevent these patients from surgery.
- Citation: Walter D, Hartmann S, Herrmann E, Peveling-Oberhag J, Bechstein WO, Zeuzem S, Hansmann ML, Friedrich-Rust M, Albert JG. Eosinophilic cholangitis is a potentially underdiagnosed etiology in indeterminate biliary stricture. World J Gastroenterol 2017; 23(6): 1044-1050
- URL: https://www.wjgnet.com/1007-9327/full/v23/i6/1044.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i6.1044
Sclerosing cholangitis (SC) can arise from various underlying diseases, such as infectious, immunological, toxic or ischemic etiology as well as due to mechanical obstruction. Different types of SC may look alike at cross sectional imaging and radiography of the bile ducts, unified by a characteristic narrowing of intrahepatic and/or extrahepatic bile duct system. For clinical management, especially differentiation of benign from malignant strictures is fundamental. However, a definite diagnosis can solely be established by histopathological analysis of the bile duct alterations. Clinical data together with percutaneous ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and endoscopic retrograde cholangiopancreatography (ERCP) contribute to a probable diagnosis, not equaling the security of histopathology. Differentiating indeterminate biliary strictures (IBS) and establishing the diagnosis of SC vs cholangiocarcinoma (CCA) is challenging in many patients. This is represented by studies reporting a benign diagnosis in patients with IBS after surgery in up to 17%[1-3].
In recent years, an increasing number of cases were reported with SC caused by eosinophilic cholangitis (EC): a benign condition first described by Leegaard in 1980[4]. EC is characterized by (1) a wall thickening or stenosis of the biliary system; (2) histopathological findings of eosinophilic infiltration; and (3) reversibility of biliary abnormalities without treatment or following steroid treatment[5]. Peripheral eosinophilia was observed often but not necessarily in case reports (65%[6]). EC as cause of SC is of special interest, since it can appear as Klatskin-mimicking lesion and is often only diagnosed after bile duct resection, although conservative treatment leads to resolution of the stricture. However, an underlying cause of EC could not be identified to date and data on prevalence of EC are lacking.
In the present study, we performed a retrospective analysis to evaluate prevalence of EC and IgG4-RD in patients with IBS and inconclusive histopathological findings.
Patients with diagnosis of SC (according to ICD-10 Code) between 2005 and 2015 at University Hospital Frankfurt were screened and all patients with histopathological specimen available from biopsies or surgical resections were included. Thereby, patients with IBS that were surgically treated for suspicion of malignancy were included as well as inconclusive findings at biopsies. To evaluate the subsequent clinical course of the patients with inconclusive histopathological findings, electronic medical records were investigated and standardized. Extracted data were: age, gender and final diagnosis from clinical documents.
In all patients with inconclusive histological and clinical findings after full diagnostic work-up, hematoxylin-eosin stained slides of surgical or bioptical specimens were reevaluated by an expert pathologist. Eosinophilic granulocytes were counted per high power field (HPF) in areas of cholangitis with the highest density up to an eosinophilic count of 30/HPF. All cases with ≥ 15 eosinophilic granulocytes/HPF were assessed as positive according to the threshold for eosinophilic esophagitis[7]. In addition, a representative block was chosen and staining with an IgG4-antibody (Mouse anti-IgG4, Zytomed Systems, Berlin, Germany) was performed. Cases were considered as IgG4-positive, when > 30% of plasma cells stained positive for IgG4.
Furthermore, all patients with inconclusive findings after full diagnostic work-up were reviewed for presence and appearance of biliary stricture in cross sectional imaging (CT, MRI) and ERCP.
After the review of histopathological and clinical data, patients were classified into consistent with EC, consistent with IgG4-RD or not consistent with either EC or IgG4-RD.
To evaluate eosinophilic infiltration in primary sclerosing cholangitis (PSC) and non-inflammatory bile ducts, samples with histopathological reports of biopsies or surgical specimens including the diagnosis PSC and liver transplant-donors were investigated as well.
For ERCP, standard duodenoscopes (Olympus V-Scopes, TJF 160VF, TJF-Q180 V; Olympus Europe, Hamburg, Germany) were used and the short-wire technique with locking the wire at the distal end of the duodenoscope was applied. In patient 4, cholangioscopy was used as well (duodenoscope TJF - Q180V, Olympus Medical, Tokyo, Japan).
Descriptive statistics were calculated using BiAS (version 11.01, BiAS for Windows; Epsilon-Verlag, Frankfurt, Germany). The study protocol was approved by the institutional review board (No. 478/15) of the local ethics committee of the University Hospital Frankfurt.
In total, 135 histopathological specimens were identified of patients treated for SC between 2005 and 2015. Mean age of the patients at the date of initial histopathological investigation was 54.0 (SD 15.2, range 22-86) and 87/135 (64%) of the patients were men. Histopathological reports were based on liver biopsies (n = 64), intraductal bile duct biopsies (n = 33), biliary brush cytology (n = 16), surgical specimen from liver or bile duct resection (n = 15), liver transplant (n = 6) or autopsy (n = 1).
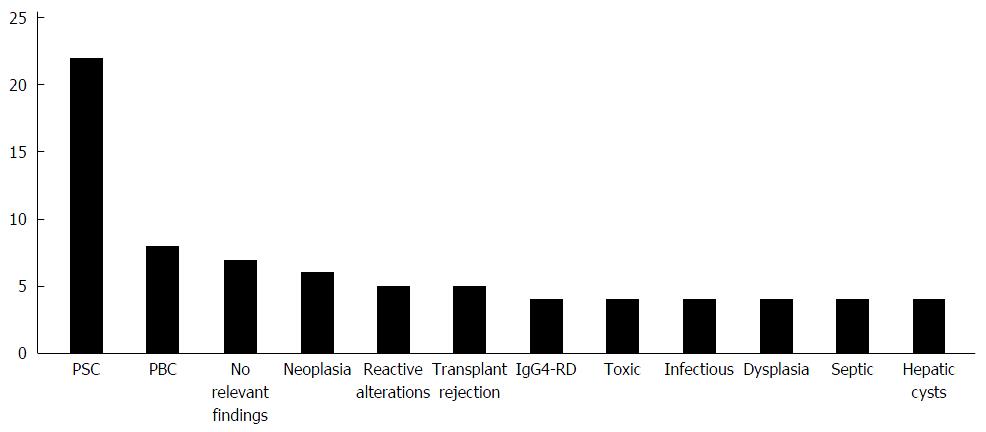
In 69 of 135 patients (51%), the histopathological investigation reported a strong suspicion of a specific disease or a definite diagnosis (all diagnoses are shown in Figure 1). In 7 patients, no relevant pathological findings were reported. 6/7 of these patients were clinically diagnosed with PSC and one patient with CCA in further clinical course.
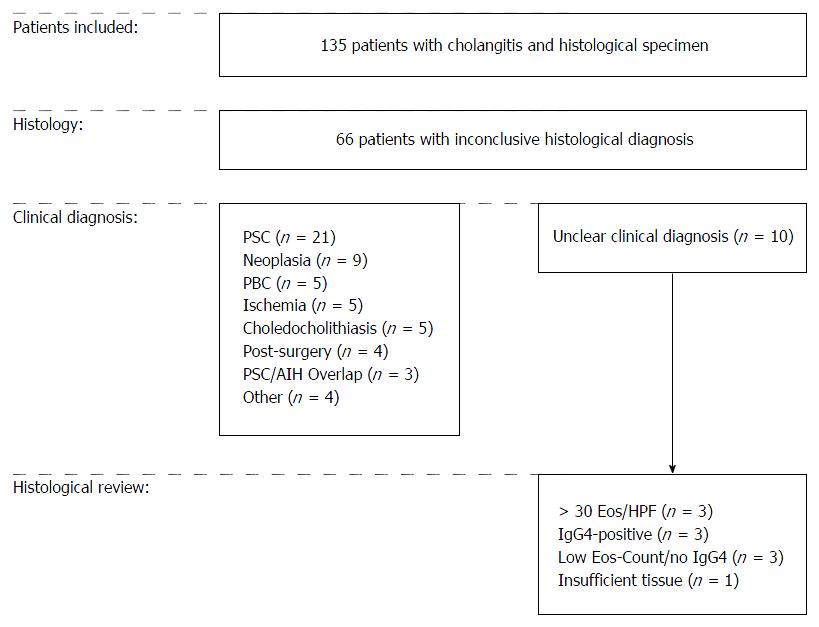
In the group of patients with inconclusive histopathological diagnosis, investigation of clinical reports revealed a specific diagnosis in 56/66 patients (85%). In the remaining ten patients (15%), clinical diagnosis was either unclear cholangitis (n = 4) or patients had surgery with suspicion for malignancy but inconclusive findings in histopathological examination (n = 6). The study design, including final diagnoses, is documented in Figure 2.
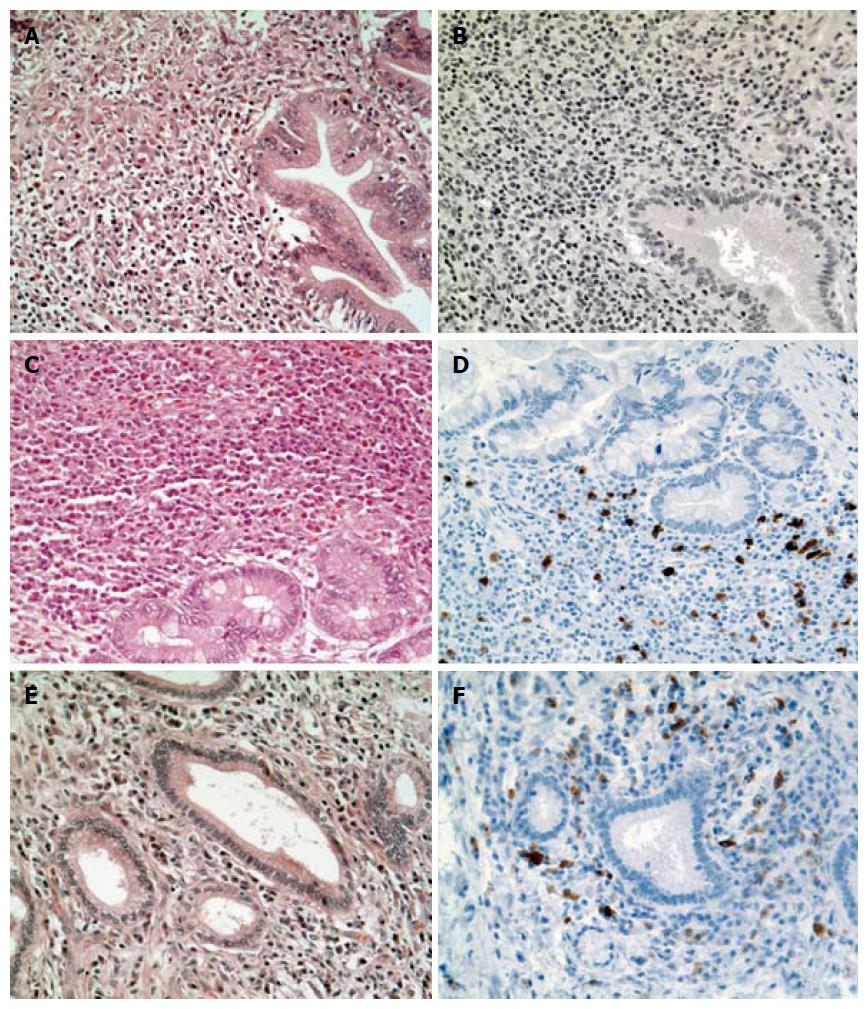
To further characterize these ten patients with unclear clinical as well as histopathological diagnosis and to exclude EC and IgG4-RD as possible underlying etiology, we performed a histological review quantifying eosinophilic infiltration and determining IgG4-status. Thereby, we identified 3/10 (30%) patients with dense eosinophilic infiltrate (≥ 30 eosinophilic granulocytes/HPF) but without IgG4-positive plasma cells. We also found 3/10 patients (30%) to have a distinct infiltration of plasma cells with positive IgG4 staining. Notably, 2 of these 3 patients additionally showed as well a dense eosinophilic infiltrate (> 30/HPF; Figure 3). Of the remaining 4 patients, 3 did neither have an elevated eosinophilic count nor a positive staining for IgG4 and 1 patient had insufficient material for a reliable histopathological review.
Since 2/3 newly diagnosed IgG4-positive samples had a marked eosinophilic infiltrate, a review of the 4 routinely diagnosed IgG4-RD cases was performed. Notably, 3/4 of these patients had > 30 eosinophilic granulocytes/HPF, whereas one patient had 25 eosinophilic granulocytes/HPF. Thus, of all cases with IgG4-RD, 6/7 (85.7%) showed a marked infiltration with eosinophilic granulocytes.
All six patients with suspicion of EC or IgG4-RD were investigated for alterations in the biliary tract. Patients with suspicion of EC had strictures in both the extrahepatic and intrahepatic biliary tree, whereas all strictures of patients with suspicion of IgG4-RD were located in the perihilar region. Besides anatomical location of the stricture, further clinical course of these patients was investigated. 5/6 of these patients underwent surgical resection, whereas one patient with EC was treated conservatively with oral steroids. Clinical data of the 6 patients consistent with diagnoses of EC or IgG4-RD are shown in Table 1.
| Patient ID | Age | Sex | Year | Eos (histology) | Eos (serum) | IgG4-staining | Biliary stenosis | Clinical course |
| Pat1 | 40 | M | 2008 | + | - | + | Perihilar | Hemihepatectomy |
| Pat2 | 52 | M | 2011 | + | - | + | Perihilar | Hemihepatectomy |
| Pat3 | 73 | M | 2005 | - | NA | + | Perihilar | CBD-resection |
| Pat4 | 40 | F | 2015 | + | - | - | Distal | Steroids |
| Pat5 | 63 | M | 2008 | + | - | - | Distal | PPPD |
| Pat6 | 65 | F | 2006 | + | - | - | Perihilar | CBD-resection |
Thereby, after histological and clinical review, prevalence of cases consistent with EC was 3/135 (2%) in all patients with cholangitis and biopsy or surgical specimen, 3/66 (5%) in cases with inconclusive histological findings and 3/10 (30%) in patients with no clear clinical diagnosis in further course after full diagnostic work-up. Further clinical course of these patients was investigated as well: the two patients with EC treated with bile duct resection had several episodes of transient cholestasis or cholangitis after surgery, which were managed with temporary stent placement if necessary. The patient treated with oral steroids is currently off treatment since 12 mo and has not experienced a relapse so far.
Taking into account the growing knowledge about IgG4-RD during the period of this study, the date of histological examination of this entity was investigated as well: Samples with IgG4-RD being diagnosed only within the retrospective histopathological review in this study were taken from 2005-2011, whereas patients with diagnosis of IgG4-RD in initial histopathological examination were taken between 2012 and 2014.
To further characterize EC in comparison to other potential differential diagnoses, eosinophilic infiltration was analyzed in 14 patients with PSC (mean histological stage was 2.0, range 1-3). Mean eosinophilic count/HPF was 1.8 (range 0-6, SD 2.0). In addition, non-inflammatory biliary tissue from liver transplant donors was investigated for degree of eosinophilic infiltration: 27 samples were examined and mean eosinophilic count was 0.1/HPF (range 0-3, SD 0.6; Supplementary Figure 1).
Management of patients presenting with IBS is a frequent challenge for the clinician and the strategic choice of whether and when to perform surgery might have an important effect on these patients' quality of live. In the present study we report results from a retrospective prevalence study on EC, a benign and conservatively treatable differential diagnosis of IBS, which so far has only been described in case reports.
We evaluated all patients with diagnosis of sclerosing cholangitis and available histopathological specimen and found a prevalence of EC of 3/135 (2.2%) as well as 3/10 (30%) in the group of patients without clear clinical diagnosis in further course. 2/3 patients with EC were treated surgically. Of note, also in case reports of EC, 14/35 (40%) patients had cholecystectomy and 10/35 (28.6%) patients were treated with major bile duct resections due to suspicion for malignancy (Supplementary Table 1). The considerable part of patients being retrospectively diagnosed with EC in our study combined with the high rate of surgeries in literature indicates that EC is most likely an underdiagnosed disease, which is often only identified after possibly avoidable bile duct resection. These data stress the important role EC plays as potential underlying disease of SC and point out that surgeons, interventional endoscopists and gastrointestinal pathologists should be aware of this conservatively treatable condition in cases with IBS.
All cases consistent with EC in this study had a dense infiltrate of eosinophilic granulocytes with ≥ 30 per HPF which was considerably higher than the threshold of ≥ 15 eosinophilic granulocytes/HPF we defined according to diagnostic criteria for eosinophilic esophagitis[7]. In view of the frequent submucosal infiltration with eosinophilic granulocytes, we recommend taking preferably intraductal biopsies instead of biliary brushings in case of suspicion for EC. Moreover, as recommended in IBS and eosinophilic esophagitis, we suggest to take multiple biopsies to reduce risk of false negative results[8,9]. Since some cases with EC were reported to be associated with eosinophilic infiltration in other parts of the gastrointestinal tract, taking additional biopsies in the stomach or colon should be considered as well[10-12]. In addition, cholangioscopy can be another helpful tool to diagnose EC[13].
IgG4-RD was identified to be the underlying disease of SC in 7/135 (5.2%) cases. Three of these cases were diagnosed only after retrospective histopathological review within this study. Of note, these three cases were originally diagnosed 2011 and earlier whereas the four routinely diagnosed cases of IgG4-RD were between 2012 and 2014, pointing out the growing knowledge about this disease during the period of our study. Of note, since prevalence data about IgG4-related cholangitis in western countries is very limited, a comparison of our data with other studies is hindered[14,15].
Notably, 6/7 (85.7%) cases with IgG4-RD additionally showed a marked eosinophilic infiltration suggesting a potential overlap in pathogenesis of these two conditions. This finding correlates to a prospective study from 2014, where a cohort of patients with eosinophilic esophagitis was shown to have significantly elevated IgG4-positive plasma cells compared to a control group[16]. Furthermore, IgG4-positive plasma cells and serum levels reacted to specific foods suggesting an IgG4-associated pathogenesis of eosinophilic esophagitis. However, three patients with diagnosis of EC in our study did not show IgG4-positive plasma cells indicating that common features in pathogenesis of EC and IgG4-RD need further characterization.
Another entity important to differentiate from EC is PSC. For instance, in a Japanese study, 27% of PSC patients were reported to have peripheral eosinophilia and hepatic infiltration with eosinophilic granulocytes was described in a patient with PSC[17,18]. Even so, in the present study we performed a histological investigation of 27 cases of PSC and did not find evidence for eosinophilic infiltration in this cohort suggesting a rather easy differentiation of these entities in western patients by histopathological investigation.
To interpret the data of this study it has to be considered that our study was designed retrospectively and monocentric including mostly Caucasian patients. Moreover, Frankfurt University Hospital is a tertiary referral center, which might have raised the number of patients with inconclusive findings.
In conclusion, the findings of this study suggest that to date, EC is most likely an underdiagnosed etiology of SC, which is important to have in mind in patients presenting with IBS.
The excellent technical assistance of Yvonne Michel, Katharina Sandkühler and Damaris Zaubzer is greatly acknowledged.
Differentiation of benign and malignant biliary stenosis in indeterminate biliary stricture (IBS) is a common interdisciplinary challenge. Data on eosinophilic cholangitis as a potential cause of IBS are lacking.
This study represents the first retrospective analysis on prevalence of eosinophilic cholangitis.
This study revealed eosinophilic cholangitis as a most likely underdiagnosed differential diagnosis of sclerosing cholangitis.
This study suggests that eosinophilic cholangitis has to be kept in mind in patients presenting with IBS. In suspicious cases, multiple biopsies should be obtained and cholangioscopy should be considered.
Eosinophil granulocytes are a subgroup of leukocytes. IgG4 is a subgroup of immunoglobulin G which represents the majority of serum antibodies. Infiltration of the bile duct with eosinophilic granulocytes or IgG4-positive plasma cells can lead to sclerosing cholangitis and thereby mimic biliary cancer.
Excellent effort in contributing important information on the challenging problem of IBS. As the authors learn more about EC and similar entities from contributions such as this the authors will be able to offer more specific treatment and avoid surgery that is not needed.
| 1. | Erdogan D, Kloek JJ, ten Kate FJ, Rauws EA, Busch OR, Gouma DJ, van Gulik TM. Immunoglobulin G4-related sclerosing cholangitis in patients resected for presumed malignant bile duct strictures. Br J Surg. 2008;95:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Domagk D, Poremba C, Dietl KH, Senninger N, Heinecke A, Domschke W, Menzel J. Endoscopic transpapillary biopsies and intraductal ultrasonography in the diagnostics of bile duct strictures: a prospective study. Gut. 2002;51:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Juntermanns B, Kaiser GM, Reis H, Saner FH, Radunz S, Vernadakis S, Heuer M, Kuehl H, Paul A, Treckmann J. Klatskin-mimicking lesions: still a diagnostical and therapeutical dilemma? Hepatogastroenterology. 2011;58:265-269. [PubMed] |
| 4. | Leegaard M. Eosinophilic cholecystitis. Acta Chir Scand. 1980;146:295-296. [PubMed] |
| 5. | Matsumoto N, Yokoyama K, Nakai K, Yamamoto T, Otani T, Ogawa M, Tanaka N, Iwasaki A, Arakawa Y, Sugitani M. A case of eosinophilic cholangitis: imaging findings of contrast-enhanced ultrasonography, cholangioscopy, and intraductal ultrasonography. World J Gastroenterol. 2007;13:1995-1997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Nashed C, Sakpal SV, Shusharina V, Chamberlain RS. Eosinophilic cholangitis and cholangiopathy: a sheep in wolves clothing. HPB Surg. 2010;2010:906496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Kavitt RT, Hirano I, Vaezi MF. Diagnosis and Treatment of Eosinophilic Esophagitis in Adults. Am J Med. 2016;129:924-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Walter D, Peveling-Oberhag J, Schulze F, Bon D, Zeuzem S, Friedrich-Rust M, Albert JG. Intraductal biopsies in indeterminate biliary stricture: Evaluation of histopathological criteria in fluoroscopy- vs. cholangioscopy guided technique. Dig Liver Dis. 2016;48:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Nielsen JA, Lager DJ, Lewin M, Rendon G, Roberts CA. The optimal number of biopsy fragments to establish a morphologic diagnosis of eosinophilic esophagitis. Am J Gastroenterol. 2014;109:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Schoonbroodt D, Horsmans Y, Laka A, Geubel AP, Hoang P. Eosinophilic gastroenteritis presenting with colitis and cholangitis. Dig Dis Sci. 1995;40:308-314. [PubMed] [DOI] [Full Text] |
| 11. | Jimenez-Saenz M, Villar-Rodriguez JL, Torres Y, Carmona I, Salas-Herrero E, Gonzalez-Vilches J, Herrerias-Gutierrez JM. Biliary tract disease: a rare manifestation of eosinophilic gastroenteritis. Dig Dis Sci. 2003;48:624-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Sussman DA, Bejarano PA, Regev A. Eosinophilic cholangiopathy with concurrent eosinophilic colitis in a patient with idiopathic hypereosinophilic syndrome. Eur J Gastroenterol Hepatol. 2008;20:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Walter D, Hartmann S, Albert JG. Indeterminate biliary stricture with suspicion for malignancy unmasked as eosinophilic cholangitis by cholangioscopy. Gastrointest Endosc. 2017;85:265-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Hubers LM, Maillette de Buy Wenniger LJ, Doorenspleet ME, Klarenbeek PL, Verheij J, Rauws EA, van Gulik TM, Oude Elferink RP, van de Graaf SF, de Vries N. IgG4-associated cholangitis: a comprehensive review. Clin Rev Allergy Immunol. 2015;48:198-206. [PubMed] [DOI] [Full Text] |
| 15. | Joshi D, Webster GJ. Biliary and hepatic involvement in IgG4-related disease. Aliment Pharmacol Ther. 2014;40:1251-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, Lowichik A, Chen X, Emerson L, Cox K. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 17. | Takikawa H, Manabe T. Primary sclerosing cholangitis in Japan--analysis of 192 cases. J Gastroenterol. 1997;32:134-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Watanabe H, Ohira H, Kuroda M, Takagi T, Ishikawa H, Nishimaki T, Kasukawa R, Takahashi K. Primary sclerosing cholangitis with marked eosinophilic infiltration in the liver. J Gastroenterol. 1995;30:524-528. [PubMed] [DOI] [Full Text] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hokuto D, Jacob H, Peng SY S- Editor: Gong ZM L- Editor: A E- Editor: Liu WX