Published online Dec 14, 2017. doi: 10.3748/wjg.v23.i46.8235
Peer-review started: August 1, 2017
First decision: September 6, 2017
Revised: September 28, 2017
Accepted: November 2, 2017
Article in press: November 2, 2017
Published online: December 14, 2017
Processing time: 134 Days and 4.7 Hours
To optimize the efficacy of noninvasive evaluations in monitoring the endoscopic activity of inflammatory bowel disease (IBD).
Fecal calprotectin (FC), clinical activity index (CDAI or CAI), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and procalcitonin (PCT) were measured for 136 IBD patients. Also, FC was measured in 25 irritable bowel syndrome (IBS) patients that served as controls. Then, endoscopic activity was determined by other two endoscopists for colonic or ileo-colonic Crohn’s disease (CICD) with the “simple endoscopic score for Crohn’s disease” (SES-CD), CD-related surgery patients with the Rutgeerts score, and ulcerative colitis (UC) with the Mayo score. The efficacies of these evaluations to predict the endoscopic disease activity were assessed by Mann-Whitney test, χ2 test, Spearman’s correlation, and multiple linear regression analysis.
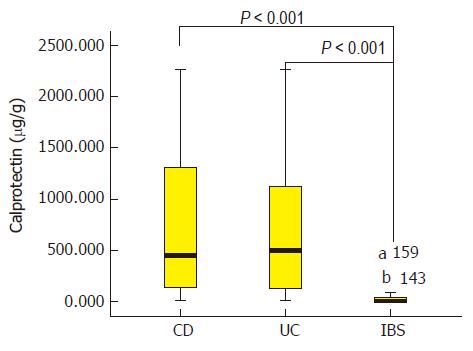
The median FC levels in CD, UC, and IBS patients were 449.6 (IQR, 137.9-1344.8), 497.9 (IQR, 131.7-118.0), and 9.9 (IQR, 049.7) μg/g, respectively (P < 0.001). For FC, CDAI or CAI, CRP, and ESR differed significantly between endoscopic active and remission in CICD and UC patients, but not in CD-related surgery patients. The SES-CD correlated closely with levels of FC (r = 0.802), followed by CDAI (r = 0.734), CRP (r = 0.658), and ESR (r = 0.557). The Mayo score also correlated significantly with FC (r = 0.837), CAI (r = 0.776), ESR (r = 0.644), and CRP (r = 0.634). For FC, a cut-off value of 250 μg/g indicated endoscopic active inflammation with accuracies of 87.5%, 60%, and 91.1%, respectively, for CICD, CD-related surgery, and UC patients. Moreover, clinical FC activity (CFA) calculated as 0.8 × FC + 4.6 × CDAI showed higher area under the curve (AUC) of 0.962 for CICD and CFA calculated as 0.2 × FC + 50 × CAI showed higher AUC (0.980) for UC patients than the FC. Also, the diagnostic accuracy of FC in identifying patients with mucosal inflammation in clinical remission was reflected by an AUC of 0.91 for CICD and 0.96 for UC patients.
FC is the most promising noninvasive evaluation for monitoring the endoscopic activity of CICD and UC. CFA might be more accurate for IBD activity evaluation.
Core tip: This was a prospective study conducted in China to assess the efficacy of noninvasive markers, including fecal calprotectin, clinical activity index (CDAI or CAI), CRP, ESR, and procalcitonin (PCT) for monitoring disease activity in colonic or ileo-colonic Crohn’s disease (CICD), CD-related surgery patients, and UC patients and further to optimize the accuracy of those noninvasive biomarkers in detecting active residual mucosal inflammation in IBD patients in clinical remission.
- Citation: Chen JM, Liu T, Gao S, Tong XD, Deng FH, Nie B. Efficacy of noninvasive evaluations in monitoring inflammatory bowel disease activity: A prospective study in China. World J Gastroenterol 2017; 23(46): 8235-8247
- URL: https://www.wjgnet.com/1007-9327/full/v23/i46/8235.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i46.8235
Inflammatory bowel disease (IBD) is a chronic, destructive inflammatory disease of the gastrointestinal tract with unknown etiology[1]. Generally, it occurs most commonly at a young age and most patients need life-long medicine treatment, thus causing work disabilities and imposing heavy social and economic burdens[2]. IBD is no more than a “Western disease”; according to a multicenter study, IBD in Asia might be as severe as or worse than in the West, and China has the highest incidence of IBD in Asia[3]. Two recent prospective population-based studies also demonstrated that IBD has a high incidence and wide geographical coverage in China[4,5].
Because IBD has a chronic relapsing-remitting course, patients and clinicians need a monitoring technique to detect an imminent flare for timely tailoring therapy regimen. To date, most clinicians monitor IBD activity and guide therapeutic decisions based on clinical activity indexes[6]. However, emerging data showed that IBD patients with a clinically quiescent disease may still have residual mucosal inflammation and remission of symptoms may not be an available predictor of long-term favorable outcome in IBD patients[7,8]. Mucosal healing (MH) has been proven to be a strong predictor of disease-related outcomes and has become a new therapeutic goal in IBD[9-11]. An endoscopic procedure for IBD can determine the disease location and mucosal lesions precisely and is considered the gold standard for assessing disease activity. However, endoscopy is invasive, uncomfortable, expensive, and not well tolerated by patients; therefore, regular monitoring of disease activity using endoscopy is not feasible[12]. Furthermore, some patients are terrified by painful endoscopy experiences and they are reluctant to visit clinicians until the disease manifests as rectal bleeding or obstruction, which affects the prognosis. A simple, acceptable, and specific evaluation is needed to play an adjunctive role in the assessment of disease activity, enabling the most cost-effective use of medical resources[13,14].
Clinical assessment indexes correlate poorly with endoscopic activity and remission of symptoms may not indicate remission of IBD[11,15]. Also, for patients with clinically overt relapse, their recent history and symptoms will give sufficient reasons for further endoscopic exploration or intensification of treatment, and there is no need to wait for the results of inflammatory biomarkers. Therefore, finding an evaluation that can detect increased endoscopic disease activity earlier before any clinical symptoms have occurred is an unmet clinical need.
The aim of this study, therefore, was to assess the efficacy of noninvasive evaluations, including fecal calprotectin (FC), clinical activity index (CDAI or CAI), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and procalcitonin (PCT) for monitoring disease activity in colonic or ileo-colonic Crohn’s disease (CICD), CD-related surgery patients, and UC patients and further to optimize the accuracy of those noninvasive evaluations in detecting active residual mucosal inflammation in IBD patients in clinical remission.
This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (NFEC-2014-065). In this prospective study, we consecutively recruited 136 adult outpatients and inpatients with previously confirmed diagnoses of IBD referred for colonoscopy at the Department of Gastroenterology of Nanfang Hospital. The diagnosis of IBD was based on clinical, ileocolonoscopic, histopathological, and radiological findings. A second cohort of 25 IBS patients, defined according to the Rome III criteria, served as controls. Of the 136 IBD patients, six had undergone colonoscopy twice to evaluate therapy efficiency, and the interval between the two endoscopies was longer than 2 mo. First, 35 IBD patients were excluded because we could not timely collect a fecal sample before bowel preparation (n = 10), a full colonoscopy was not performed due to a stricture or without a properly prepared colon compatible with adequate endoscopic assessment (n = 7), the age was < 18 years (n = 4), blood samples were missed (n = 5), patients used non-steroidal anti-inflammatory drugs (NSAIDs) (n = 3), patients had poor compliance with fecal sampling (n = 4), or patients had concomitant colorectal cancer (n = 2). Outpatients were provided with a fecal collection tube when they attended the endoscopy center to make an appointment with the physician and were asked to return a fecal sample before the endoscopic examination. In inpatients, the fecal collection set was prepared by a trained nurse and then sent to the laboratory.
For IBD patients, the day before the endoscopy, blood samples were taken to measure CRP, ESR, and PCT in the IBD Unit at Nanfang Hospital. At the same time, the patients were asked to complete a case report to record their demographic data, symptoms, and current medication usage.
A firm diagnosis of IBD must have been made or confirmed based on clinical, ileocolonoscopic, histopathological, and radiological findings at our institution. Other inclusion criteria were complete colonoscopy (ileum or neo-terminal ileum was included), age between 18 and 85 years, and fecal samples delivered from 1 to 3 d before bowel preparation.
The exclusion criteria included terminal ileum or neo-terminal ileum not reached; history of extensive bowel resection unrelated to IBD; indeterminate colitis (IC); pregnancy; age < 18 years or > 85 years; other deceptive reasons for elevated CRP/EST/PCT, such as infection (within one month), malignancy (current), rheumaimmune systemic diseases not related to IBD, trauma or surgery (within one month); and use of NSAIDs within three months before colonoscopic examination.
The endoscopic activities of IBD patients were graded according to validated endoscopic score tools. Simple endoscopic score for Crohn’s disease” (SES-CD) was used for the CICD patients[16]. For the SES-CD, the four endoscopic parameters selected were ulcers, proportion of the surface covered by ulcers, proportion of the surface with any other lesions, and stenosis. Each variable was scored from 0 to 3 in each segment (the ileum, the right, transverse, and left colon, and the rectum). The SES-CD activity levels were graded as follows: inactive (remission), 0-3; mild activity, 4-10; moderate activity, 11-19; and severe activity, ≥ 20, according to Schoepfer et al[17]. The Rutgeerts score is a well-established endoscopic scoring system for assessing the neo-terminal ileum for patients having prior CD-related surgery[18]: i0, no lesion; i1, < 5 aphthous lesions; i2, > 5 aphthous lesions with normal mucosa between lesions, or skip areas of larger lesions or lesions confined to the ileo-colonic anastomosis; i3, diffuse aphthous ileitis with diffusely inflamed mucosa; and i4, diffuse inflammation with larger ulcers, nodules, and/or narrowing (i0-i1: endoscopic remission; ≥ i2: endoscopic recurrence). The disease severity of UC was evaluated according to the Mayo score scale (02: remission; 35: mild; 610: moderate; 1112: severe)[19].
All endoscopies were performed by two experienced gastroenterologists with at least 5 years of experience in performing colonoscopies. The endoscopists completed an endoscopic scoring sheet immediately after the colonoscopic examination. To minimize the subjective nature of the scoring tools, we recorded a video of the endoscopy procedure. The two endoscopists analyzed the videos together and disagreements were resolved by discussion to realize consistent scoring. To avoid bias, the endoscopists performing the endoscopies were unaware of the FC, clinical activity index, CRP, ESR, and PCT results.
Upon arrival of stool samples at the laboratory, we sampled from six sites in each stool sample and mixed the sample with a stirrer. One volume of feces was diluted with 49 volumes of extraction buffer. After homogenizing by vigorous shaking for 30 min, the sample was centrifuged at 3000 g for 10 min and the supernatant was stored at 20 °C until analysis. The Bühlmann Calprotectin ELISA kit (Bühlmann, Schönenbuch, Switzerland) was designed for the quantitative determination of FC in stool samples. The assay was performed according to the manufacturer’s instructions. Briefly, after a short extraction procedure, we diluted the stool extracts 1:150 with incubation buffer. Then, the calprotectin antigen was measured by sandwich ELISA; the ELISA plates were read at 450 nm using a Spectra mini-reader. All results were normalized to stool wet weight (in grams), and FC concentrations are expressed in μg/g. The researcher carrying out the analyses was blinded to the identity of the patients and their clinical or endoscopic findings.
For CD patients, clinical activity was assessed based on the “Crohn’s disease activity index” (CDAI; ≤ 150: clinical remission; 150-220: mild clinical activity; 220-450: moderate clinical activity; ≥ 450: severe clinical activity)[20]. For UC patients, clinical activity was assessed by the “clinical colitis activity index” (CAI; ≤ 4: clinical remission; 5-10: mild clinical activity; 11-17: moderate clinical activity; ≥ 18: severe clinical activity) according to Rachmilewitz[21]. Serological biomarkers included CRP (upper limit of normal, 5 mg/L), ESR (upper limit of normal, 10 mm/h), and PCT (lower limit of range, 0.02 ng/mL; upper limit of normal, 0.05 ng/mL).
Data were recorded in an Excel sheet (Microsoft Excel 2007) and analyzed using SPSS software (ver. 15.0 for Windows; SPSS, China). The normality of the distribution of data was tested using the Kolmogorov-Smirnov test. The Mann-Whitney test was used to assess differences between groups. Non-parametric data were presented as medians and interquartile ranges (IQR) or ranges. Non-parametric tests were used when data were not normally distributed. The χ2 test was used to assess associations of categorical data in two independent groups. A Bonferroni adjustment was carried out in multiple testing of noninvasive parameters according to endoscopic activity grade (inactive/mild/moderate/severe) and correlations of parameters with disease location (L1/L2/L3 or E1/E2/E3) in CD and UC patients. Associations between endoscopic disease activity and laboratory parameters were assessed using Spearman’s correlation. The true positive rate (sensitivity) was plotted as a function of the false positive rate (1specificity) for various cut-off points to plot a receiver operating characteristic (ROC) curve. The area under the ROC curve (AUC) was used to measure the ability of parameters to predict endoscopic severity. Multiple linear regression analysis with stepwise deletion was performed based on FC, CDAI/CAI, CRP, ESR, and PCT in order to construct a combined score that best predicted the endoscopy activity.
In total, 136 consecutive IBD patients and 25 recruited IBS patients who met the inclusion and exclusion criteria were enrolled between August 2014 and January 2015. During the study period, six patients (three CICD, two CD-related surgery, and one UC patients) underwent colonoscopy twice. Thus, a total of 142 endoscopies were performed in the 136 IBD patients. FC, CDAI/CAI, CRP and ESR were successfully measured in all included IBD patients. PCT was measured in 67 cases with CD, 18 cases with CD-related surgery, and 41 cases with UC. No adverse events happened when performing the ileo-colonoscopy. The demographic characteristics of the included patients are shown in Table 1.
| CD | UC | IBS | |
| Number of patients, n | 92 | 44 | 25 |
| Male | 54 (58.7) | 26 (59.1) | 11 (44) |
| Median age at test (range) | 29.5 (18-62) | 38 (19-70) | 35 (21-52) |
| Age at diagnosis (yr) | NA | ||
| A1 (< 16) | 7 (7.6) | 2 (4.5) | |
| A2 (17-40) | 69 (75.0) | 27 (61.4) | |
| A3 (> 40) | 16 (17.4) | 15 (34.1) | |
| Disease location | NA | ||
| Ileum (L1) | 22 (23.9) | NA | |
| Colonic (L2) | 10 (10.9) | NA | |
| Ileum-Colonic (L3) | 60 (65.2) | NA | |
| Upper GI (L4) | 9 (9.8) | NA | |
| Rectum (E1) | NA | 15 (34.1) | |
| Distal colitis (E2) | NA | 14 (31.8) | |
| Extensive colitis (E3) | NA | 15 (34.1) | |
| Concomitant medications1 | NA | ||
| No medication | 9 (9.8) | 3 (6.8) | |
| 5-ASA | 28 (30.4) | 37 (84.1) | |
| Corticosteroids | 22 (23.9) | 9 (20.5) | |
| Immunosuppressants | 39 (42.4) | 1 (2.31) | |
| Anti-TNF therapy | 36 (39.1) | 4 (9.1) | |
| Previous IBD-related surgery: no/yes | 69/23 | NA | NA |
Because the small bowel is not accessible in conventional colonoscopy, small-bowel CD patients are routinely determined to be endoscopically in remission according to SES-CD, but are shown to have active lesion by double-balloon or capsule endoscopy. We hypothesized that the evaluations could correlate more closely with the SES-CD in CICD patients. Later in this article we performed the endoscopy disease activity analysis mainly in three subgroup patients: CICD patients, CD-related surgery patients, and UC patients. Also, we included small bowel CD patients in the analysis of the correlation of non-invasive disease-activity parameters stratified according to disease location.
Baseline FC, clinical activity index (CDAI or CAI), and laboratory indexes (CRP, ESR, and PCT) according to endoscopy-based classification of active and inactive IBD patients and IBS patients are presented in Table 2. The median FC levels in CD, UC, and IBS patients were 449.6 (IQR, 137.9-1344.8), 497.9 (IQR, 131.7-118.0), and 9.9 (IQR, 049.7) μg/g, respectively (Figure 1). IBS patients had significantly lower FC levels than the three subgroups of endoscopically active IBD patients (P < 0.001). Indeed, IBS patients also had significantly lower levels of FC when compared with the three subgroups of IBD patients without endoscopic inflammation (P < 0.05, Table 2). After the IBD patients were grouped into CICD, CD-related surgery, and UC subgroups, the median FC values were 695.0 (IQR, 147.1-1805.0), 188.5 (IQR, 72.06559.7), and 497.9 (IQR, 131.71198.0) μg/g, respectively (P < 0.01). Within each IBD subgroup, the evaluations were compared between the endoscopically active and inactive patients, and FC yielded a significant difference in the CICD and UC subgroups. But in the CD-related surgery patients, FC values showed no difference based on Rutgeerts score-classified endoscopic inflammation. Also as shown in Table 2, differences in CDAI/CAI, CRP, and ESR according to endoscopy-based inflammation status were observed in CICD patients and UC patients, but not in CD-related surgery patients. With regard to PCT, no significant difference was detected in the three groups of IBD patients.
| CICD | CD-related surgery | UC | IBS | ||||
| Inactive, n = 21 | Active, n = 35 | Inactive, n = 15 | Active, n = 10 | Inactive, n = 12 | Active, n = 33 | n = 25 | |
| FC (µg/g) | |||||||
| Median | 131.7213 | 1795.812 | 142.973 | 229.272 | 45.3113 | 690.612 | 9.923 |
| Range | (0-658.71) | (264.64-2266.07) | (0.02-1805.0) | (0-1805.0) | (6.7-189.42) | (74.93-2266.01) | (0-385.4) |
| CDAI/CAI | NA | ||||||
| Median | 63.121 | 168.451 | 81 | 135.64 | 11 | 61 | |
| Range | (32.2-155.0) | (46.8-364.0) | (37.8-192.8) | (38. 8-327.1) | (0-4) | (1-17) | |
| CRP (mg/L) | NA | ||||||
| Median | 0.481 | 20.571 | 2.1 | 4.37 | 0.391 | 41 | |
| Range | (0.10-12.88) | (0.2-79.8) | (0.13-134.6) | (0.10-41.20) | (0.04-2.1) | (0.08-65.40) | |
| ESR (mm/h) | NA | ||||||
| Median | 8.01 | 30.01 | 9 | 17 | 4.31 | 141 | |
| Range | (1.0-57.0) | (5.0-123.0) | (1.0-92.0) | (2.0-41.0) | (2.0-15.0) | (2.0-100.0) | |
| PCT (ng/mL) | NA | ||||||
| Median | 0.037 | 0.043 | 0.028 | 0.025 | 0.028 | 0.025 | |
| Range | (0-4.39) | (0-5.02) | (0-0.082) | (0-0.058) | (0-0.082) | (0-0.058) | |
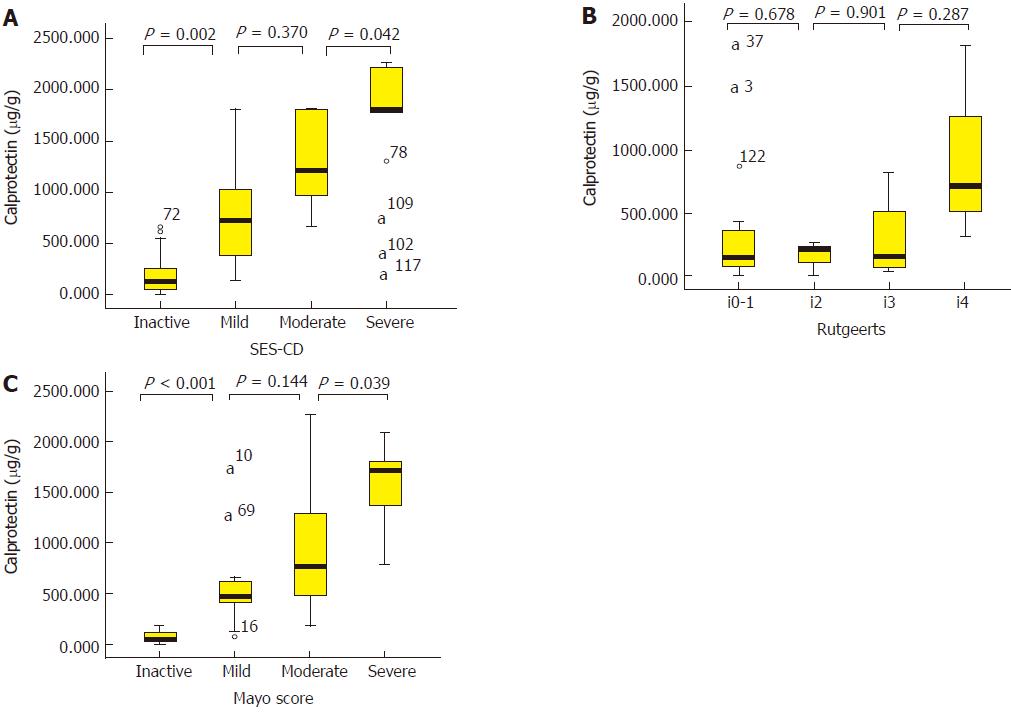
Tables 3 and 4 summarize the median noninvasive parameter values for inactive, mild, moderate, and severe endoscopic grades in CICD and UC patients. In the two subgroups of patients, FC had the clinical usage to distinguish inactive from mild and moderate from severe endoscopic activity; however, FC failed to distinguish mild from moderate endoscopic activity. For clinical activity index, CDAI not only could distinguish inactive from mild endoscopic activity, but also moderate from severe endoscopic activity in CICD patients. For CAI, a significant difference could be detected between inactive vs mild and mild vs moderate endoscopic activity. CRP and ESR could only distinguish moderate from severe endoscopic activity in UC patients. Also, no Rutgeerts score-based endoscopic activity grade was individually distinguishable by noninvasive parameters. Median FC values according to endoscopic activity grade in three subgroups of IBD patients are vividly illustrated in Figure 2.
| Inactive, n1 = 21 | Mild activity, n2 = 8 | Moderate activity, n3 = 9 | Severe activity, n4 = 18 | |||||
| FC, median (range) | 125.7(0-658.7) | 717.8 (137.7-1805.0) | 1211.8 (660.8-1805.0) | 1805.0 (264.6-2266.1) | ||||
| P value | < 0.05 | NS | < 0.05 | |||||
| CDAI, median (range) | 63.1 (32.2-155.0) | 125.4 (46.8-157.8) | 145.1 (75.0-234.9) | 201.4 (64.7-364.0) | ||||
| P value | < 0.05 | NS | < 0.05 | |||||
| CRP, median (range) | 0.48(0.1-12.9) | 5.1 (0.20-48.1) | 15.0 (0.6-70.8) | 30.6 (1.1-79.8) | ||||
| P value | NS | NS | < 0.05 | |||||
| ESR, median (range) | 8.0(1.0-57.0) | 22.0 (5.0-68.0) | 28.0 (8.9-95.0) | 37.0 (5.5-123.0) | ||||
| P value | NS | NS | < 0.05 | |||||
| PCT, median (range) | 0.037 (0-4.39) | 0.038 (0.021-0.064) | 0.038 (0-0.137) | 0.049 (0.02-5.02) | ||||
| P value | NS | NS | NS | |||||
| Inactive, n1 = 12 | Mild activity, n2 = 12 | Moderate activity, n3 = 16 | Severe activity, n4 = 5 | |||||
| FC, median (range) | 45.3 (6.7-189.42) | 474.8 (74.9-1805.0) | 769.9 (186.8-2266.1) | 1704.6 (788.4-2092.4) | ||||
| P value | < 0.001 | NS | < 0.05 | |||||
| CAI, median (range) | 1 (0-4) | 2.5 (1-8) | 6.5 (2-17) | 9 (8-15) | ||||
| P value | < 0.05 | < 0.001 | NS | |||||
| CRP, median (range) | 0.39 (0.04-2.10) | 1.9 (0.26-65.40) | 4.7 (0.08-28.19) | 16.4 (7.1-53.2) | ||||
| P value | NS | NS | < 0.05 | |||||
| ESR, median (range) | 4.3 (2.0-15.0) | 8.5 (2.0-40.0) | 14.0 (2.0-88.0) | 45.0 (24.0-100.0) | ||||
| P value | NS | NS | < 0.05 | |||||
| PCT, median (range) | 0.03 (0-0.041) | 0.031 (0.023-0.127) | 0.044 (0.021-0.538) | 0.035 (0.026-0.085) | ||||
| P value | NS | NS | NS | |||||
Base on CDAI/CAI, 34 CICD, 19 CD-related surgery, and 25 UC patients were in clinical remission (CDAI ≤ 150/CAI ≤ 4). Among the three subgroups of IBD patients in clinical remission, 14/34, 7/19, and 13/25 patients showed active endoscopic inflammation, respectively (SESCD ≥ 4/Rutgeerts ≥ 2/Mayo ≥ 3). The noninvasive biomarker values in the three subgroups of IBD patients in clinical remission with and without evidence of endoscopic inflammation are shown in Supplementary Table 1. For CICD and UC patients in clinical remission, FC, CRP, and ESR differed significantly between the endoscopically active and inactive patients, being higher in the group with endoscopic active disease. However, no noninvasive biomarker in the 19 CD-related surgery patients in clinical remission differed significantly between active and inactive disease at ileo-colonoscopy.
The sensitivities and specificities of FC at a cut-off value of 250 μg/g, CRP ≥ 5 mg/L, and ESR ≥ 10 mm/h in discriminating patients with active disease at ileo-colonoscopy in CICD and UC patients in clinical remission are shown in Table 5. AUCs for FC to detect active endoscopic inflammation were 0.91 and 0.96, for the two subgroups of IBD patients in clinical remission, respectively, suggesting that FC is the most accurate biomarker to predict preclinical active inflammation. In CICD patients in clinical remission, FC at the cut-off value 250 μg/g had the highest sensitivity (93%) and a moderate specificity (70%) to predict active lesion, CRP had the highest specificity (83.3%) but lowest sensitivity (46.2%). ESR had a mild sensitivity and specificity of 61.5%, 66.7%, respectively. In UC patients in clinical remission, both FC and CRP had a 100% specificity to identify active disease, but the sensitivity of CRP was much lower (23.1%) compared with FC (84.6%). Also, all the noninvasive biomarkers were not useful as diagnostic tests for mucosal inflammation in CD-related surgery patients in clinical remission.
| FC ≥ 250 μg/g | CRP ≥ 5 mg/L | ESR ≥ 10 mm/h | |
| Colonic or ileum-colonic CD (%) | |||
| Sensitivity | 93 | 46.2 | 61.5 |
| Specificity | 70 | 83.3 | 66.7 |
| PPV | 68.4 | 75.0 | 53.3 |
| NPV | 93.3 | 73.1 | 73.7 |
| AUC | 0.91 | 0.76 | 0.71 |
| P value | < 0.001 | 0.011 | 0.042 |
| UC | |||
| Sensitivity | 84.6 | 23.1 | 46.2 |
| Specificity | 100 | 100 | 83.3 |
| PPV | 100 | 100 | 75.0 |
| NPV | 85.7 | 54.5 | 58.8 |
| AUC | 0.96 | 0.84 | 0.76 |
| P value | < 0.001 | 0.004 | 0.028 |
Of 56 CICD patients, the SES-CD correlated significantly with levels of FC (Spearman’s rank correlation coefficient, r = 0.802), CDAI (r = 0.734), CRP (r = 0.658), and ESR (r = 0.557) (P < 0.01 for all). However, PCT failed to significantly correlate with the SES-CD (r = 0.209, P = 0.19). In the 25 CD-related surgery patients, the median time between surgery and the endoscopic examination at entry was 432 (IQR, 266-755) d. There was no significant correlation between endoscopic score and the time from surgery to the endoscopic examination (P = 0.76). Noninvasive evaluations had no significant correlation with Rutgeerts score. In 45 UC patients, the Mayo score correlated closely with FC (r = 0.837), followed by CAI (r = 0.776), ESR (r = 0.644), and CRP (r = 0.634) (P < 0.001 for all).
Supplementary Table 2 describes the relationship between noninvasive evaluations and disease location in CD and UC patients. According to CD disease location, there were 22 (22.7%) cases with L1 (ileal) disease, 11 (11.3%) with L2 (colonic) disease, and 64 (66%) with L3 (ileo-colonic) disease. There was no significant difference between FC levels and disease location in CD patients (P = 0.361). FC also did not differ between those with L2 or L3 disease vs those with L1 disease (P = 0.24). In UC patients, extensive colitis UC (E3) was associated with significantly higher FC levels compared with distal colitis (E2; P = 0.001) and left-sided colitis (E1; P < 0.001) UC patients.
The performance data of FC, clinical activity index (CDAI or CAI), CRP, ESR, and CAF in predicting the presence of endoscopic activity in the three categories of IBD patients are listed in Table 6. In CICD patients, the ROC curves revealed that the AUCs were 0.93 and 0.85 for FC and CDAI, respectively, for the detection of endoscopic active disease, followed by elevated CRP (AUC = 0.81) and ESR (AUC = 0.77), as shown in Supplementary Figure 1A. We tested a FC threshold of 250 μg/g, determined by our previous meta-analysis, to indicate endoscopic remission, with a 97.1% sensitivity and 71.4% specificity (PPV: 85.0%; NPV: 93.8%). As a non-invasive biomarker, FC had the highest sensitivity, while CDAI had the highest specificity. We also constructed a Spearman’s correlation based on the sums of differently emphasized FC, CDAI, CRP, ESR, and PCT. The scores based on the FC and CDAI proved consequently to be superior to other combinations of parameters or FC alone. The highest Spearman’s correlation, 0.839, was obtained when using the following score: 0.8 × FC + 4.6 × CDAI. The sensitivity for this score was 91.4% and the specificity was 90.5%, when using its resulting sum 850 as a cut-off value for endoscopic remission. The coefficients of multiple linear regression models to construct CFA are showed in Supplementary Table 3.
| FC ≥ 250 μg/g | CDAI > 150/CAI > 4 | CRP ≥ 5 mg/L | ESR ≥ 10 mm/h | CFA ≥ 850 or ≥ 150 | |
| Colonic or ileum-colonic CD (%) | |||||
| Sensitivity | 97.1 | 60 | 71.4 | 82.8 | 91.4 |
| Specificity | 71.4 | 95.2 | 90.5 | 57.1 | 90.5 |
| PPV | 85 | 95.5 | 92.6 | 76.3 | 94.1 |
| NPV | 93.8 | 58.8 | 65.5 | 66.7 | 86.4 |
| Accuracy | 87.5 | 73.2 | 78.6 | 73.2 | 91.1 |
| CD-related surgery (%) | |||||
| Sensitivity | 50.0 | 40.0 | 50.0 | 60.0 | NA |
| Specificity | 66.7 | 73.3 | 73.3 | 53.3 | NA |
| PPV | 50 | 50.0 | 55.6 | 46.2 | NA |
| NPV | 66.7 | 64.7 | 68.8 | 66.7 | NA |
| Accuracy | 60 | 60.0 | 64.0 | 56.0 | NA |
| UC | |||||
| Sensitivity | 87.9 | 60.6 | 42.4 | 63.6 | 92.8 |
| Specificity | 100 | 100 | 100 | 83.3 | 91.7 |
| PPV | 100 | 100 | 100 | 91.3 | 96.8 |
| NPV | 75.0 | 48.0 | 38.7 | 45.5 | 78.6 |
| Accuracy | 91.1 | 71.1 | 57.8 | 68.89 | 91.1 |
In UC, FC at a cutoff value of 250 μg/g yielded a sensitivity, specificity, PPV, and NPV of 87.9%, 100%, 100%, and 75%, respectively. The ROC curves revealed that the AUCs were 0.96 for FC, 0.88 for CAI, 0.86 for CRP, and 0.81 for ESR, as shown in Supplementary Figure 1B. FC and CAI both had a 100% specificity to predict active mucosal lesion, but CAI had much lower sensitivity. For UC patients, we also constructed the score: 0.2 × FC + 50 × CAI (Spearman’s correlation r = 0.868). With a cut-off value of 150, the sensitivity for this score was 92.8% and the specificity was 91.7%. Supplementary Table 3 describes the coefficients of multiple linear regression models to construct CFA.
However, FC, CDAI, CRP, ESR, and PCT were not useful as diagnostic tests for mucosal inflammation in CD-related surgery patients, with AUCs of 0.58 (P = 0.52), 0.69 (P = 0.11), 0.53 (P = 0.82), 0.51 (P = 0.93), and 0.49 (P = 1.00), respectively. Table 6 shows the sensitivities and specificities of FC, CDAI, CRP, and ESR for the prediction of endoscopic recurrence.
In this study, we found that FC was a more useful noninvasive marker of intestinal inflammation compared with the other evaluations in CICD and UC patients. However, FC did not discriminate between endoscopic recurrent and remission in CD-related surgery patients, based on the Rutgeerts score. Our data confirmed the findings that IBD could be differentiated from IBS using FC. We also found that both FC and clinical activity index had a good correlation with SES-CD and Mayo score, and a combined score (CFA) of FC with the clinical activity index had better diagnostic accuracy to detect endoscopic active disease. In addition, we demonstrated that considerable IBD patients in clinical remission actually had active endoscopic inflammation, and FC had better diagnostic accuracy in detecting preclinical mucosal inflammation in CICD and UC patients.
Why the FC was better than the others? Active lesion in IBD was associated with an acute inflammatory reaction and migration of leukocytes to the gut, resulting in considerable protective factors released to blood and stool[22]. For CRP in serum, its short half-life made it a valuable biomarker to detect disease activity in IBD, but the low sensitivity to detect active inflammation, especially in UC patients, limited its clinical usage[23]. ESR would take several days to respond or decrease when inflammation status was changed, so the ESR also appeared to be a less accurate measure to disease activity in IBD compared with CRP[22]. In this study, our results that elevated CRP and ESR had moderate efficiency to detect active endoscopic inflammation in IBD patients are consistent with previous studies. For PCT, to date only two studies, with small simple size, evaluated the correlation between PCT and endoscopic activity score, and controversial results were achieved[24,25]. We tested the PCT level and no significant difference was detected in patients with active endoscopic disease in comparison with the three subgroups of IBD patients with inactive disease. This result suggested that PCT is a more specific marker of bacterial infection, however, active IBD mainly involves the disorder of the immune system and defensive deficiency of the mucosa. FC is a surrogate marker of neutrophil turnover in the digestive tract and might become a better biomarker in measuring bowel inflammation for researchers and clinicians[26]. Unlike “traditional” serological biological markers, FC had higher specificity for the assessment of gastrointestinal inflammatory conditions. Indeed, IBS patients still had significantly lower levels of FC when compared with IBD patients in endoscopic remission. A high level of FC could be a reliable marker of persistent active microscopic inflammation. FC also had the advantage of showing excellent stability in feces at room temperature, for up to 3 d[27]. Those characteristics allowed patients themselves to retrieve samples in their homes and mail them to a hospital where they were frozen, allowing for batching of samples.
In Western countries, it was reported that FC was a reliable marker of endoscopic activity in both CD and UC. Several prospective population-based studies showed that disease location and disease behavior differed between Western and Asian countries, especially for CD[3-5,28]. As CD is characterized by patchy and segmental inflammation, it is not reasonable to use SES-CD to quantify disease activity in small bowel CD and CD-related surgery patients. Schoepfer et al[17], Bjokesten et al[29], Sipponen et al[30], and Vieira et al[31] used SES-CD or CDEIS to quantify CD-related surgery patients and ileal CD patients. All of these studies also demonstrated that FC showed a correlation with disease location, but using the SES-CD to qualify disease activity in isolated ileum disease was inappropriate because the small bowel was not accessible to routine endoscopic techniques. Sipponen et al then made an improvement[32]: they used the terminal ileal SES-CD to assess the small bowel CD activity. As the terminal ileum was only the window of the small intestine, they failed to correlate FC with terminal ileal SES-CD. Thus, in this study, we assessed only CICD disease activity using the SES-CD score. Similarly, in the current study, we found that when combining the CICD patients with the small bowel CD patients, FC appeared to be a more sensitive biomarker in CICD, with the correlation coefficient increased from 0.692 to 0.802. The cut-off point of FC at 250 μg/g was confirmed in our study by ROC curve analysis with a sensitivity of 97.1%, specificity of 71.4%, and accuracy of 87.5%. In clinical scenario, clinicians should note that 28.6% of endoscopic remission patients would be identified as false positives and receive excessive treatment. Meanwhile, treatment will be delayed in 2.9% of patients with active disease.
On the other hand, previous studies demonstrated that the correlation coefficients between endoscopic activity in UC patients and FC levels ranged from 0.49 to 0.83[31,33-35], probably due to the different endoscopic scores used in those studies. In our study, the Mayo score correlated closely with FC (r = 0.837). The accuracy rates of FC ≥ 250 μg/g and CAI > 4 to detect endoscopic inflammation were 91.1% and 71.1%, respectively. Because the CAI scores underestimated endoscopic activity and depended almost exclusively on clinical features that were often subjective and non-specific, FC was a more promising marker of endoscopic inflammation in UC patients.
The introduction of immunosuppressive and biological therapies has led to a decline in the rate of IBD-related surgery[36-38]. However, the postoperative relapse rates have been reported to be rather high. Accurate monitoring of disease activity is necessary in postoperative IBD patients. Scarpa et al[36], Lamb et al[37], and Lasson et al[38] failed to show a correlation between the FC and CD. However, Yamamoto et al[39] and Sorrentino et al[40] suggested that FC was a promising marker in postoperative CD patients. Our results suggested that it was not appropriate to predict endoscopic activity using FC in CD-related surgery patients. These varying results might be explained by the different time intervals from the surgical resection and performance of different types of resection. For IBD-related surgery patients, surgeons would resect the severe macroscopic disease and retain the normal function bowel as much as possible. Therefore, considerable patients had no macroscopic anastomotic recurrence, but actually had microscopic inflammation, which would increase the FC concentration. Also, for patients with small bowel resection, the recurrence of disease would more likely involve proximal small bowel instead of anastomosis. Those conditions would result in higher FC level but lower endoscopic activity grade. Thus, we failed to correlate the FC with Rutgeerts score, but those patients with high FC level would easily experience worse prognosis.
Although clinical activity index was subjective in our study, we found that CDAI/CAI had moderate correlation with endoscopy score, which challenged previous observations of poor correlation between clinical activity index and endoscopic score. This might be explained by the fact that we separated the small bowel CD and CD-related surgery patients from CICD patents. For assessing endoscopic activity, FC had the highest sensitivity, while CDAI had the highest specificity in CICD patients. Meanwhile, FC and CAI both had a 100% specificity to predict active mucosal lesion in UC patients, but CAI had much lower sensitivity. Clearly, no one parameter clearly outperformed the other. Thus, it is of interest to consider a comprehensive index which may allow clinicians to more reliably assess the ileo-colonoscopic inflammation. We developed a combined score (CFA) based on FC and CDAI/CAI in CICD and UC patients. The sensitivity for CFA was 91.4% and the specificity was 90.5%, when using its resulting sum 850 as a cut-off value for endoscopic remission in CICD patients. With a cut-off value of 150, the sensitivity for this score was 92.8% and the specificity was 91.7% in UC patients. In both groups, CFA had higher accuracy to identify endoscopic active disease than the FC and CDAI/CAI separately. Clinicians could identify endoscopic active disease more accurately with CFA through a short inquiry and a FC test.
Emerging data show that remission of symptoms in IBD patients may not indicate remission of mucosal inflammation at endoscopy[41,42]. In our cohort, in the three subgroups of IBD patients in clinical remission, 41% of CICD patients, 36.8% of CD-related surgery patients, and 52% of UC patients had active endoscopic inflammation. The sensitivities of CDAI > 150 and CAI > 4 were 60.0% and 60.6%, respectively, to detect active endoscopic inflammation. Thus, the clinical activity index underestimated the endoscopic inflammation. However, current ECCO guidelines emphasize that routine endoscopy for IBD patients in clinical remission is unnecessary, unless it is likely to change patient management[12]. In this setting, defining IBD disease activity using FC might discriminate between those who have preclinical relapse and those with quiescent IBD, or to protect those who dissimulate against themselves. One previous study investigated FC as a marker of inflammation in 48 IBD patients in clinical remission[43]. FC levels above a cut-off of 30 μg/g indicated endoscopic inflammation with a 93.7% sensitivity and 50% specificity. We separately analyzed the CD and UC patients with an acknowledged cut-off value of 250 μg/g, and found that FC was higher in patients with active disease (93% sensitivity/70% specificity for SES-CD ≥ 4 and 86.4% sensitivity/100% specificity for Mayo score ≥ 3), confirming the value of FC in IBD patients in clinical remission. As a screening tool in IBD patients in clinical remission, FC could be measured frequently to detect preclinical mucosal inflammation and guide clinicians timely change their clinical regimen.
Our study had several limitations. First, to more accurately reflect the efficacy of FC, we categorized IBD patients into three subgroups, resulting in a relatively small number of patients in each subgroup. Second, because of the design of the study, we did not analyze small bowel CD patients’ mucosal inflammation activity; thus, our results could not be extrapolated to small bowel CD patients. Also, the number of patients with purely ileal disease and colonic CD was limited, and we failed to detect a difference in FC levels according to disease location in CD patients, which thus needs to be further explored. Finally, the cut-off values for FC needs to be further explored. The difference between manufacture assays, heterogeneous operating conditions, no consensus definitions of endoscopic remission, and different patient spectrum could be accounted for when trying to define the optimal cut point. The optimal cut point may be different depending on if you are using this as a triage tool or as the final assessment for active disease. Thus, it may not be possible to set “an optimal cut point” for all scenarios and clinicians should form their own optimal cut-off value when implementing FC in clinical activity.
In conclusion, FC is the most promising noninvasive marker than the others for assessing mucosal inflammation in CICD and UC patients, but not in CD-related surgery patients. Furthermore, CFA calculated as 0.8 × FC + 4.6 × CDAI for colonic (ileo-colonic) CD or 0.2 × FC + 50 × CAI for UC patients might be more accurate for IBD activity evaluation. FC also has the ability to detect active residual mucosal inflammation in IBD patients in clinical remission to guide clinicians to timely change their clinical regimen. With the increasing recognition of the clinical value of biomarkers, the next step is implementation of marker-guided treatment in patients with IBD. To achieve this, we should attempt to improve the standardization of pre-analytical procedures and further clinical trials are warranted to demonstrate its value in clinical practice.
Inflammatory bowel disease (IBD) is characterized by periods of relapsing-remitting. At present, most clinicians monitor IBD activity and guide therapeutic decisions based on clinical activity indexes. However, emerging data show that clinical assessment indexes correlate poorly with endoscopic activity and IBD patients with clinically quiescent disease may still have residual mucosal inflammation. An endoscopic procedure is considered the gold standard for assessing disease activity. However, the endoscopy is invasive, uncomfortable, and expensive.
A simple, acceptable, and specific evaluation is needed to play an adjunctive role in the assessment of IBD activity. The specific and noninvasive evaluation could instruct clinicians to timely choose reasonable therapy regimen and predict prognosis. Furthermore, a new evaluation that can detect increased disease activity earlier before any clinical symptoms have occurred could change disease course, enabling the most cost-effective use of medical resources.
The main objective of this study was to assess the efficacy of noninvasive evaluations for the disease activity in colonic or ileo-colonic Crohn’s disease (CICD), CD-related surgery, and ulcerative colitis (UC) patients and further to optimize the accuracy of those noninvasive evaluations in detecting active residual mucosal inflammation in IBD patients in clinical remission. In our study we confirmed the efficacy of fecal calprotectin (FC) and the new clinical FC activity (CFA) in assessing disease activity in CICD and UC patients. In future, clinicians and researchers could use FC to recognize an imminent endoscopic flare. What’s more, FC could be measured frequently as a clinical activity index to detect preclinical mucosal inflammation in clinical remission patients.
In total, 136 consecutive IBD patients and 25 recruited IBS patients were enrolled. For all IBD patients, the day before the endoscopy, fecal and blood samples were collected to measure FC, CRP, ESR, and PCT. At the same time, the patients were asked to complete a case report to calculate their clinical activity index (CDAI/CAI). Then, endoscopic activity was determined for CICD patients with the “simple endoscopic score for Crohn’s disease” (SES-CD), CD-related surgery patients with the Rutgeerts score, and UC patients with the Mayo score. The efficacies of these evaluations to predict the endoscopic activity were assessed by Mann-Whitney test, χ2 test, Spearman’s correlation, and multiple linear regression analysis. In our study, multiple linear regression analysis with stepwise deletion was performed based on FC, CDAI/CAI, CRP, ESR, and PCT to construct a combined score, clinical FC activity (CFA), which could best predict the endoscopy activity. In clinical scenario, clinicians could identify endoscopic active disease more accurately with CFA through a short inquiry and a FC test.
We found that FC and clinical FC activity (CFA) are useful, non-invasive, and sensitive stool markers for gut inflammation in both CICD and UC patients. However, the standard collection of fecal sample and best cutoff to predict endoscopic activity are needed to be solved.
This was the first study performed in China for disease activity analysis in the three groups of IBD patients separately. We also constructed a clinical FC activity (CFA) index to more accurately assess disease activity. Moreover, we found that FC had ability to detect active residual mucosal inflammation in IBD patients in clinical remission. Indeed, we also found that IBD patients in endoscopic remission still had significantly higher levels of FC when compared with IBS patients. A high level of FC could be a reliable marker of persistent active microscopic inflammation. Then FC remission may indicate deep remission at histopathology level, which was proven to be a strong predictor of favorable prognosis in IBD. In future, the next step is to use FC to guide the clinicians to adjust the treatment regimen. We can schedule regular FC measurement and compare the change from baseline level to reflect the degree of response to treatment.
In our study, we confirmed the efficacy of FC in assessing disease activity in IBD patients. In future, we recommend periodic FC measurements instead of a single measurement in monitoring disease activity and deciding the treatment regimen. The clinical remission and biomarker healing could be the new therapeutic goals in IBD patients. To achieve those goals, multicenter, large-sample, randomized clinical trials are warranted to prove their value in clinical practice.
| 1. | Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2894] [Cited by in RCA: 3412] [Article Influence: 179.6] [Reference Citation Analysis (12)] |
| 2. | Ananthakrishnan AN, Weber LR, Knox JF, Skaros S, Emmons J, Lundeen S, Issa M, Otterson MF, Binion DG. Permanent work disability in Crohn’s disease. Am J Gastroenterol. 2008;103:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Ng SC, Tang W, Ching JY, Wong M, Chow CM, Hui AJ, Wong TC, Leung VK, Tsang SW, Yu HH, Li MF, Ng KK, Kamm MA, Studd C, Bell S, Leong R, de Silva HJ, Kasturiratne A, Mufeena MN, Ling KL, Ooi CJ, Tan PS, Ong D, Goh KL, Hilmi I, Pisespongsa P, Manatsathit S, Rerknimitr R, Aniwan S, Wang YF, Ouyang Q, Zeng Z, Zhu Z, Chen MH, Hu PJ, Wu K, Wang X, Simadibrata M, Abdullah M, Wu JC, Sung JJ, Chan FK; Asia-Pacific Crohn’s and Colitis Epidemiologic Study (ACCESS) Study Group. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158-165.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 615] [Article Influence: 47.3] [Reference Citation Analysis (2)] |
| 4. | Zhao J, Ng SC, Lei Y, Yi F, Li J, Yu L, Zou K, Dan Z, Dai M, Ding Y. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of “western” disease. Inflamm Bowel Dis. 2013;19:1839-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Zeng Z, Zhu Z, Yang Y, Ruan W, Peng X, Su Y, Peng L, Chen J, Yin Q, Zhao C. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong Province, China: a prospective population-based study. J Gastroenterol Hepatol. 2013;28:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (3)] |
| 6. | Schoepfer AM, Vavricka S, Zahnd-Straumann N, Straumann A, Beglinger C. Monitoring inflammatory bowel disease activity: clinical activity is judged to be more relevant than endoscopic severity or biomarkers. J Crohns Colitis. 2012;6:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 7. | Baars JE, Nuij VJ, Oldenburg B, Kuipers EJ, van der Woude CJ. Majority of patients with inflammatory bowel disease in clinical remission have mucosal inflammation. Inflamm Bowel Dis. 2012;18:1634-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (1)] |
| 8. | Berrill JW, Green JT, Hood K, Campbell AK. Symptoms of irritable bowel syndrome in patients with inflammatory bowel disease: examining the role of sub-clinical inflammation and the impact on clinical assessment of disease activity. Aliment Pharmacol Ther. 2013;38:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | af Björkesten CG, Nieminen U, Sipponen T, Turunen U, Arkkila P, Färkkilä M. Mucosal healing at 3 months predicts long-term endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2013;48:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 890] [Article Influence: 46.8] [Reference Citation Analysis (1)] |
| 11. | Meucci G, Fasoli R, Saibeni S, Valpiani D, Gullotta R, Colombo E, D'Incà R, Terpin M, Lombardi G; IG-IBD. Prognostic significance of endoscopic remission in patients with active ulcerative colitis treated with oral and topical mesalazine: a prospective, multicenter study. Inflamm Bowel Dis. 2012;18:1006-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 602] [Article Influence: 46.3] [Reference Citation Analysis (1)] |
| 13. | van Deen WK, van Oijen MG, Myers KD, Centeno A, Howard W, Choi JM, Roth BE, McLaughlin EM, Hollander D, Wong-Swanson B. A nationwide 2010-2012 analysis of U.S. health care utilization in inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:1747-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Gauss A, Geib T, Hinz U, Schaefert R, Zwickel P, Zawierucha A, Stremmel W, Klute L. Quality of Life Is Related to Fecal Calprotectin Concentrations in Colonic Crohn Disease and Ulcerative Colitis, but not in Ileal Crohn Disease. Medicine (Baltimore). 2016;95:e3477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Bodelier AG, Jonkers D, van den Heuvel T, de Boer E, Hameeteman W, Masclee AA, Pierik MJ. High Percentage of IBD Patients with Indefinite Fecal Calprotectin Levels: Additional Value of a Combination Score. Dig Dis Sci. 2017;62:465-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Daperno M, D’Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, Sostegni R, Rocca R, Pera A, Gevers A. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 1382] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 17. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 430] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 18. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1226] [Cited by in RCA: 1272] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 19. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2325] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 20. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439-444. [PubMed] |
| 21. | Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 813] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 22. | Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 654] [Cited by in RCA: 650] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 23. | Saverymuttu SH, Hodgson HJ, Chadwick VS, Pepys MB. Differing acute phase responses in Crohn’s disease and ulcerative colitis. Gut. 1986;27:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 104] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Oussalah A, Laurent V, Bruot O, Guéant JL, Régent D, Bigard MA, Peyrin-Biroulet L. Additional benefit of procalcitonin to C-reactive protein to assess disease activity and severity in Crohn’s disease. Aliment Pharmacol Ther. 2010;32:1135-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Koido S, Ohkusa T, Takakura K, Odahara S, Tsukinaga S, Yukawa T, Mitobe J, Kajihara M, Uchiyama K, Arakawa H. Clinical significance of serum procalcitonin in patients with ulcerative colitis. World J Gastroenterol. 2013;19:8335-8341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Summerton CB, Longlands MG, Wiener K, Shreeve DR. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14:841-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Burri E, Beglinger C. IBD: Faecal calprotectin testing--the need for better standardization. Nat Rev Gastroenterol Hepatol. 2014;11:583-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Vester-Andersen MK, Prosberg MV, Jess T, Andersson M, Bengtsson BG, Blixt T, Munkholm P, Bendtsen F, Vind I. Disease course and surgery rates in inflammatory bowel disease: a population-based, 7-year follow-up study in the era of immunomodulating therapy. Am J Gastroenterol. 2014;109:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 29. | af Björkesten CG, Nieminen U, Turunen U, Arkkila P, Sipponen T, Färkkilä M. Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-TNF-treated luminal Crohn’s disease. Scand J Gastroenterol. 2012;47:528-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 370] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 31. | Vieira A, Fang CB, Rolim EG, Klug WA, Steinwurz F, Rossini LG, Candelária PA. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res Notes. 2009;2:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 33. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Renzulli P, Seibold F. Ulcerative colitis: correlation of the Rachmilewitz endoscopic activity index with fecal calprotectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. 2009;15:1851-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 34. | Schoepfer AM, Beglinger C, Straumann A, Safroneeva E, Romero Y, Armstrong D, Schmidt C, Trummler M, Pittet V, Vavricka SR. Fecal calprotectin more accurately reflects endoscopic activity of ulcerative colitis than the Lichtiger Index, C-reactive protein, platelets, hemoglobin, and blood leukocytes. Inflamm Bowel Dis. 2013;19:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 35. | Önal İK, Beyazit Y, Şener B, Savuk B, Özer Etık D, Sayilir A, Öztaş E, Torun S, Özderın Özın Y, Tunç Demırel B, Ülker A, Dağli Ü. The value of fecal calprotectin as a marker of intestinal inflammation in patients with ulcerative colitis. Turk J Gastroenterol. 2012;23:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Scarpa M, D’Incà R, Basso D, Ruffolo C, Polese L, Bertin E, Luise A, Frego M, Plebani M, Sturniolo GC. Fecal lactoferrin and calprotectin after ileocolonic resection for Crohn’s disease. Dis Colon Rectum. 2007;50:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 37. | Lamb CA, Mohiuddin MK, Gicquel J, Neely D, Bergin FG, Hanson JM, Mansfield JC. Faecal calprotectin or lactoferrin can identify postoperative recurrence in Crohn’s disease. Br J Surg. 2009;96:663-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 38. | Lasson A, Strid H, Ohman L, Isaksson S, Olsson M, Rydström B, Ung KA, Stotzer PO. Fecal calprotectin one year after ileocaecal resection for Crohn’s disease--a comparison with findings at ileocolonoscopy. J Crohns Colitis. 2014;8:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 39. | Yamamoto T, Shiraki M, Bamba T, Umegae S, Matsumoto K. Faecal calprotectin and lactoferrin as markers for monitoring disease activity and predicting clinical recurrence in patients with Crohn’s disease after ileocolonic resection: A prospective pilot study. United European Gastroenterol J. 2013;1:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 40. | Sorrentino D, Paviotti A, Terrosu G, Avellini C, Geraci M, Zarifi D. Low-dose maintenance therapy with infliximab prevents postsurgical recurrence of Crohn’s disease. Clin Gastroenterol Hepatol. 2010;8:591-599.e1; quiz e78-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Ferreiro-Iglesias R, Barreiro-de Acosta M, Lorenzo-Gonzalez A, Dominguez-Muñoz JE. Accuracy of Consecutive Fecal Calprotectin Measurements to Predict Relapse in Inflammatory Bowel Disease Patients Under Maintenance With Anti-TNF Therapy: A Prospective Longitudinal Cohort Study. J Clin Gastroenterol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Assa A, Bronsky J, Kolho KL, Zarubova K, de Meij T, Ledder O, Sladek M, van Biervliet S, Strisciuglio C, Shamir R. Anti-TNFα Treatment After Surgical Resection for Crohn’s Disease Is Effective Despite Previous Pharmacodynamic Failure. Inflamm Bowel Dis. 2017;23:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Voiosu T, Benguş A, Dinu R, Voiosu AM, Bălănescu P, Băicuş C, Diculescu M, Voiosu R, Mateescu B. Rapid fecal calprotectin level assessment and the SIBDQ score can accurately detect active mucosal inflammation in IBD patients in clinical remission: a prospective study. J Gastrointestin Liver Dis. 2014;23:273-278. [PubMed] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Jadallah KA, Manguso F, Sergi CM S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Ma YJ