Published online Dec 28, 2017. doi: 10.3748/wjg.v23.i48.8553
Peer-review started: October 7, 2017
First decision: October 25, 2017
Revised: November 14, 2017
Accepted: November 27, 2017
Article in press: November 27, 2017
Published online: December 28, 2017
Processing time: 81 Days and 23.7 Hours
To evaluate the safety and efficacy of totally laparoscopic total gastrectomy (TLTG) with esophagojejunostomy using a linear stapler compared with laparoscopic-assisted total gastrectomy (LATG) using a circular stapler in gastric cancer patients.
We retrospectively reviewed 687 patients who underwent laparoscopic total gastrectomy for gastric cancer at a single institution from August 2008 to August 2014. The patients were divided into two groups according to the type of operation: 421 patients underwent TLTG and 266 underwent LATG. Clinicopathologic characteristics and surgical outcomes in the two groups were compared and analyzed.
The TLTG group had higher mean ages at the time of operation (57.78 ± 11.20 years and 55.69 ± 11.96 years, P = 0.020) and more histories of abdominal surgery (20.2% and 12.4%, P = 0.008) compared with the LATG group. Surgical outcomes such as intraoperative and postoperative transfusions, combined operations, pain scores and administration of analgesics, and complications were similar between the two groups. However, compared with the LATG group, the TLTG group required a shorter operation time (149 min vs 170 min, P < 0.001), had lower postoperative hematocrit change (3.49% vs 4.04%, P = 0.002), less intraoperative events (3.1% vs 10.2%, P < 0.001), less intraoperative anastomosis events (2.4% vs 7.1%, P = 0.003), faster postoperative recovery such as median time to first flatus (3.30 d vs 3.60 d, P < 0.001), faster median commencement of soft diet (4.30 d vs 4.60 d, P < 0.001) and shorter length of postoperative hospital stay (6.75 d vs 7.02 d, P = 0.005).
The intracorporeal method for reconstruction of esophagojejunostomy using a linear stapler may be considered a feasible procedure comparing with extracorporeal anastomosis using circular stapler because TLTG is simpler and more straightforward than LATG. Therefore, TLTG can be recommended as an appropriate procedure for gastric cancer.
Core tip: There are many studies that compared totally laparoscopic total gastrectomy (TLTG) with laparoscopic-assisted total gastrectomy (LATG). Moreover, various modified methods of intracorporeal esophagojejunostomy have been presented, but standardized methods have not been established. Our results show that TLTG by esophagojejunostomy intracorporeal anastomosis using linear stapler is an easier and more straightforward procedure compared with LATG by extracorporeal anastomosis using circular stapler.
- Citation: Gong CS, Kim BS, Kim HS. Comparison of totally laparoscopic total gastrectomy using an endoscopic linear stapler with laparoscopic-assisted total gastrectomy using a circular stapler in patients with gastric cancer: A single-center experience. World J Gastroenterol 2017; 23(48): 8553-8561
- URL: https://www.wjgnet.com/1007-9327/full/v23/i48/8553.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i48.8553
Asian countries, especially South Korea and Japan, have the highest incidences of gastric cancer in the world[1]. Gastric cancer is the second most common cause of cancer-related deaths worldwide[2,3], and surgery is the only curative modality for primary treatment of resectable gastric cancer[4,5]. The proportion of early gastric cancer patients has increased in Korea and Japan as a result of improved nationwide surveillance[6,7], and now accounts for nearly 60% of all cases. The incidence of upper and middle body gastric cancer has also increased. Consequently, the demand for minimally invasive treatments for upper body early gastric cancer has grown, and there is more need for new therapeutic methods and modalities. Laparoscopic gastrectomy has become one of the most popular modalities because of less postoperative pain, rapid postoperative recovery, lower blood loss, better cosmetic outcomes, and fewer complications compared with open gastrectomy[5,8-10]. In addition, the oncologic outcomes of laparoscopic gastrectomy for gastric cancer are acceptable as well[11,12].
Intracorporeal anastomosis has advantages over extracorporeal anastomosis because the former creates a smaller wound, provides a larger workspace and is less invasive[13-19]. Totally laparoscopic total gastrectomy (TLTG) using various types of intracorporeal anastomosis methods has been developed due to improvements in surgical devices and the accumulation of operative experience, but an optimal method for TLTG is yet to be established because of the technical challenges, especially for reconstruction of the esophagojejunostomy (EJ). Since 2008, TLTG using endoscopic linear staplers has been performed in our institute on more than 400 patients by expert surgeons with much experience of laparoscopic surgery, and we have developed a secure and effective technique for reconstructing the EJ[15-17].
In this study, we aimed to evaluate the surgical safety and efficacy of TLTG for treating gastric cancer of the upper third of the stomach by comparing its outcomes with those of laparoscopic-assisted total gastrectomy (LATG) using a circular stapler.
We reviewed the retrospectively collected data of 687 consecutive patients who underwent total gastrectomy by LATG (266 patients) and TLTG (421 patients), for gastric cancer in the upper and middle stomach, between August 2008 and August 2014 at Asan Medical Center in Seoul, South Korea. The diagnosis was based on preoperative examinations including esophagogastroduodenoscopy, endoscopic ultrasound (EUS), and computed tomography (CT). Patients with gastric cancer were selected by preoperative diagnostic test under T3N2M0 according to the American Joint Committee on Cancer (AJCC) - International Union for Cancer Control (UICC) 7th edition[20]. Based on operative findings, patients with serosa-exposed advanced gastric cancer were converted to open surgery and were not included in this study. All patients were managed by clinical pathway after surgery[21]. The study was approved by the Institutional Review Board of Asan Medical Center.
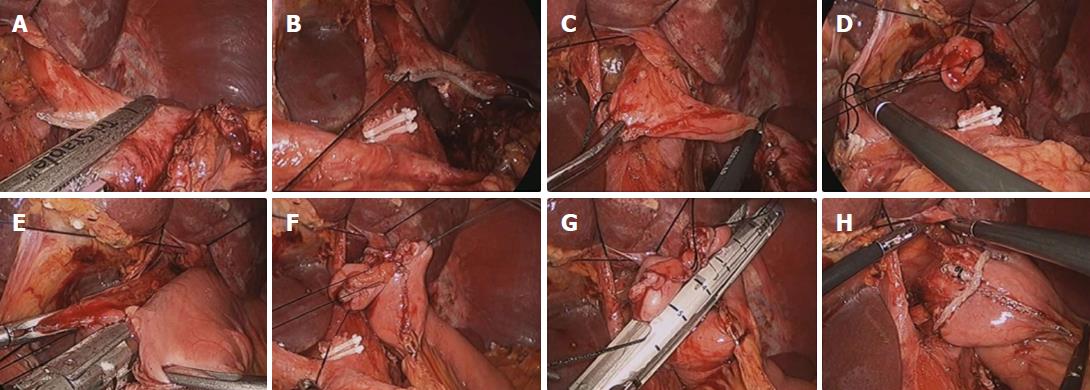
Partial omentectomy with either D1+ or D2 lymphadenectomy for early gastric cancer and total omentectomy with either D2 lymphadenectomy for advanced gastric cancer were performed using an ultrasonic scalpel according to the treatment guidelines published by the Japanese Gastric Cancer Association[22]. EJ was performed with a circular stapler (DST EEATM 25 single use stapler with 3.5 staples; Covidien, North Haven, CT, United States) via mini-laparotomy in LATG and with an endoscopic linear stapler (Endo GIATM 60 mm and 45 mm Articulating Medium/Thick Reload with Tri-StapleTM Technology; Covidien) in TLTG (Figure 1). Finally, defects in the transmesentery and transcolon were closed via suturing. Details of the technique of TLTG have been described previously[16,17]. In case of advanced gastric cancer or with spleen hilar lymph node swelling, hilar lymph node was harvested and intraoperative frozen biopsy was carried out. If the frozen biopsy result was positive, then splenectomy was also carried out.
Data obtained from medical records included patient age, sex, body mass index (BMI), American Society of Anesthesiologist (ASA) score, history of previous abdominal surgery, operative time, pre- and postoperative hematocrit, time to first flatus, day of commencement of soft diet, pain score by visual analogue scale, number of analgesics administered, intra- and postoperative transfusion, intraoperative events, postoperative hospital stay, tumor size, number of retrieved lymph nodes, resection margins and cancer stage according to the AJCC/UICC 7th edition. Intraoperative events included jejunojejunostomy site kicking or narrowing, emphysema, and injury to organs such as pancreas, spleen, colon, small bowel, liver and major vessels. Intraoperative anastomosis events-related EJ refers to all unexpected events related to the EJ anastomosis, such as leakage after anastomosis, small bowel or esophagus injury caused by small diameter, pseudo-lumen stapling, sticking together of the crus muscle, etc.
Postoperative pain control consisted of intravenous, patient-controlled analgesia (fentanyl 2500 µg, ketorolac tromethamine 180 mg, and ondansetron hydrochloride 16 mg) and intermittent analgesic infusions. The amount of postoperative pain was assessed by visual analogue scale and by the number of additional doses of analgesics required until hospital discharge. A postoperative complication was defined as any event that required conservative or surgical treatment after surgery. Early complications were defined as events occurring within 30 d, and late complications as those occurring after 30 d. These complications were examined and classified by the Clavien-Dindo system[23].
Statistical analyses were performed with SPSS v18.0. Categorical variables were compared using the chi-square test or Fisher’s exact test. All continuous variables were analyzed using the Mann-Whitney test, the t-test or the chi-square test, depending on the data. A P-value of less than 0.05 was considered statistically significant.
The clinical characteristics of the LATG and TLTG groups are presented in Table 1. The LATG and TLTG groups consisted of 266 and 421 patients, respectively. Their mean ages at the time of operation were 55.69 ± 11.96 years and 57.78 ± 11.20 years, respectively (P = 0.020). There were no significant differences between the two groups for sex (P = 0.583), ASA score (P = 0.064) or BMI (P = 0.883). Frequencies of abdominal surgery were 12.4% and 20.2% (P = 0.008) in LATG and TLTG groups, respectively. In summary, the TLTG group was slightly older and had more histories of abdominal surgery than LATG group.
| Variable | LATG, n = 266 | TLTG, n = 421 | P value |
| Age in years, mean ± SD | 55.69 ± 11.96 | 57.78 ± 11.20 | 0.020 |
| Sex | 0.583 | ||
| Male | 167 (62.8) | 273 (64.8) | |
| Female | 99 (37.2) | 148 (35.2) | |
| ASA score | 0.064 | ||
| I | 181 (68.0) | 249 (59.1) | |
| II | 68 (25.6) | 145 (34.4) | |
| III | 17 (6.4) | 27 (6.4) | |
| BMI in kg/m2 | 0.883 | ||
| < 23 | 119 (44.7) | 198 (47.0) | |
| ≥ 23, < 25 | 70 (26.3) | 103 (24.5) | |
| ≥ 25, < 30 | 69 (25.9) | 110 (26.1) | |
| ≥ 30 | 8 (3.0) | 10 (2.4) | |
| History of abdominal surgery | 33 (12.4) | 85 (20.2) | 0.008 |
Table 2 presents the pathologic results for the LATG and TLTG groups. The mean numbers of retrieved lymph nodes were 34.91 ± 13.92 and 40.04 ± 15.59 in the LATG and TLTG groups, respectively, indicating that lymph node dissection was adequate in both groups. The remaining pathological characteristics did not differ significantly between the groups, except for proximal resection margin length (LATG: 3.85 ± 3.11 cm and TLTG: 2.68 ± 2.62 cm, P < 0.001).
| Variable | LATG, n = 266 | TLTG, n = 421 | P value |
| Tumor size in cm | 3.72 ± 2.47 | 3.95 ± 2.90 | 0.302 |
| Retrieved lymph nodes, n | 34.91 ± 13.92 | 40.04 ± 15.59 | < 0.001 |
| metastatic lymph nodes, n | 0.63 ± 2.59 | 0.82 ± 3.20 | 0.421 |
| Proximal margin in cm | 3.85 ± 3.11 | 2.68 ± 2.62 | < 0.001 |
| Distal margin in cm | 12.72 ± 4.86 | 12.79 ± 4.67 | 0.870 |
| TNM (AJCC/UICC) staging | 0.395 | ||
| IA | 202 (75.9) | 285 (67.7) | |
| IB | 26 (9.8) | 52 (12.4) | |
| IIA | 19 (7.1) | 40 (9.5) | |
| IIB | 8 (3.0) | 22 (5.2) | |
| IIIA | 5 (1.9) | 8 (1.9) | |
| IIIB | 4 (1.5) | 11 (2.6) | |
| IIIC | 2 (0.8) | 3 (0.7) |
Table 3 shows the early surgical outcomes. There were significant differences in operation time (LATG: 170 (range, 65-453) min and TLTG: 149 (range, 75-342) min, P < 0.001), postoperative hematocrit change (LATG: 4.05% (range, -3.8%-15.2%) and TLTG: 3.50% (range, -4.9%-18.6%), P = 0.002), intraoperative events (LATG: 27 cases (10.2%) and TLTG: 13 cases (3.1%), P < 0.001), and intraoperative anastomosis events related to EJ (LATG: 19 cases (7.1%) and TLTG: 10 cases (2.4%), P < 0.001). There were no significant differences in postoperative transfusions, combined operations, pain scores or administration of analgesics. Combined operations were appendectomy, cholecystectomy, distal pancreatectomy and splenectomy, etc. There were three splenectomy cases in the TLTG group. Splenectomy was carried out in two cases in order to control splenic bleeding and in one case because of metastasis found in splenic hilar lymph node biopsy. However, the median time to first flatus [LATG: 3.60 (range, 1-7) d and TLTG: 3.30 (range, 1-7) d, P < 0.001] and to median commencement of soft diet (LATG: 4.61 (range, 2-68) d and TLTG: 4.30 (range, 3-36) d, P < 0.001), as well as length of post-operative hospital stay [LATG: 7.02 (range, 5-1117) and TLTG: 6.75 (range, 4-82), P = 0.005] were significantly longer in LATG than in TLTG.
| Variables | LATG, n = 266 | TLTG, n = 421 | P value |
| Operation time in min | 170 (65-453) | 149 (75-342) | < 0.001 |
| Hematocrit change in (%) | 4.04 (-3.8-15.2) | 3.49 (-4.9-18.6) | 0.002 |
| Intra-operative transfusion | 1 (0.4) | 1 (0.2) | 1.000 |
| Post operative transfusion | 28 (10.5) | 55 (13.1) | 0.32 |
| Intra-operative event | 27 (10.2) | 13 (3.1) | < 0.001 |
| Intra-operative anastomosis event | 19 (7.1) | 10 (2.4) | 0.003 |
| Combined operation | 17 (6.4) | 27 (6.4) | 1.000 |
| Time to first flatus in d (range) | 3.60 (1-7) | 3.30 (1-7) | < 0.001 |
| Time to soft diet in d (range) | 4.61 (2-68) | 4.30 (3-36) | < 0.001 |
| Pick of pain score, score (range) | 7.11 (2-10) | 6.96 (3-10) | 0.912 |
| 8AM Pain socre of POD #1, score (range) | 3.45 (0-10) | 3.49 (0-10) | 0.841 |
| 8AM Pain socre of POD #3, score (range) | 2.44 (0-9) | 2.54 (0-7) | 0.529 |
| 8AM Pain socre of POD #5, score (range) | 1.75 (0-10) | 1.51 (0-8) | 0.055 |
| Number of administration of analgesics, n (range) | 2.49 (0-69) | 2.86 (0-67) | 0.131 |
| Post-operative hospital stay in d (range) | 7.02 (5-1117) | 6.75 (4-82) | 0.005 |
Early and late postoperative complications are presented in Table 4. There was no significant difference in Clavien-Dindo classification between the groups. Overall, early postoperative complications were observed in 53 (19.9%) patients in the LATG group and 87 (20.7%) in the TLTG group (P = 0.447). There were 21 (7.9%) and 37 (8.8%) overall late postoperative complications in the LATG and TLTG groups, respectively (P = 0.681). In addition, the occurrence rate of EJ-related early complications, such as leakage, did not significantly differ between the two groups (LATG: 14 cases (5.3%) and TLTG: 14 cases (3.3%), P = 0.211). Late complications related to EJ were also similar in the two groups (LATG: 4 cases (0.9%) and TLTG: cases (0.7%), P = 0.439). The classes of the postoperative complications are given in Table 4, and the types of complications, including bleeding, leakage, stricture, intraabdominal fluid collection, internal hernia, ileus and wound infection, are presented in Table 5. Early complications following TLTG classified as Clavien-Dindo classification grade ≥ III were observed in 85 (8.3%) patients, and late complications classified as Clavien-Dindo classification grade ≥ III were observed in 18 (4.3%) patients.
| Early complications | Late complications | |||||
| LATG, n = 266 | TLTG, n = 421 | P value | LATG, n = 266 | TLTG, n = 421 | P value | |
| CDC | 0.447 | 0.681 | ||||
| 0 | 213 (80.1) | 334 (79.3) | 245 (92.1) | 384 (91.2) | ||
| 1 | 24 (9.0) | 26 (6.2) | 10 (3.8) | 15 (3.6) | ||
| 2 | 13 (4.9) | 26 (6.2) | 0 (0) | 4 (1.0) | ||
| 3 | 12 (4.5) | 33 (7.8) | 11 (4.1) | 18 (4.3) | ||
| 4 | 4 (1.5) | 2 (0.5) | 0 (0) | 0 (0) | ||
| Cx of EJ | 0.211 | 0.439 | ||||
| None | 252 (94.7) | 407 (96.7) | 262 (98.5) | 418 (99.3) | ||
| Leakage | 14 (5.3) | 14 (3.3) | 1 (0.4) | 1 (0.2) | ||
| Stricture | 0 (0.0) | 0 (0.0) | 3 (1.1) | 2 (0.5) | ||
| LATG, n = 266 | TLTG, n = 421 | |
| Bleeding | 4 (1.50) | 8 (1.90) |
| EJ leakage | 15 (5.64) | 15 (3.56) |
| EJ stricture | 3 (1.13) | 2 (0.48) |
| Intraabdominal fluid collection | 8 (3.01) | 26 (6.18) |
| Internal hernia | 5 (1.88) | 12 (2.85) |
| Mechanical ileus | 10 (3.76) | 28 (6.65) |
| Paralytic ileus | 3 (1.13) | 7 (1.66) |
| Wound infection | 18 (6.77) | 9 (2.14) |
| Other surgical complications | 4 (1.50) | 8 (1.90) |
| Medical complications | 4 (1.50) | 2 (0.48) |
Various modified methods of TLTG have been developed, but no standard method for upper and middle gastric cancer has been established because the reconstruction of intracorporeal EJs requires a high level of technical proficiency and is difficult, even for experienced surgeons[24-28]. We have recently reported a TLTG method developed for intracorporeal EJ using an endoscopic linear stapler, and we believe that it could become a standard method for these patients[16,17]. Although extracorporeal EJ anastomosis using a circular stapler is the generally accepted method for laparoscopic total gastrectomy, the anastomosis is often difficult to complete because of the limited working space formed by the mini-laparotomy[24]. Furthermore, an extended laparotomy incision is sometimes required, but this may reduce the benefits of the laparoscopic approach.
In a study on distal gastrectomy, TLTG without a mini-laparotomy was unaffected by obesity and could, thus, be a safe procedure for avoiding the impact of obesity[18,19]. Similarly, TLTG helps the surgeon easily resect and reconstruct the anastomosis without limiting the surgeon’s view. In a previous study, TLTG produced similar early surgical outcomes to LATG, although the BMI was higher in the TLTG group[29]. In the present retrospective study, the TLTG patients had similar BMIs and tended to be slightly older, with more histories of abdominal surgery compared with LATG patients. Nevertheless, TLTG was superior to LATG in terms of operation time, postoperative hematocrit change, intraoperative events, bowel movements, and postoperative hospital stays. Although TLTG is less invasive than LATG, there was no significant difference in pain score, which was probably due to the use of active pain control, such as patient-controlled analgesia.
Chen et al[5] found in their meta-analysis that the number of lymph nodes harvested in TLTG was marginally higher than in LATG (P = 0.06). In our study, lymphadenectomy seems to have been adequate in both groups, despite the significant difference in the number of retrieved lymph nodes in the LATG and TLTG group (34.91 ± 13.92 and 40.04 ± 15.59, respectively, P < 0.001). The reason for this difference is unclear since the lymphadenectomy procedure is the same in both LATG and TLTG. There was a significant difference between the LATG and TLTG groups with regard to the length of the resection margin (LATG: 3.85 ± 3.11 cm and TLTG: 2.68 ± 2.62 cm, P < 0.001). This could be attributed to the fact that linear staplers are often placed on either side of the resection line, and might hinder accurate histopathologic evaluation of the surgical margin of the resected specimen. Linear staplers generally have four or six rows of staples and form two or three staple lines on a margin of length approximately 4-5 mm as exempted staples on the resection line, in contrast to conventional circular staplers[30]. Moreover, the linear staplers used in TLTG require a substantial length of esophagus for anastomosis. On the other hand, the circular stapler used in LATG allows the esophagus to be transected more proximally and does not need a long esophageal stump. EJ anastomosis using a circular stapler, thus, allows higher anastomosis in patients with tumors at the gastroesophageal junction, or in the upper stomach and invading the esophagus[31,32].
In the current study, the TLTG group was older and had more histories of abdominal surgery. However, the operation time for TLTG was shorter than for LATG. Our TLTG experience suggests several factors that may contribute to this shorter operation time. First, TLTG provides a wider view than LATG. Second, reconstruction in TLTG carried out with a linear stapler is easy, rapid and requires no hand-sewn reinforcement procedure. Finally, opening and closing a mini-laparotomy is not required. Incision for a mini-laparotomy may take especially long additional incision in obese patients. Moreover, our data show that TLTG has superior surgical outcomes in terms of postoperative hematocrit change, intraoperative events, time to first flatus, soft diet and postoperative hospital stay because the intracorporeal method has a wider view and causes less surgical trauma.
Postoperative morbidity after LATG has been reported to range from 17% to 27%[33-38]. In our study, early complication occurring within 30 d following LATG and TLTG classified as Clavien-Dindo classification grade ≥ III were observed in 16 (6.0%) and 35 (8.3%) patients, respectively; late complications developing after 30 d following LATG and TLTG were observed in 11 (4.1%) and 18 (4.3%) patients, respectively. These results show that there were no significant differences between LATG and TLTG in terms of postoperative complications.
Patient characteristics such as age and obesity are risk factors for postoperative complications in laparoscopic gastrectomy. ASA scores may be influenced by age and comorbidity because these factors reinforce each other. Most of all, being overweight is a potent risk factor for poor surgical outcomes[39,40]. Delayed bowel movement, increased postoperative pain, and prolonged hospital stay can occur in obese patients, as we have suggested in a previous report on laparoscopic distal gastrectomy[40]. We found no significant differences in early and late complications between LATG and TLTG, even though the TLTG group patients were much older and had more histories of abdominal surgery. In addition, there was no significant difference between the two groups in complications related to EJ. However, TLTG makes a wide operating space and carries out EJ construction safely, and several investigators have insisted that the anastomotic site should be further secured and have a wider diameter when using a linear stapler than when using a circular stapler[5,16,41].
The procedure of LATG and TLTG differ in many ways. First, TLTG is less invasive, and requires a smaller incision than does LATG. Second, the wider working space in TLTG ensures safe reconstruction of the EJ. Therefore, laparoscopic surgeons are more comfortable with the intracorporeal than the extracorporeal one. Furthermore, using a linear stapler in TLTG has another advantage in that whereas circular staplers have only two staggered rows, endoscopic linear staplers have three staggered rows and provide better staple line security.
We conclude that TLTG requires a shorter operation time and permits faster postoperative recovery than LATG, while having similar surgical outcomes and complications. Therefore, TLTG using a linear stapler may be considered a more appropriate procedure than LATG using a circular stapler, and may be recommended for the treatment of gastric cancer of the upper third of the stomach.
This study has certain limitations. It is a retrospective study from a single institution and the baseline clinical characteristic of the two groups were different. Although the pathologic results for the patients in the LATG and TLTG groups were similar, the LATG and TLTG operations were performed at different periods of time. In addition, cancer recurrence and long-term survival rates were not analyzed because approximately half the patients underwent surgery, and 5 years had not yet passed. Therefore, data on long-term outcomes are still needed in order to compare the oncological adequacy of these two methods.
In Korea and Japan, the incidence of upper and middle body gastric cancer has increased as a result of improved nationwide surveillance. Furthermore, the indication of laparoscopic gastrectomy has also extended. Therefore, the demand for minimally invasive surgery for upper body gastric cancer has grown, and there is more need for new therapeutic methods and modalities.
Intracorporeal anastomosis and extracorporeal anastomosis in laparoscopic total gastrectomy has developed due to improvements in surgical devices and the accumulation of operative experience, but an optimal method for laparoscopic total gastrectomy has yet to be established due to the difficulties of esophagojejunostomy.
We aimed to evaluate the surgical safety and efficacy of intracorporeal anastomosis using linear stapler for treating gastric cancer of the upper third of the stomach by comparing its outcomes with those of extracorporeal anastomosis using circular stapler.
From August 2008 to August 2014, 687 consecutive patients who underwent total gastrectomy (266 laparoscopic-assisted total gastrectomy (LATG) patients and 421 totally laparoscopic total gastrectomy (TLTG) patients) were reviewed retrospectively. Data obtained from medical records included patient age, sex, body mass index, American Society of Anesthesiologist score, history of abdominal surgery, operative time, pre- and postoperative hematocrit, time to first flatus, day of commencement of soft diet, pain score by visual analogue scale, number of analgesics administered, intra- and postoperative transfusion, intraoperative events, postoperative hospital stay, tumor size, number of retrieved lymph nodes, resection margins and cancer stage according to the American Joint Committee on Cancer - International Union for Cancer Control 7th edition.
The TLTG group had higher mean age at time of operation, and more histories of abdominal surgery. However, the TLTG group required a shorter operation time, lower postoperative hematocrit change, less intraoperative events, less intraoperative anastomosis events, and permitted faster postoperative recovery, such as median time to first flatus, median commencement of soft diet and length of postoperative hospital stay.
TLTG may be considered a feasible procedure, as compared to LATG. Because TLTG provides a wider view than TLTG, reconstruction in TLTG carried out with a linear stapler is easy, rapid and requires no hand-sewn reinforcement procedure, and TLTG does not need additional mini-laparotomy. Furthermore, TLTG had superior surgical outcomes in terms of operation time, postoperative hematocrit change, intraoperative events and postoperative recovery.
Based on our results, we can consider TLTG as a feasible and straightforward procedure. But this study has certain limitations. It is a retrospective study from a single institution, and although the pathologic results in the LATG and TLTG groups were similar, data on long-term outcomes are still needed to compare the oncological adequacy of these two methods.
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21463] [Article Influence: 1951.2] [Reference Citation Analysis (6)] |
| 2. | Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, Ryu SW, Cho GS, Song KY, Ryu SY. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | Cunningham D, Chua YJ. East meets west in the treatment of gastric cancer. N Engl J Med. 2007;357:1863-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Wu CW, Lo SS, Shen KH, Hsieh MC, Lui WY, P’eng FK. Surgical mortality, survival, and quality of life after resection for gastric cancer in the elderly. World J Surg. 2000;24:465-472. [PubMed] |
| 5. | Chen K, Pan Y, Cai JQ, Wu D, Yan JF, Chen DW, Yu HM, Wang XF. Totally laparoscopic versus laparoscopic-assisted total gastrectomy for upper and middle gastric cancer: a single-unit experience of 253 cases with meta-analysis. World J Surg Oncol. 2016;14:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011;11:69-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 7. | Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 243] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 8. | Kim EY, Choi HJ, Cho JB, Lee J. Totally Laparoscopic Total Gastrectomy Versus Laparoscopically Assisted Total Gastrectomy for Gastric Cancer. Anticancer Res. 2016;36:1999-2003. [PubMed] |
| 9. | Treitl D, Hochwald SN, Bao PQ, Unger JM, Ben-David K. Laparoscopic Total Gastrectomy with D2 Lymphadenectomy and Side-to-Side Stapled Esophagojejunostomy. J Gastrointest Surg. 2016;20:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Corcione F, Pirozzi F, Cuccurullo D, Angelini P, Cimmino V, Settembre A. Laparoscopic total gastrectomy in gastric cancer: our experience in 92 cases. Minim Invasive Ther Allied Technol. 2013;22:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N; Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 520] [Article Influence: 27.4] [Reference Citation Analysis (1)] |
| 12. | Bracale U, Rovani M, Bracale M, Pignata G, Corcione F, Pecchia L. Totally laparoscopic gastrectomy for gastric cancer: meta-analysis of short-term outcomes. Minim Invasive Ther Allied Technol. 2012;21:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Ikeda O, Sakaguchi Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, Ohga T, Adachi E, Toh Y, Okamura T. Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009;23:2374-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Chen K, Pan Y, Cai JQ, Xu XW, Wu D, Mou YP. Totally laparoscopic gastrectomy for gastric cancer: a systematic review and meta-analysis of outcomes compared with open surgery. World J Gastroenterol. 2014;20:15867-15878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Kim HS, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and open total gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech A. 2013;23:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Kim HS, Kim BS, Lee S, Lee IS, Yook JH, Kim BS. Reconstruction of esophagojejunostomies using endoscopic linear staplers in totally laparoscopic total gastrectomy: report of 139 cases in a large-volume center. Surg Laparosc Endosc Percutan Tech. 2013;23:e209-e216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Kim HS, Kim MG, Kim BS, Yook JH, Kim BS. Totally laparoscopic total gastrectomy using endoscopic linear stapler: early experiences at one institute. J Laparoendosc Adv Surg Tech A. 2012;22:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Kim MG, Kawada H, Kim BS, Kim TH, Kim KC, Yook JH, Kim BS. A totally laparoscopic distal gastrectomy with gastroduodenostomy (TLDG) for improvement of the early surgical outcomes in high BMI patients. Surg Endosc. 2011;25:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Kim MG, Kim KC, Kim BS, Kim TH, Kim HS, Yook JH, Kim BS. A totally laparoscopic distal gastrectomy can be an effective way of performing laparoscopic gastrectomy in obese patients (body mass index≥30). World J Surg. 2011;35:1327-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | TNM|UICC [Internet] Cited 2016-12-7. Available from: http://www.uicc.org/resources/tnm. |
| 21. | Kim HS, Kim SO, Kim BS. Use of a clinical pathway in laparoscopic gastrectomy for gastric cancer. World J Gastroenterol. 2015;21:13507-13517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2947] [Article Influence: 196.5] [Reference Citation Analysis (1)] |
| 23. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 26109] [Article Influence: 1186.8] [Reference Citation Analysis (2)] |
| 24. | Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc. 2009;23:2624-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Bracale U, Marzano E, Nastro P, Barone M, Cuccurullo D, Cutini G, Corcione F, Pignata G. Side-to-side esophagojejunostomy during totally laparoscopic total gastrectomy for malignant disease: a multicenter study. Surg Endosc. 2010;24:2475-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Wang ZQ, Yu PW, Qian F, Chen J, Luo HX, Lei X. [A modified method of laparoscopic side-to-side esophagojejunal anastomosis after laparoscopic total gastrectomy: a report of 12 cases]. Zhonghua Wei Chang Wai Ke Za Zhi. 2007;10:323-325. [PubMed] |
| 27. | Hirahara N, Tanaka T, Yano S, Yamanoi A, Minari Y, Kawabata Y, Ueda S, Hira E, Yamamoto T, Nishi T. Reconstruction of the gastrointestinal tract by hemi-double stapling method for the esophagus and jejunum using EEA OrVil in laparoscopic total gastrectomy and proximal gastrectomy. Surg Laparosc Endosc Percutan Tech. 2011;21:e11-e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Ziqiang W, ZhiMin C, Jun C, Xiao L, Huaxing L, PeiWu Y. A modified method of laparoscopic side-to-side esophagojejunal anastomosis: report of 14 cases. Surg Endosc. 2008;22:2091-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Kim HS, Kim MG, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Comparison of totally laparoscopic total gastrectomy and laparoscopic-assisted total gastrectomy methods for the surgical treatment of early gastric cancer near the gastroesophageal junction. J Laparoendosc Adv Surg Tech A. 2013;23:204-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Tsujimoto H, Tsuda H, Hiraki S, Nomura S, Ito N, Kanematsu K, Horiguchi H, Aosasa S, Yamamoto J, Hase K. In vivo evaluation of a modified linear stapling device designed to facilitate accurate pathologic examination of the surgical margin. Gastric Cancer. 2016;19:666-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Hiyoshi Y, Oki E, Ando K, Ito S, Saeki H, Morita M, Baba H, Maehara Y. Outcome of esophagojejunostomy during totally laparoscopic total gastrectomy: a single-center retrospective study. Anticancer Res. 2014;34:7227-7232. [PubMed] |
| 32. | Kinoshita T, Gotohda N, Kato Y, Takahashi S, Konishi M, Okazumi S, Katoh R, Kinoshita T. Laparoscopic transhiatal resection for Siewert type II adenocarcinoma of the esophagogastric junction: operative technique and initial results. Surg Laparosc Endosc Percutan Tech. 2012;22:e199-e203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Jeong GA, Cho GS, Kim HH, Lee HJ, Ryu SW, Song KY. Laparoscopy-assisted total gastrectomy for gastric cancer: a multicenter retrospective analysis. Surgery. 2009;146:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, Cho SJ, Lee JY, Kim CG, Choi IJ. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol. 2009;100:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Mochiki E, Toyomasu Y, Ogata K, Andoh H, Ohno T, Aihara R, Asao T, Kuwano H. Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc. 2008;22:1997-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 36. | Tanimura S, Higashino M, Fukunaga Y, Kishida S, Ogata A, Fujiwara Y, Osugi H. Laparoscopic gastrectomy with regional lymph node dissection for upper gastric cancer. Br J Surg. 2007;94:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Usui S, Yoshida T, Ito K, Hiranuma S, Kudo SE, Iwai T. Laparoscopy-assisted total gastrectomy for early gastric cancer: comparison with conventional open total gastrectomy. Surg Laparosc Endosc Percutan Tech. 2005;15:309-314. [PubMed] |
| 38. | Jeong O, Ryu SY, Zhao XF, Jung MR, Kim KY, Park YK. Short-term surgical outcomes and operative risks of laparoscopic total gastrectomy (LTG) for gastric carcinoma: experience at a large-volume center. Surg Endosc. 2012;26:3418-3425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Lee KG, Lee HJ, Yang JY, Oh SY, Bard S, Suh YS, Kong SH, Yang HK. Risk factors associated with complication following gastrectomy for gastric cancer: retrospective analysis of prospectively collected data based on the Clavien-Dindo system. J Gastrointest Surg. 2014;18:1269-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 40. | Kim HS, Kim MG, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Analysis of predictive risk factors for postoperative complications of laparoscopy-assisted distal gastrectomy. J Laparoendosc Adv Surg Tech A. 2013;23:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Umemura A, Koeda K, Sasaki A, Fujiwara H, Kimura Y, Iwaya T, Akiyama Y, Wakabayashi G. Totally laparoscopic total gastrectomy for gastric cancer: literature review and comparison of the procedure of esophagojejunostomy. Asian J Surg. 2015;38:102-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Guner A, Inokuchi M, Koch TR, Umemura A, Ziogas DE S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Huang Y