Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8562
Peer-review started: August 2, 2017
First decision: August 10, 2017
Revised: August 19, 2017
Accepted: November 22, 2017
Article in press: November 22, 2017
Published online: December 28, 2017
Processing time: 309 Days and 16 Hours
To assess whether elevated serum carcinoembryonic antigen (CEA) is in the inferior prognosis for pathological lymph node-negative (pN0) gastric cancer (GC) patients who underwent D2 gastrectomy.
About 469 pN0 GC patients, who received D2 radical gastrectomy were retrospectively analyzed. The X-tile plots cut-off point for CEA were 30.02 ng/mL using minimum P-value from log-rank χ2 statistics, and pN0 GC patients were assigned to two groups: those more than 30.02 ng/mL (n = 48; CEA-high group) and those less than 30.02 ng/mL (n = 421; CEA-low group). Clinicopathologic characteristics were compared using Pearson's χ2 or Fisher’s exact tests, and survival curves were so manufactured using the Kaplan-Meier method. Univariate and multivariate analysis were carried out using the logistic regression method.
The percentage of vessel carcinoma embolus (31.35% vs 17.1%) and advanced GC (T2-4b) (81.25% vs 65.32%) were higher in CEA-high group than CEA-low group. The CEA-positive patients had a significantly poorer prognosis than the CEA-nagetive patients in terms of overall survival (57.74% vs 90.69%, P < 0.05), and no different was found between subgroup of T category, differentiation, nerve invasion, and vessel carcinoma embolus (all P > 0.05). Multivariate survival analysis showed that CEA (OR = 4.924), and T category (OR = 2.214) were significant prognostic factors for stage pN0 GC (all P < 0.05). Besides, only T category (OR = 1.962) was an independent hazard factor in the CEA-high group (P < 0.05).
Those pretreatment serum CEA levels over 30.02 ng/mL on behalf of worse characteristics and unfavourable tumor behavior, and a poor prognosis for a nearly doubled risk of mortality in GC patients.
Core tip: Currently, the survival rate for gastric cancer (GC) is still unsatisfactory. Reliable biomarker such as carcinoembryonic antigen (CEA) is necessary to improve the management of GC and pathological lymph node-negative (pN0) represents a group of reliable biological status. About 469 pN0 GC patients, who received D2 radical gastrectomy were retrospectively analyzed, and an optimal cut-off value of CEA was reset, and we found that pretreatment serum CEA levels over 30.02 ng/mL on behalf of worse characteristics and unfavourable tumor behavior, and a poor prognosis for a nearly doubled risk of mortality in staging pN0 GC patients.
- Citation: Xiao J, Ye ZS, Wei SH, Zeng Y, Lin ZM, Wang Y, Teng WH, Chen LC. Prognostic significance of pretreatment serum carcinoembryonic antigen levels in gastric cancer with pathological lymph node-negative: A large sample single-center retrospective study. World J Gastroenterol 2017; 23(48): 8562-8569
- URL: https://www.wjgnet.com/1007-9327/full/v23/i48/8562.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i48.8562
Currently, the therapeutic effect for gastric cancer (GC) is still dispiriting[1], especially in China. This reason may be partly ascribed to the delayed diagnosis of GC. In addition to tumor-nodes-metastasis (TNM) stage and selection of treatment, the survival rate of GC patients may be hit by other factors such as differentiation, behavior and genetic mutation[2].
Pathological lymph node-negative (pN0) represents a group of reliable biological status, however, the survival for patients can unending changes, even when they share the same clinical stage[3]. Therefore, clinicians and researchers keep looking for other survival factors that might be able to help in the selection of a suitable treatment strategy.
Carcinoembryonic antigen (CEA), an acknowledged as an intracellular adhesion molecule, is one of the most common markers used in GC[2]. Up to now, many studies showed that extremely elevated serum CEA, which is closely related to an awful prognosis[4]. Numerous studies have been in favor of preoperative CEA levels as predictive marker for the survival situation of GC[5-8]. However, other studies have reported the opposite results[9-13]. Inconsistent views can be partly explained by different cutoff values of CEA, limited number of eligible cases and study endpoints, and the inadequate statistical power.
To solve the above-mentioned problem, we performed a large sample retrospective study and reset an optimal cut-off value of CEA and to explore the relationship between preoperative serum CEA and clinicopathological traits and prognostic information.
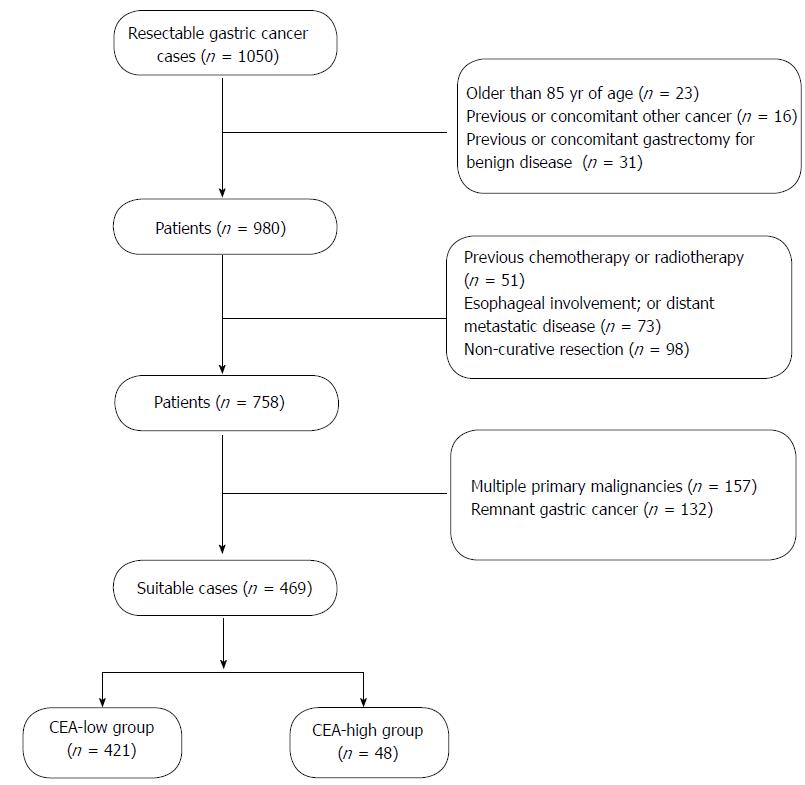
From January 2000 to December 2010, a retrospective analysis was conducted of 1801 consecutive patients with GC who underwent D2 lymphadenectomy, at the Department of gastrointestinal surgery, Fujian tumor hospital. Among them, 469 pN0 resectable GC patients suffered from stage pTxN0M0 GC according to the 7th edition of the TNM classification. Data from these patients were enrolled into a prospectively maintained database.
The inclusion criteria were as follows: (1) pN0 resectable GC; (2) Adenocarcinoma confirmed by histopathology; (3) Physical fitness suitable for surgery; (4) D2 lymphadenectomy; and (5) no prior history of any type of adjunctive therapy.
The exclusion criteria were as follows: (1) older than 85 years of age; (2) previous or concomitant other cancer; (3) previous or concomitant gastrectomy for benign disease; (4) previous chemotherapy or radiotherapy; (5) esophageal involvement; or (6) distant metastatic disease; (7) non-curative resection; (8) multiple primary malignancies; (9) remnant GC; and (10) mortality within 30 d after surgery (Figure 1).
All of the above patients were followed up by posting letters or by telephone interviews. The last follow-up was 1 January 2017. The cardiopathy logical and follow-up findings were collected and recorded in the database. All subjects gave written informed consent to the study protocol, which was approved by the Ethical Committees of Fujian Provincial Tumor Hospital.
According to the 7th edition NCCN guidelines[2], surgery with lymph node (LN) dissection is the primary treatment option for medically fit patients with resectable T1b, any N tumors. All patients in the study underwent standard total or distal gastrectomy, depending on the location and macroscopic appearance of the primary tumor (Table 1). The strategy for LN dissections was determined using a standardized technique according to the guidelines of the 2010 Japanese Classification of Gastric Cancer and Gastric Cancer Treatment Guidelines edited by the Japanese Gastric Cancer Association[14].
| Characteristics | CEA-Low group(n = 421) | CEA-High group( n = 48) | P value |
| Age (yr), mean ± SD | 58.74 ± 10.98, 60 (20-83) | 60.4 ± 11.55, 61 (31-78) | |
| Gender | |||
| Female | 118 (28) | 12 (25) | 0.657 |
| Male | 303 (72) | 36 (75) | |
| Male-to-female ratio | 2.81:1 | 3:01 | |
| Family history | |||
| Positive | 8 (1.9) | 1 (2.01) | 0.930 |
| Negative | 413 (98.1) | 47 (97.99) | |
| HP infection status | |||
| Positive | 37 (8.8) | 5 (10.4) | 0.708 |
| Negative | 384 (91.2) | 43 (89.6) | |
| BMI (kg/m2) | |||
| Less than 18.5 | 28 (6.65) | 4 (8.33) | 0.358 |
| 18.5-24.99 | 304 (72.21) | 38 (79.17) | |
| More than 25 | 89 (21.14) | 6 (12.5) | |
| Differentiation degree | |||
| Well | 226 (53.7) | 22 (45.8) | 0.302 |
| Poor | 195 (46.3) | 26 (54.2) | |
| Location | |||
| Upper | 113 (26.8) | 16 (33.33) | 0.779 |
| Middle | 129 (30.64) | 10 (20.83) | |
| Lower | 168 (39.9) | 21 (43.75) | |
| Mixed | 11 (2.61) | 1 (2.08) | |
| Lauren classification | |||
| Intestinal type | 105 (24.94) | 10 (20.83) | 0.668 |
| Diffuse type | 270 (64.13) | 31 (64.59) | |
| Mixed type | 46 (10.93) | 7 (14.58) | |
| T category | |||
| T1a | 68 (16.2) | 4 (8.3) | 0.033a |
| T1b | 78 (18.5) | 5 (10.4) | |
| T2 | 89 (21.1) | 13 (27.1) | |
| T3 | 65 (15.4) | 14 (29.2) | |
| T4a | 116 (27.6) | 10 (20.8) | |
| T4b | 5 (1.2) | 2 (4.2) | |
| T1 | 146 (34.68) | 9 (18.75) | 0.026a |
| T2-4b | 275 (65.32) | 39 (81.25) | |
| Nerve invasion | |||
| Positive | 70 (16.6) | 11 (22.92) | 0.275 |
| Negative | 351 (83.4) | 37 (77.08) | |
| Vessel carcinoma embolus | |||
| Positive | 72 (17.1) | 15 (31.35) | 0.017a |
| Negative | 349 (82.9) | 33 (68.75) |
The clinicopathological findings, including depth of tumor invasion and LN metastases, were utilized to stage tumors according to the 7th edition NCCN guidelines[2]. LNs were dissected and described according to the Japanese Classification of Gastric Carcinoma[14], which was also used to classify the location, histological type, and lymphatic invasion of tumors.
Statistical analyses were conducted using Statistical Product for Social Sciences (SPSS) 19.0 software (SPSS, Inc, Chicago, IL, United States). The distribution of baseline characteristics was compared by using either Fisher’s exact test or the chi-square test. The CEA cut-off points were produced and analyzed using the X-tile program which identified the cut-off with the minimum P values from log-rank χ2 statistics for the categorical CEA in terms of survival. Meaningful factors were extracted for further analysis, which was conducted by using the logistic regression method. The overall cumulative probability of survival was calculated by the Kaplan-Meier method, and differences were evaluated by using the log-rank test. A P value less than 0.05 was regarded as statistically significant.
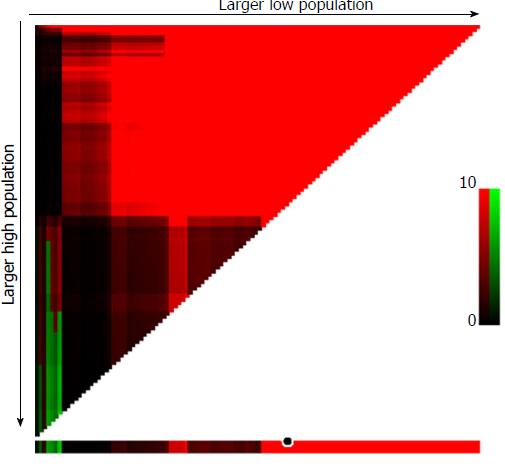
X-tile plots, constructed in Figure 2, illustrated that the optimal cut-off point for CEA was 30.02 ng/mL, and GC patients with in stage pN0 were assigned to two groups: those more than 30.02 ng/mL (n = 48; CEA-high group) and those less than 30.02 ng/mL (n = 421; CEA-low group), with the strongest discriminatory capacity, with a χ2 value of 85.15 and a relative risk ratio of 1:2.15.
Depending on the 7th editions of the TNM system, a total of 469 pN0 GC patients were recruited in this study. Patient demographic data are summarized in Table 1. Overall, no observably difference was found in these characteristics, including gender, age, family history (FH), HP infection status, BMI, location, and lauren classification (all P > 0.05).
A slightly higher proportion of male patients constituted in the CEA-high patients (76% vs 64.04%), and male-to-female ratio was 3:1 among the CEA-high compare to 2.81:1 with CEA-low patients. In the CEA-high group, the proportion of was slightly higher than the negative group in poor differentiation (54.2% vs 46.3%), and nerve invasion (22.92% vs 16.6%). What is more, percentage was dramatically higher in CEA-high group than CEA-low counterparts in stage of T2-4b (81.25% vs 65.32%, P = 0.026), vessel carcinoma embolus (31.35% vs 17.1%, P = 0.017) among the CEA-positive goup.
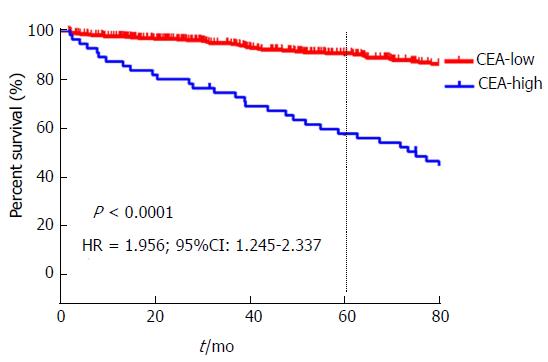
The 5-year OS of stage pN0 GC patients with high level of CEA was significantly inferior than CEA-low groups (57.74% vs 90.69%, P < 0.05, Figure 3).
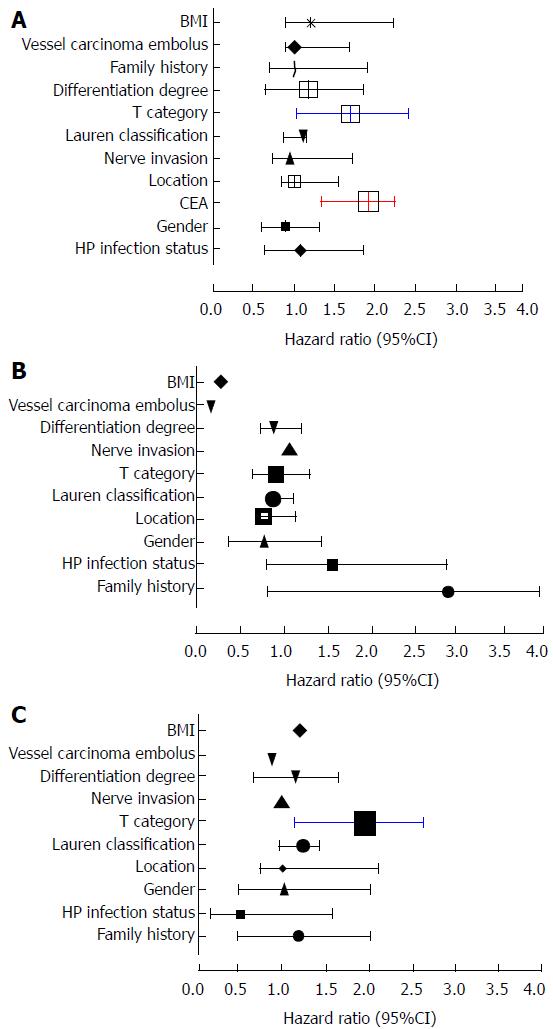
Univariate analysis exhibited that FH of GC, HP infection status, gender, CEA, T category, differentiation degree, location, and lauren classification, nerve invasion, vessel carcinoma embolus, and BMI; among which T category (OR = 1.906), CEA (OR = 1.919), vessel carcinoma embolus (OR = 1.764), and gender (OR = 1.716) were independent hazard prognostic factors(all P < 0.05, Table 2, Figure 4A).
| Univariate analysis | Multivariate analysis | |||||||
| P value | Exp(B) | 95%CI used for Exp (B) | P value | Exp (B) | 95%CI used for Exp (B) | |||
| Lower | Upper | Lower | Upper | |||||
| Family history | 0.912 | 0.923 | 0.765 | 1.311 | 0.069 | 1.017 | 0.72 | 1.896 |
| HP infection status | 0.209 | 0.832 | 0.781 | 1.226 | 0.754 | 1.088 | 0.643 | 1.840 |
| Gender | 0.000a | 1.716 | 1.316 | 2.553 | 0.590 | 0.898 | 0.608 | 1.327 |
| CEA | 0.000a | 1.919 | 1.319 | 2.352 | 0.000a | 1.924 | 1.353 | 2.232 |
| Location | 0.245 | 0.841 | 0.792 | 1.234 | 0.749 | 1.012 | 0.861 | 1.531 |
| Lauren classification | 0.241 | 0.851 | 0.814 | 1.091 | 0.711 | 1.109 | 0.891 | 1.154 |
| T category | 0.000a | 1.906 | 1.659 | 2.271 | 0.009a | 1.714 | 1.050 | 2.403 |
| Differentiation degree | 0.279 | 0.932 | 0.881 | 1.126 | 0.784 | 1.188 | 0.663 | 1.640 |
| Nerve invasion | 0.971 | 0.801 | 0.731 | 1.145 | 0.097 | 0.951 | 0.7768 | 1.655 |
| Vessel carcinoma embolus | 0.000a | 1.764 | 1.321 | 2.562 | 0.983 | 0.994 | 0.895 | 1.660 |
| BMI | 0.732 | 0.812 | 0.729 | 1.234 | 0.356 | 1.228 | 0.912 | 2.229 |
Further multivariate analysis showed that CEA (OR = 1.924), T category (OR = 1.714) were significant prognostic factors for pN0 GC (all P < 0.05, Table 2, Figure 4B). In the CEA-high sub-group, T category (OR = 1.962) was an independent hazard factor in CEA-high group by multivariate analysis (P < 0.05, Table 3, Figure 4C).
| CEA-Low group (n = 421) | CEA-High group (n = 48) | |||||||
| P value | Exp(B) | 95%CI | P value | Exp(B) | 95%CI | |||
| Lower | Upper | Lower | Upper | |||||
| Family history | 0.077 | 2.978 | 0.888 | 3.986 | 0.512 | 1.191 | 0.501 | 2.019 |
| HP infection status | 0.140 | 1.590 | 0.858 | 2.947 | 0.247 | 0.522 | 0.174 | 1.570 |
| Gender | 0.478 | 0.834 | 0.504 | 1.378 | 0.919 | 1.036 | 0.527 | 2.037 |
| Location | 0.482 | 0.831 | 0.764 | 1.124 | 0.897 | 1.012 | 0.752 | 2.102 |
| Lauren classification | 0.831 | 0.911 | 0.891 | 1.103 | 0.843 | 1.245 | 0.984 | 1.435 |
| T category | 0.647 | 0.941 | 0.725 | 1.222 | 0.001a | 1.962 | 1.139 | 2.629 |
| Differentiation degree | 0.879 | 0.931 | 0.811 | 1.176 | 0.884 | 1.148 | 0.673 | 1.641 |
| Nerves invaded | 0.811 | 1.090 | 0.539 | 2.205 | 0.987 | 0.993 | 0.438 | 2.251 |
| Vessel carcinoma embolus | 0.064 | 0.315 | 0.093 | 1.068 | 0.883 | 0.889 | 0.685 | 2.281 |
| BMI | 0.392 | 0.424 | 0.851 | 1.124 | 0.356 | 1.228 | 0.912 | 2.229 |
As we known, CEA is part of the most familiarly used cancer biomarkers, and high preoperative CEA are closely associated with tumor load[10,15-19]. However, there had been few literatures regarding the treatment outcome of evaluating the prognostic significance of CEA, in particularly to those pN0 GC patients. Previous studies have offered ambivalent testimony on the survival value of pretreatment CEA levels in resectable GC.
At the present stage, there existed no unified and well-recognized cut-off points[2]. Tied to various objective factors such as the sample size, different follow-up periods, ethnicities and different tumor stage, it leaded to inconsistent bias. To strengthen the statistical power, we collected a large sample analysis, and the number of eligible patients on the basis of similar endpoints. In the present study, the cut-off point was applied to 30.02 ng/mL.
In addition, study characteristics that miscellaneous large span studies might have influenced the effect size in GC patients. To confirm this synergistic effect, we performed subgroup analyses by clinicopathologic baseline. Firstly, in the CEA-high group, the proportion of was slightly higher than the negative group in poor differentiation (54.2% vs 46.3%), and nerve invasion (22.92% in vs 16.6%), showing that CEA-high GC patients with stage pN0 may be at higher risk, and it should be remunerated meticulous attention to the crowd.
Although the biological actions of CEA are not fully understood, the close link of preoperative CEA to cancer aggressiveness has been known for many years[20]. Specifically, the patients with a high level of CEA were consulted more frequently in the presence of a advanced stage (T2-4b: 81.25% vs 65.32%, P = 0.026), vessel carcinoma embolus (31.35% vs 17.1%, P = 0.017). The baseline data supported the view that a high level of CEA in stage pN0 patients were identified as having worse biological behavior and more aggressive baseline conditions, which might be fastened to a potential genetic susceptibility and infaust living habits[21-24].
In consideration of worse characteristics and unfavourable tumor behavior, CEA-high patients’ survival rate was poor. In the data, the 5-year OS of patients with high expression of CEA was strikingly inferior (57.74% vs 90.69%, P < 0.05). The data added weight to show that preoperative prominent CEA correlates with more aggressive and poor survival, and the point above had to be in conformity with a former research[25].
Further verification was tested by multivariate analysis, the findings highlighted that CEA (OR = 1.924), T category (OR = 1.714) were significant prognostic factors for GC cases with stage of pN0, suggesting that these two factors were closely associated with the survival and multicollinearity might exist between them. The view was in accordance with many scholars[2] who had found that elevated serum CEA was involved in tumor depth (T category), lymphatic metastasis, and TNM stage, and liver metastasis[26,27], and it was an independent prognostic risk factor.
Further analysis show that T category (OR = 1.962) was an objective hazard factor in the CEA-high group for pN0 GC patients. It was found consistently in the aforementioned studies, which were at the root of the CEA was substituted for T category in the current TNM staging system to come up with a modified staging system.
To our knowledge, this analysis is one of the relatively few that have been reported. However, there were several limitations inherent in this study. First, it was intended to serve as a retrospective study and a clinical bias could potentially occur. Also, follow-ups were achieved through phone calls and a recall bias existed.
In spite of the assistance brought by the optimal cut-off value for serum CEA level in clinical practice, there exists limitations. Firstly, the possibility of patient selection introducing bias was inherent, which can affect surgical outcomes. Secondly, the number of CEA-high patients was relatively small, which reducing the intensity of statistics. What’s more, the data come from a single hospital, so the results may not represent the Chinese population well.
In conclusion, the CEA, categorized by cut-off points of 30.02 ng/mL, could produce the best prognostic discriminatory ability, and increased pretreatment CEA levels nearly doubled the risk of mortality in pN0 GC patients.
The survival value of carcinoembryonic antigen (CEA) for gastric cancer patients remains obscure. This study aims at assessing whether elevated serum CEA is a partner in the inferior prognosis for pathological lymph node-negative (pN0) patients.
CEA, an acknowledged as an intracellular adhesion molecule, is one of the most common markers used in GC. Numerous studies have been in favour of preoperative CEA levels as biomarker for the survival of GC. However, other studies have reported the opposite results. The X-tile plot has been recently elaborated to establish cut-off point for biomarkers in cancer. we performed a large sample retrospective study and reset an optimal cut-off value.
The authors found that the CEA, categorized by cut-off points of 30.02 ng/mL could develop the best prognostic discriminatory ability and predictive accuracy for staging pN0 GC patients. Increased pretreatment serum CEA levels (> 30.02 ng/mL) nearly doubled the risk of mortality in in pN0 GC patients.
This study results suggest that those pretreatment serum CEA levels over 30.02 ng/mL on behalf of worse characteristics and unfavourable tumor behavior.
The authors examined subjects with pretreatment serum CEA > 30.02 ng/mL have a poor prognosis in terms of survival, vascular invasion and transmural invasion. Clinicopathologic factors affecting outcome were evaluated. Their results show that those pretreatment serum CEA levels over 30.02 ng/mL on behalf of worse characteristics and unfavourable tumor behavior, and a poor prognosis for a nearly doubled risk of mortality in GC patients.
| 1. | Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 798] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 2. | Tsai YC, Hsiao WH, Lin SH, Yang HB, Cheng HC, Chang WL, Lu CC, Sheu BS. Genomic single nucleotide polymorphisms in the offspring of gastric cancer patients predispose to spasmolytic polypeptide-expressing metaplasia after H. pylori infection. J Biomed Sci. 2015;22:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077-3079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 820] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 4. | Bagaria B, Sood S, Sharma R, Lalwani S. Comparative study of CEA and CA19-9 in esophageal, gastric and colon cancers individually and in combination (ROC curve analysis). Cancer Biol Med. 2013;10:148-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (2)] |
| 5. | Shimizu K, Ueda Y, Yamagishi H. Titration of serum p53 antibodies in patients with gastric cancer: a single-institute study of 40 patients. Gastric Cancer. 2005;8:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Mihmanli M, Dilege E, Demir U, Coskun H, Eroglu T, Uysalol MD. The use of tumor markers as predictors of prognosis in gastric cancer. Hepatogastroenterology. 2004;51:1544-1547. [PubMed] |
| 7. | Tachibana M, Takemoto Y, Nakashima Y, Kinugasa S, Kotoh T, Dhar DK, Kohno H, Nagasue N. Serum carcinoembryonic antigen as a prognostic factor in resectable gastric cancer. J Am Coll Surg. 1998;187:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Tian SB, Yu JC, Kang WM, Ma ZQ, Ye X, Cao ZJ, Yan C. Combined detection of CEA, CA 19-9, CA 242 and CA 50 in the diagnosis and prognosis of resectable gastric cancer. Asian Pac J Cancer Prev. 2014;15:6295-6300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Ychou M, Duffour J, Kramar A, Gourgou S, Grenier J. Clinical significance and prognostic value of CA72-4 compared with CEA and CA19-9 in patients with gastric cancer. Dis Markers. 2000;16:105-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Ucar E, Semerci E, Ustun H, Yetim T, Huzmeli C, Gullu M. Prognostic value of preoperative CEA, CA 19-9, CA 72-4, and AFP levels in gastric cancer. Adv Ther. 2008;25:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Galizia G, Lieto E, De Vita F, Romano C, Orditura M, Castellano P, Imperatore V, Infusino S, Catalano G, Pignatelli C. Circulating levels of interleukin-10 and interleukin-6 in gastric and colon cancer patients before and after surgery: relationship with radicality and outcome. J Interferon Cytokine Res. 2002;22:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S. Serum interleukin-6 and -10 levels in patients with gastric cancer. Gastric Cancer. 2009;12:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Wang Z, Si X, Xu A, Meng X, Gao S, Qi Y, Zhu L, Li T, Li W, Dong L. Activation of STAT3 in human gastric cancer cells via interleukin (IL)-6-type cytokine signaling correlates with clinical implications. PLoS One. 2013;8:e75788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Sano T. [Evaluation of the gastric cancer treatment guidelines of the Japanese Gastric Cancer Association]. Gan To Kagaku Ryoho. 2010;37:582-586. [PubMed] |
| 15. | Deng K, Yang L, Hu B, Wu H, Zhu H, Tang C. The prognostic significance of pretreatment serum CEA levels in gastric cancer: a meta-analysis including 14651 patients. PLoS One. 2015;10:e0124151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Feng F, Sun L, Liu Z, Liu S, Zheng G, Xu G, Guo M, Lian X, Fan D, Zhang H. Prognostic values of normal preoperative serum cancer markers for gastric cancer. Oncotarget. 2016;7:58459-58469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Huang ZB, Zhou X, Xu J, Du YP, Zhu W, Wang J, Shu YQ, Liu P. Prognostic value of preoperative serum tumor markers in gastric cancer. World J Clin Oncol. 2014;5:170-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Wu YC, Lv P, Han J, Yu JL, Zhu X, Hong LL, Zhu WY, Yu QM, Wang XB, Li P. Enhanced serum methylated p16 DNAs is associated with the progression of gastric cancer. Int J Clin Exp Pathol. 2014;7:1553-1562. [PubMed] |
| 19. | Dilege E, Mihmanli M, Demir U, Ozer K, Bostanci O, Kaya C, Aksakal O, Sakiz D. Prognostic value of preoperative CEA and CA 19-9 levels in resectable gastric cancer. Hepatogastroenterology. 2010;57:674-677. [PubMed] |
| 20. | Chen S, Chen YB, Li YF, Feng XY, Zhou ZW, Yuan XH, Qian CN. Normal carcinoembryonic antigen indicates benefit from perioperative chemotherapy to gastric carcinoma patients. World J Gastroenterol. 2012;18:3910-3916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Raza Y, Khan A, Khan AI, Khan S, Akhter S, Mubarak M, Ahmed A, Kazmi SU. Combination of Interleukin 1 Polymorphism and Helicobacter pylori Infection: an Increased Risk of Gastric Cancer in Pakistani Population. Pathol Oncol Res. 2017;23:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Hua RX, Zhuo ZJ, Zhu J, Jiang DH, Xue WQ, Zhang SD, Zhang JB, Li XZ, Zhang PF, Jia WH. Association between genetic variants in the XPG gene and gastric cancer risk in a Southern Chinese population. Aging (Albany NY). 2016;8:3311-3320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Petrovchich I, Ford JM. Genetic predisposition to gastric cancer. Semin Oncol. 2016;43:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Choi YJ, Kim N. Gastric cancer and family history. Korean J Intern Med. 2016;31:1042-1053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 25. | Park SH, Ku KB, Chung HY, Yu W. Prognostic significance of serum and tissue carcinoembryonic antigen in patients with gastric adenocarcinomas. Cancer Res Treat. 2008;40:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Wada N, Kurokawa Y, Miyazaki Y, Makino T, Takahashi T, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. The characteristics of the serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels in gastric cancer cases. Surg Today. 2017;47:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Chae HD, Kim IH. Prognostic significance of CEA expression by RT-PCR in peritoneal wash from patients with gastric cancer: result of a 5-year follow-up after curative resection. Scand J Gastroenterol. 2016;51:956-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Eleftheriadis NP, Ierardi E S- Editor: Gong ZM L- Editor: A E- Editor: Ma YJ