Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8582
Peer-review started: July 21, 2017
First decision: August 30, 2017
Revised: October 21, 2017
Accepted: November 28, 2017
Article in press: November 28, 2017
Published online: December 28, 2017
Processing time: 36 Days and 2.2 Hours
To explore the features and prognostic value of lymph node metastasis in patients with T1-stage colorectal cancer (CRC).
In all, 321 cases of T1-stage CRC were selected from 10132 patients with CRC who received surgical therapy in six large-scale hospitals in China and were retrospectively analyzed. Univariate and multivariate analyses were performed to analyze the risk factors for lymphatic metastasis. A survival analysis was then performed to analyze the prognostic value of lymph node metastasis.
The occurrence rate of T1 stage was 3.17% (321/10132); of these patients, the lymph node metastasis rate was 8.41% (27/321), and the non-lymph node metastasis rate was 91.59% (294/321). Univariate analysis showed that preoperative serum CEA, preoperative serum CA199, preoperative serum CA724, vascular invasion, and degree of differentiation were associated with lymph node metastasis in T1-stage CRC (P < 0.05 for all). Multivariate analysis indicated that preoperative serum CA724, vascular invasion, and degree of differentiation were closely related to lymph node metastasis (P < 0.05 for all). Log-rank survival analysis showed that age, preoperative serum CEA, preoperative serum CA199, vascular invasion, degree of differentiation, and lymph node metastasis (χ2 = 24.180, P < 0.001) were predictors of 5-year overall survival (OS) (P < 0.05 for all). COX regression analysis demonstrated that preoperative serum CA199 and lymph node metastasis (HR = 5.117; P < 0.05; 95%CI: 0.058-0.815) were independent prognostic indicators of 5-year OS in patients with T1-stage CRC (P < 0.05 for both).
The morbidity of T1-stage CRC was 3.17% for all CRC cases. Preoperative serum CA724, vascular invasion, and degree of differentiation are independent risk factors for lymph node metastasis. Lymph node metastasis is an independent prognostic factor for OS in patients with T1-stage CRC.
Core tip:The high morbidity of patients with colorectal cancer (CRC) is caused by the likelihood of recurrence and metastasis. This study focused on the features and prognostic value of lymph node metastasis in patients with T1-stage CRC. According to the statistical analysis, we found a very low morbidity in patients with T1-stage CRC. Moreover, our findings confirm that preoperative serum CA724, vascular invasion, and degree of differentiation were independent risk factors for lymph node metastasis, which was demonstrated to be an independent prognostic factor for 5-year OS in patients with T1-stage CRC.
- Citation: Sun ZQ, Ma S, Zhou QB, Yang SX, Chang Y, Zeng XY, Ren WG, Han FH, Xie X, Zeng FY, Sun XT, Wang GX, Li Z, Zhang ZY, Song JM, Liu JB, Yuan WT. Prognostic value of lymph node metastasis in patients with T1-stage colorectal cancer from multiple centers in China. World J Gastroenterol 2017; 23(48): 8582-8590
- URL: https://www.wjgnet.com/1007-9327/full/v23/i48/8582.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i48.8582
Colorectal cancer (CRC) is one of the most common malignancies worldwide[1]. With economic development and changes in dietary history, CRC has shown a steadily increasing incidence and is now the fifth leading cause of cancer-related death in China[2,3]. Due to adverse treatment-related side effects and the poor prognosis of this disease, which results from easy recurrence and metastasis, oncotherapy for CRC has posed a dilemma[4]. In addition, lymph node metastasis is the main type of metastasis in advanced CRC. The occurrence rate of T1-stage CRC has been reported to be approximately 3.51%[5,6]. When the tumor is completely removed, patients with T1-stage CRC generally have a good prognosis. However, because metastasis does not often occur in lymph nodes in T1-stage CRC, lymph node metastasis is often overlooked during the process of diagnosis and treatment. Nevertheless, lymph node metastasis is one of the most essential prognostic risk factors. Chock et al[7] reported that the incidence of lymph node metastasis was 5.6% in T1-stage CRC, whereas Gao et al[8] demonstrated that the occurrence of lymph node metastasis was 5.5% in T1-stage CRC. Zheng et al[9] reported that elevated serum levels of tumor markers indicated a high risk of cancer recurrence and poor survival, yet the relationship between tumor markers and lymph node metastasis in T1-stage CRC remains unknown. Our study found that the incidence of lymph node metastasis was 8.41% in T1-stage CRC. What is the detailed prognostic value of lymph node metastasis in T1-stage CRC? This question has received increased attention in clinical practice, but as of now, no definite answer has been provided.
In this study, 321 cases of T1-stage CRC were selected from 10132 patients with CRC who received surgical therapy in six large-scale hospitals in China and were retrospectively analyzed. A statistical analysis was performed to analyze the features of lymph node metastasis and to evaluate its risk factors and prognostic value in patients with T1-stage CRC. These data will provide a theoretical basis for more effective treatment of patients with T1-stage CRC.
In all, 321 cases of T1-stage CRC were screened from 10132 patients with CRC who received surgical therapy in six large-scale hospitals in China (the First Affiliated Hospital of Zhengzhou University, the Affiliated Tumor Hospital of Xinjiang Medical University, Sun Yat-sen Memorial Hospital of Sun Yat-sen University, the First Affiliated Hospital of Xinjiang Medical University, the Third Xiangya Hospital of Central South University, and the Affiliated Hospital of Traditional Chinese Medicine of Xinjiang Medical University) from June 2001 to June 2011. These cases consisted of 172 males and 149 females. The mean patient age was 61.37 ± 13.41 years. Prior to participation, a diagnosis of CRC was confirmed by histopathology for all patients. The tumor-node-metastasis (TNM) stage was determined according to the American Joint Committee on Cancer/International Union Against Cancer TNM staging system for colorectal cancer (2010, 7th edition). No patient received preoperative chemotherapy, radiotherapy, or immunotherapy. The following exclusion criteria were used: cases with incomplete clinical data, those who were inappropriate for statistical analysis, cases who had other malignant tumors, and cases who were treated by endoscopic resection.
All tissues were approved by the Ethics Review Committees of the First Affiliated Hospital of Zhengzhou University before they were used for research purposes. All the patients who provided clinical material signed an informed consent form.
After surgery, the patients were assessed once a month for the first 6 mo, once every 3 mo from 6 mo to 2 years, once every 6 mo from 2 years to 5 years, and finally, once a year after 5 years. Follow-ups were conducted either by outpatient or inpatient review or by telephone. Forty patients did not participate in the follow-up analyses because they did not communicate with the physicians after surgery. In addition, 16 patients developed dysthymia and were unable to cooperate for the remainder of the study, two patients committed suicide, and 21 patients did not participate in the follow-up for unknown reasons. Therefore, the total follow-up rate in the study was 75.39%.
According to the NCCN Guidelines, CRC with lymph node metastasis is defined as stage III disease, but postoperative chemotherapy should be performed in CRC patients with lymph node metastasis, regardless of T stage. FOLFOX6 was used as the first-line adjuvant or neoadjuvant therapy regimen for CRC patients with stage III disease. CapeOX was used as either a first- or second-line adjuvant or neoadjuvant chemotherapy regimen for patients with stage III CRC, those with drug resistance, or those with postoperative recurrence. FOLFIRI was used as the chemotherapy regimen for CRC patients with postoperative recurrence, metastasis, or drug resistance.
Radical surgery was performed according to complete mesocolic excision for patients with colon cancer and total mesorectal excision for patients with rectal cancer. All the patients received scheduled surgery (i.e., not emergency surgery). More than 12 lymph nodes were removed during surgery.
All statistical analyses were performed with SPSS version 18.0. Graphs were constructed with GraphPad Prism software. Univariate analysis was performed using the χ2 test to analyze the association between lymph node metastasis and clinicopathological parameters. Kaplan-Meier survival curves and the log-rank test were used to compare the group with lymph node metastasis and the group without lymph node metastasis. The multivariate survival analysis was performed using the Cox regression model to determine the relative risk (RR) and 95% CI. Statistical significance was defined as P < 0.05.
In all, 321 patients with T1-stage CRC were divided into a lymph node metastasis group (27 cases) and a non-lymph node metastasis group (294 cases). The occurrence rate of lymph node metastasis was 8.41%. The univariate analysis showed that lymph node metastasis was associated with preoperative CEA, preoperative CA199, preoperative CA724, vascular invasion, and degree of differentiation (P < 0.05, for all parameters; Table 1). Lymph node metastasis was not associated with gender, age, smoking status, absolute granulocyte count, D-dimer value, preoperative hemoglobin level, tumor location, tumor size, general tumor type, or tumor tissue type (P > 0.05 for all).
| Clinicopathological characteristic | n | Lymph node metastasis | χ2 | P value | |
| Yes | No | ||||
| Gender | 1.955 | 0.162 | |||
| Male | 172 | 11 | 161 | ||
| Female | 149 | 16 | 133 | ||
| Age (yr) | 0.436 | 0.509 | |||
| ≥ 60 | 174 | 13 | 161 | ||
| < 60 | 147 | 14 | 133 | ||
| Smoking | 0.766 | 0.382 | |||
| No | 279 | 22 | 257 | ||
| Yes | 42 | 5 | 37 | ||
| Preoperative CEA (ng/mL) | 5.994 | 0.014 | |||
| < 5 | 284 | 20 | 264 | ||
| ≥ 5 | 37 | 7 | 30 | ||
| Preoperative CA199 (ng/mL) | 5.015 | 0.025 | |||
| < 9 | 173 | 9 | 164 | ||
| ≥ 9 | 148 | 18 | 130 | ||
| Preoperative CA724 (ng/mL) | 12.275 | 0.000 | |||
| < 2 | 163 | 5 | 158 | ||
| ≥ 2 | 158 | 22 | 136 | ||
| Granulocyte absolute value | 0.771 | 0.380 | |||
| < 2.2 | 32 | 4 | 28 | ||
| ≥ 2.2 | 289 | 23 | 266 | ||
| D-dimer | 2.227 | 0.136 | |||
| < 0.21 | 158 | 17 | 141 | ||
| ≥ 0.21 | 163 | 10 | 153 | ||
| Preoperative hemoglobin | 1.504 | 0.220 | |||
| < 132 | 154 | 16 | 138 | ||
| ≥ 132 | 167 | 11 | 156 | ||
| Vascular invasion | 18.421 | 0.000 | |||
| No | 313 | 23 | 290 | ||
| Yes | 8 | 4 | 4 | ||
| Tumor location | 1.184 | 0.277 | |||
| Rectum | 170 | 17 | 153 | ||
| Colon | 151 | 10 | 141 | ||
| Tumor size (cm) | 1.526 | 0.217 | |||
| < 3 | 190 | 19 | 171 | ||
| ≥ 3 | 131 | 8 | 123 | ||
| Differentiation degree | 7.723 | 0.005 | |||
| High/moderate | 307 | 23 | 284 | ||
| Low | 14 | 4 | 10 | ||
| Tumor general type | 0.219 | 0.640 | |||
| Ulcer | 238 | 19 | 219 | ||
| Non-ulcer | 83 | 8 | 75 | ||
| Tumor tissue type | 2.293 | 0.130 | |||
| Non-adenocarcinoma | 9 | 2 | 7 | ||
| Adenocarcinoma | 312 | 25 | 287 | ||
The multivariate analysis showed that lymph node metastasis was associated with preoperative CA724, vascular invasion, and degree of differentiation (P < 0.05 for all parameters; Table 2). Lymph node metastasis was not associated with gender, age, smoking status, preoperative CEA, preoperative CA199, absolute granulocyte count, D-dimer value, preoperative hemoglobin level, tumor location, tumor size, general tumor type, or tumor tissue type (P > 0.05 for all).
| Clinicopathological characteristic | HR | P value | 95%CI | |
| Lower bound | Upper bound | |||
| Gender, Male vs Female | 1.636 | 0.201 | 0.698 | 5.518 |
| Age (yr), < 60 vs ≥ 60 | 0.063 | 0.801 | 0.357 | 2.218 |
| Smoking No vs Yes | 0.880 | 0.348 | 0.529 | 6.077 |
| Preoperative CEA (ng/mL) < 5 vs ≥ 5 | 1.188 | 0.276 | 0.603 | 5.904 |
| Preoperative CA199 (ng/mL) < 9 vs ≥ 9 | 2.765 | 0.096 | 0.869 | 5.541 |
| Preoperative CA724 (ng/mL) < 2 vs ≥ 2 | 5.461 | 0.019 | 1.232 | 10.783 |
| Granulocyte absolute value, < 2.2 vs ≥ 2.2 | 0.004 | 0.951 | 0.285 | 3.797 |
| D-dimer < 0.21 vs ≥ 0.21 | 1.398 | 0.237 | 0.651 | 5.652 |
| Preoperative hemoglobin < 132 vs ≥ 132 | 0.437 | 0.508 | 0.249 | 1.992 |
| Tumor size (cm), < 3 vs ≥ 3 | 2.090 | 0.148 | 0.185 | 1.290 |
| Vascular invasion, No vs Yes | 8.461 | 0.004 | 2.358 | 81.596 |
| Tumor location, Rectum vs Colon | 0.264 | 0.607 | 0.485 | 3.444 |
| Differentiation degree, High/moderate vs Low | 4.204 | 0.040 | 1.057 | 11.956 |
| Tumor general type, Non-ulcer vs Ulcer | 0.686 | 0.407 | 0.556 | 4.239 |
| Tissue type, Non-adenocarcinoma vs Adenocarcinoma | 0.897 | 0.344 | 0.043 | 2.989 |
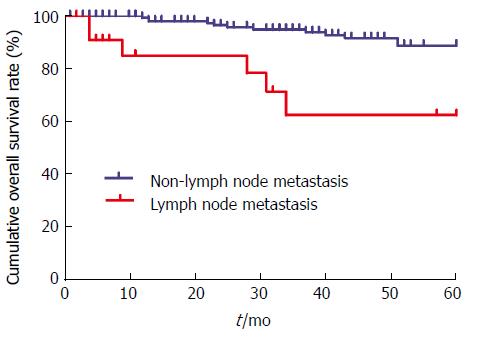
As shown in Table 3, the univariate survival analysis demonstrated that age, preoperative CEA, preoperative CA199, vascular invasion, degree of differentiation, and lymph node metastasis (χ2 = 24.180, P < 0.001) were associated with 5-year OS (P < 0.05 for all). Gender, smoking status, preoperative CA724, absolute granulocyte count, D-dimer value, preoperative hemoglobin level, tumor location, tumor size, general tumor type, and tissue type were not associated with 5-year OS (P > 0.05 for all). The Kaplan-Meier curve showed that the 5-year OS of patients in the lymph node metastasis group was lower than that of patients in the non-lymph node metastasis group (Figure 1).
| Clinicopathological characteristic | n | OS rate | χ2 | P value |
| Gender | 1.225 | 0.268 | ||
| Male | 172 | 90.90% | ||
| Female | 149 | 90.10% | ||
| Age (yr) | 6.103 | 0.013 | ||
| ≥ 60 | 174 | 86.50% | ||
| < 60 | 147 | 95.50% | ||
| Smoking | 0.429 | 0.513 | ||
| No | 279 | 91.00% | ||
| Yes | 42 | 87.50% | ||
| Preoperative CEA (ng/mL) | 13.594 | 0.000 | ||
| < 5 | 284 | 92.70% | ||
| ≥ 5 | 37 | 78.40% | ||
| Preoperative CA199 (ng/mL) | 7.229 | 0.007 | ||
| < 9 | 173 | 94.70% | ||
| ≥ 9 | 148 | 85.70% | ||
| Preoperative CA724 (ng/mL) | 0.012 | 0.912 | ||
| < 2 | 163 | 87.40% | ||
| ≥ 2 | 158 | 93.20% | ||
| Granulocyte absolute value | 1.613 | 0.204 | ||
| < 2.2 | 32 | 85.00% | ||
| ≥ 2.2 | 289 | 91.00% | ||
| D-dimer | 0.001 | 0.979 | ||
| < 0.21 | 158 | 88.80% | ||
| ≥ 0.21 | 163 | 91.90% | ||
| Preoperative hemoglobin | 0.907 | 0.341 | ||
| < 132 | 154 | 89.10% | ||
| ≥ 132 | 167 | 91.70% | ||
| Vascular invasion | 12.955 | 0.000 | ||
| No | 313 | 91.60% | ||
| Yes | 8 | 40.00% | ||
| Tumor location | 1.121 | 0.290 | ||
| Rectum | 170 | 89.80% | ||
| Colon | 151 | 91.90% | ||
| Tumor size (cm) | 0.210 | 0.647 | ||
| < 3 | 190 | 90.00% | ||
| ≥ 3 | 131 | 91.20% | ||
| Differentiation degree | 6.825 | 0.009 | ||
| High/moderate | 307 | 91.30% | ||
| Low | 14 | 78.60% | ||
| Tumor general type | 3.149 | 0.207 | ||
| Protruded | 228 | 91.80% | ||
| Infiltration | 10 | 100.00% | ||
| Ulcer | 83 | 86.20% | ||
| Tumor tissue type | 1.828 | 0.176 | ||
| Non-adenocarcinoma | 9 | 75.00% | ||
| Adenocarcinoma | 312 | 91.30% | ||
| Lymph node metastasis | 24.180 | 0.000 | ||
| No | 294 | 93.20% | ||
| Yes | 27 | 65.20% |
The multivariate survival analysis showed that preoperative CA199 level and lymph node metastasis (RR = 5.117, P < 0.05, 95%CI: 0.058-0.815) were associated with 5-year OS (P < 0.05 for all, Table 4). In contrast, gender, age, smoking status, preoperative CEA level, preoperative CA724 level, absolute granulocyte count, D-dimer value, preoperative hemoglobin level, tumor location, tumor size, general tumor type, tissue type, vascular invasion, and degree of differentiation were not associated with 5-year OS (P > 0.05 for all).
| RR | P value | 95%CI | ||
| Lower bound | Upper bound | |||
| Gender, Male vs Female | 1.784 | 0.182 | 0.695 | 6.816 |
| Age (yr), < 60 vs ≥ 60 | 3.805 | 0.051 | 0.995 | 9.034 |
| Smoking, No vs Yes | 0.590 | 0.442 | 0.413 | 7.565 |
| Preoperative CEA, (ng/mL), < 5 vs ≥ 5 | 1.121 | 0.290 | 0.580 | 6.206 |
| Preoperative CA199, (ng/mL), < 9 ≥ 9 | 6.452 | 0.011 | 1.411 | 14.481 |
| Preoperative CA724, (ng/mL), < 2 ≥ 2 | 0.935 | 0.333 | 0.201 | 1.725 |
| Granulocyte absolute value, < 2.2 vs ≥ 2.2 | 0.040 | 0.841 | 0.171 | 8.761 |
| D-dimer, < 0.21 ≥ 0.21 | 2.373 | 0.123 | 0.777 | 8.217 |
| Preoperative hemoglobin, < 132 vs ≥ 132 | 0.577 | 0.448 | 0.198 | 2.042 |
| Tumor size (cm), < 3 vs ≥ 3 | 0.581 | 0.446 | 0.261 | 21.193 |
| Vascular invasion, No vs Yes | 0.343 | 0.558 | 0.468 | 4.076 |
| Tumor location, Rectum vs Colon | 0.210 | 0.647 | 0.310 | 2.068 |
| Differentiation degree, High/moderate vs Low | 1.031 | 0.310 | 0.435 | 13.792 |
| Tumor general type, Ulcer vs Non-ulcer | 2.338 | 0.126 | 0.811 | 5.471 |
| Tumor tissue type, Non-adenocarcinoma vs Adenocarcinoma | 0.783 | 0.376 | 0.068 | 2.755 |
| Lymph node metastasis, No vs Yes | 5.328 | 0.021 | 1.264 | 17.592 |
CRC is the third most common cancer and the third leading cause of cancer-related death worldwide[10]. The 5-year OS rate of patients with colon cancer is 64.9% and is 66.5% for those with rectal cancer. T1 stage is generally early-stage CRC and has a good prognosis, but if lymph node metastasis occurs, the prognosis is usually poor. Therefore, lymph node metastasis has garnered increased attention in recent years. According to previous studies, the proportion of lymph node metastasis in patients with T1-stage CRC was reported to be 5.6% and 5.5%, which is relatively low[11,12]. In this study, the proportion of lymph node metastasis in patients with T1-stage CRC was 8.41%. The factors that affect lymph node metastasis of T1-stage CRC are multifaceted and are also interrelated with each other. Lymph node metastasis is the main basis for clinic pathological staging of CRC for the prediction of prognosis of patients and for the determination of the appropriate regimen of postoperative adjuvant therapy. Therefore, it is necessary to study the risk factors that are correlated with lymph node metastasis of CRC, as well as their influence on the prognosis of T1-stage CRC[13-15].
Some studies have reported that tumor markers had certain clinical value in the detection of postoperative recurrence and metastasis of CRC as well as in the judgment of prognosis[16-18]. In our study, univariate analysis showed that preoperative CEA, preoperative CA199, and preoperative CA724 were associated with lymph node metastasis, while multivariate analysis showed that preoperative CA724 was an independent risk factor for lymph node metastasis. CA724 has been reported to be a marker for gastrointestinal and ovarian cancers and was shown to be a better indicator for the diagnosis of gastric cancer compared with the levels of CA199 and CEA. Our study found that the CA724 level was a good indicator of lymph node metastasis, which has not been previously reported in T1-stage CRC.
Previous studies have reported that the degree of differentiation of tumor cells in rectal cancer was closely related to lymph node metastasis[19,20]. Our study demonstrated that the degree of differentiation was an independent risk factor for lymph node metastasis of T1-stage CRC. The reason for this may be that when the degree of differentiation is relatively high, the tumor cells are still in a more primitive stage, and the possibility that they may invade the lymph nodes is much lower. Poorly differentiated or undifferentiated carcinomas have a strong ability to invade the surrounding tissues, especially the lymphatic vessels. Derwinger et al[21] showed that the degree of differentiation of CRC was significantly associated with lymph node metastasis. In our study, the rates of lymph node metastasis in patients with highly/moderately and poorly differentiated T1-stage CRC were 7.5% and 28.6%, respectively. In addition, univariate survival analysis showed that the degree of tumor differentiation was a prognostic factor in patients with T1-stage CRC.
Most studies have reported that vascular invasion was an essential risk factor for lymph node metastasis in CRC[22,23]. Similarly, our study verified that vascular invasion was a positive independent risk factor for lymph node metastasis in T1-stage CRC[23]. Univariate survival analysis showed that vascular invasion was associated with the 5-year OS of patients with T1-stage CRC. However, according to the multivariate analysis, no correlation was observed between lymph node metastasis and the 5-year OS in T1-stage CRC, which may have been due to the limited case number.
In clinical practice, lymph node metastasis is a significant indicator of clinical evaluation of rectal cancer recurrence and the survival of patients, and is also the primary method used to determine the therapeutic schedule in patients with rectal cancer[24-27]. When the tumor is confined to the mucosal layer, no lymph node metastasis occurs because the layer has no lymphatic vessels. When lymphatic vessels are distributed in the submucosa, lymph node metastasis is likely to occur when the tumor invades the submucosa. When the tumor invades the deep intestinal wall, the lymph node metastasis rate will increase significantly[28,29]. In our study, the lymph node metastasis rate of this group of patients with T1-stage CRC was 8.41%, which is mostly consistent with previous reports[7,8]. In this study, the survival analysis of T1-stage CRC patients showed that patients without lymph node metastasis had a significantly higher 5-year survival rate than those with lymph node metastasis. Furthermore, our study verified that lymph node metastasis was an independent prognostic factor in patients with T1-stage CRC, which is also consistent with previous reports[30]. With the development of several new technologies, such as endoscopic mucosal resection, endoscopic submucosal dissection, and transanal endoscopic microsurgery, studies on local resection for the treatment of early rectal cancer have gradually increased[31-33]. The biggest drawback of local resection is its failure to dissect the lymph nodes in relevant drainage areas. Left metastatic lymph nodes are an important reason for postoperative recurrence, which is also the reason why caution should be taken if local resection is selected[34-36]. Consequently, if it is not clear whether preoperative lymph node metastasis is present in T1-stage CRC, radical surgery may be the most suitable choice. Moreover, the intraoperative dissection of lymph nodes should be standardized.
In conclusion, through statistical analysis, we verified that the occurrence rate of T1 stage out of all the cases of CRC was 3.17%; the lymph node metastasis rate was 8.41%, and the non-lymph node metastasis rate was 91.59%. Preoperative serum CA724 level, vascular invasion, and degree of differentiation were independent risk factors for lymph node metastasis in patients with T1-stage CRC. Lymph node metastasis was an essential prognostic factor in patients with T1-stage CRC. An accurate assessment of lymph node metastasis status is essential for decision-making regarding effective intraoperative therapeutic strategies for T1-stage CRC.
Lymph node metastasis is the primary type of metastasis seen in advanced colorectal cancer (CRC). The occurrence rate of T1-stage CRC has been reported to be approximately 3.51%[5,6]. When the tumor is completely removed, patients with T1-stage CRC generally have a good prognosis. However, since lymph node metastasis rarely occurs in T1-stage CRC, lymph node metastasis is often overlooked during the process of diagnosis and treatment. Nevertheless, lymph node metastasis is one of the most essential prognostic factors. In this study, we explored the features and prognostic value of lymph node metastasis, which will provide a theoretical basis for more effective treatment of patients with T1-stage CRC.
The main topic of this study is the exploration of whether lymph node metastasis in patients with T1-stage CRC is valuable for patient survival in multiple centers in China. The key is to find the risk factors for lymph node metastasis of CRC. The significance is the confirmation of the prognostic value of lymph node metastasis in patients with T1-stage CRC.
Studies have reported that lymph node metastasis is an essential prognostic factor for patients with CRC and that lymph node metastasis seldom occurs in T1-stage CRC. However, the definitive prognostic value of lymph node metastasis of T1-stage CRC remains elusive. The main objective of this study was to explore the features and prognostic value of lymph node metastasis in patients with T1-stage CRC.
The current research was a case-control study.
The occurrence rate of T1 stage CRC was 3.17% (321/10,132); of these cases, the lymph node metastasis rate was 8.41% (27/321), and the non-lymph node metastasis rate was 91.59% (294/321). Univariate analysis showed that preoperative serum CEA, preoperative serum CA199, preoperative serum CA724, vascular invasion, and degree of differentiation were associated with lymph node metastasis in T1-stage CRC. Multivariate analysis indicated that preoperative serum CA724, vascular invasion, and degree of differentiation were closely related to lymph node metastasis. Log-rank survival analysis showed that age, preoperative serum CEA, preoperative serum CA199, vascular invasion, degree of differentiation, and lymph node metastasis were prognostic factors for 5-year OS. COX regression analysis demonstrated that preoperative serum CA199 and lymph node metastasis were independent prognostic factors for 5-year OS of patients with T1-stage CRC.
The morbidity of T1-stage CRC was 3.17% out of all cases of CRC. Preoperative serum CA724, vascular invasion, and degree of differentiation were independent risk factors for lymph node metastasis of T1-stage CRC. Lymph node metastasis was an independent prognostic factor of OS in patients with T1-stage CRC.
T1-stage CRC is generally regarded as the early stage, easily leading to the neglect of metastasis, especially lymph node metastasis. However, the prognosis of a little part of these cases (8.41%) with lymph node metastasis will be much poorer than those without. We also analysed high risk factors of lymph node metastasis of T1-stage CRC patients. Therefore, we must pay enough attention to lymph node metastasis status of T1-stage CRC patients to guide clinic therapy. Future studies should be focused on greater verifying study to expand further clinical samples. In addition, the mechanistic study of lymph node metastasis in T1-stage CRC patients should be further explored. Multi-center prospective cohort clinical studies will be needed to further validate the conclusion. Moreover, high-throughput transcriptome or proteome screening technology will be necessary for analysing the regulators of lymph node metastasis in T1-stage CRC patients in the future.
| 1. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2937] [Article Influence: 326.3] [Reference Citation Analysis (7)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13326] [Article Influence: 1332.6] [Reference Citation Analysis (4)] |
| 3. | Zhu J, Tan Z, Hollis-Hansen K, Zhang Y, Yu C, Li Y. Epidemiological Trends in Colorectal Cancer in China: An Ecological Study. Dig Dis Sci. 2017;62:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Imai H, Sawada K, Sato A, Nishi K, Sasaki T, Takahashi T, Ohori H. [Complete resection of liver metastases of colorectal cancer after high efficacy bevacizumab, S-1, and CPT -11 combination chemotherapy]. Gan To Kagaku Ryoho. 2015;42:101-104. [PubMed] |
| 5. | Iida S, Hasegawa H, Okabayashi K, Moritani K, Mukai M, Kitagawa Y. Risk factors for postoperative recurrence in patients with pathologically T1 colorectal cancer. World J Surg. 2012;36:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Kobayashi H, Mochizuki H, Morita T, Kotake K, Teramoto T, Kameoka S, Saito Y, Takahashi K, Hase K, Oya M. Characteristics of recurrence after curative resection for T1 colorectal cancer: Japanese multicenter study. J Gastroenterol. 2011;46:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Chok KS, Law WL. Prognostic factors affecting survival and recurrence of patients with pT1 and pT2 colorectal cancer. World J Surg. 2007;31:1485-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Gao C, Li JT, Fang L, Wen SW, Zhang L, Zhao HC. Pre-operative predictive factors for intra-operative pathological lymph node metastasis in rectal cancers. Asian Pac J Cancer Prev. 2013;14:6293-6299. [PubMed] |
| 9. | Zheng CX, Zhan WH, Zhao JZ, Zheng D, Wang DP, He YL, Zheng ZQ. The prognostic value of preoperative serum levels of CEA, CA19-9 and CA72-4 in patients with colorectal cancer. World J Gastroenterol. 2001;7:431-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3032] [Cited by in RCA: 3131] [Article Influence: 208.7] [Reference Citation Analysis (0)] |
| 11. | Wada H, Shiozawa M, Katayama K, Okamoto N, Miyagi Y, Rino Y, Masuda M, Akaike M. Systematic review and meta-analysis of histopathological predictive factors for lymph node metastasis in T1 colorectal cancer. J Gastroenterol. 2015;50:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Caputo D, Caricato M, La Vaccara V, Taffon C, Capolupo GT, Coppola R. T1 colorectal cancer: poor histological grading is predictive of lymph-node metastases. Int J Surg. 2014;12:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 14. | Frattini M, Balestra D, Suardi S, Oggionni M, Alberici P, Radice P, Costa A, Daidone MG, Leo E, Pilotti S. Different genetic features associated with colon and rectal carcinogenesis. Clin Cancer Res. 2004;10:4015-4021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Kawachi H, Eishi Y, Ueno H, Nemoto T, Fujimori T, Iwashita A, Ajioka Y, Ochiai A, Ishiguro S, Shimoda T. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod Pathol. 2015;28:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Bramsen JB, Rasmussen MH, Ongen H, Mattesen TB, Ørntoft MW, Árnadóttir SS, Sandoval J, Laguna T, Vang S, Øster B. Molecular-Subtype-Specific Biomarkers Improve Prediction of Prognosis in Colorectal Cancer. Cell Rep. 2017;19:1268-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 17. | Qian Z, Zhang G, Song G, Shi J, Gong L, Mou Y, Han Y. Integrated analysis of genes associated with poor prognosis of patients with colorectal cancer liver metastasis. Oncotarget. 2017;8:25500-25512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Huang Y, Li Y, He F, Wang S, Li Y, Ji G, Liu X, Zhao Q, Li J. Metastasis-associated protein 3 in colorectal cancer determines tumor recurrence and prognosis. Oncotarget. 2017;8:37164-37171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Choi Y, Roh MS, Hong YS, Lee HS, Hur WJ. Interleukin-24 is correlated with differentiation and lymph node numbers in rectal cancer. World J Gastroenterol. 2011;17:1167-1173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Hahn-Strömberg V, Askari S, Befekadu R, Matthiessen P, Karlsson S, Nilsson TK. Polymorphisms in the CLDN1 and CLDN7 genes are related to differentiation and tumor stage in colon carcinoma. APMIS. 2014;122:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Derwinger K, Kodeda K, Bexe-Lindskog E, Taflin H. Tumour differentiation grade is associated with TNM staging and the risk of node metastasis in colorectal cancer. Acta Oncol. 2010;49:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | van Wyk HC, Roxburgh CS, Horgan PG, Foulis AF, McMillan DC. The detection and role of lymphatic and blood vessel invasion in predicting survival in patients with node negative operable primary colorectal cancer. Crit Rev Oncol Hematol. 2014;90:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Mou S, Soetikno R, Shimoda T, Rouse R, Kaltenbach T. Pathologic predictive factors for lymph node metastasis in submucosal invasive (T1) colorectal cancer: a systematic review and meta-analysis. Surg Endosc. 2013;27:2692-2703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Okugawa Y, Inoue Y, Tanaka K, Toiyama Y, Shimura T, Okigami M, Kawamoto A, Hiro J, Saigusa S, Mohri Y. Loss of the metastasis suppressor gene KiSS1 is associated with lymph node metastasis and poor prognosis in human colorectal cancer. Oncol Rep. 2013;30:1449-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Huh JW, Kim HC, Kim SH, Park YA, Cho YB, Yun SH, Lee WY, Chun HK. Mismatch repair system and p53 expression in patients with T1 and T2 colorectal cancer: predictive role of lymph node metastasis and survival. J Surg Oncol. 2014;109:848-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Wada H, Shiozawa M, Sugano N, Morinaga S, Rino Y, Masuda M, Akaike M, Miyagi Y. Lymphatic invasion identified with D2-40 immunostaining as a risk factor of nodal metastasis in T1 colorectal cancer. Int J Clin Oncol. 2013;18:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Ishihara S, Kawai K, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, Morikawa T, Watanabe T. Oncological Outcomes of Lateral Pelvic Lymph Node Metastasis in Rectal Cancer Treated With Preoperative Chemoradiotherapy. Dis Colon Rectum. 2017;60:469-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 28. | Huang C, Chen Y. Lymphangiogenesis and colorectal cancer. Saudi Med J. 2017;38:237-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Haraguchi N, Ohara N, Koseki J, Takahashi H, Nishimura J, Hata T, Mizushima T, Yamamoto H, Ishii H, Doki Y. High expression of ADAMTS5 is a potent marker for lymphatic invasion and lymph node metastasis in colorectal cancer. Mol Clin Oncol. 2017;6:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Akagi Y, Adachi Y, Kinugasa T, Oka Y, Mizobe T, Shirouzu K. Lymph node evaluation and survival in colorectal cancer: review of population-based, prospective studies. Anticancer Res. 2013;33:2839-2847. [PubMed] |
| 31. | De Ceglie A, Hassan C, Mangiavillano B, Matsuda T, Saito Y, Ridola L, Bhandari P, Boeri F, Conio M. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol. 2016;104:138-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 32. | Backes Y, Moons LM, van Bergeijk JD, Berk L, Ter Borg F, Ter Borg PC, Elias SG, Geesing JM, Groen JN, Hadithi M. Endoscopic mucosal resection (EMR) versus endoscopic submucosal dissection (ESD) for resection of large distal non-pedunculated colorectal adenomas (MATILDA-trial): rationale and design of a multicenter randomized clinical trial. BMC Gastroenterol. 2016;16:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Zhou P, Yao L, Qin X, Xu M, Zhong Y, Chen W. Endoscopic submucosal dissection for locally recurrent colorectal lesions after previous endoscopic mucosal resection. Dis Colon Rectum. 2009;52:305-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Olsen S, Jin L, Fields RC, Yan Y, Nalbantoglu I. Tumor budding in intestinal-type gastric adenocarcinoma is associated with nodal metastasis and recurrence. Hum Pathol. 2017;68:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 35. | Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg. 2017;266:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 358] [Article Influence: 39.8] [Reference Citation Analysis (1)] |
| 36. | Asano H, Kojima K, Ogino N, Fukano H, Ohara Y, Shinozuka N. Postoperative recurrence and risk factors of colorectal cancer perforation. Int J Colorectal Dis. 2017;32:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Barreto S, Giordano A, Su CC S- Editor: Chen K L- Editor: Wang TQ E- Editor: Ma YJ