Published online Dec 7, 2017. doi: 10.3748/wjg.v23.i45.8097
Peer-review started: September 15, 2017
First decision: October 10, 2017
Revised: October 25, 2017
Accepted: November 1, 2017
Article in press: November 1, 2017
Published online: December 7, 2017
Processing time: 80 Days and 21.8 Hours
We report the first use of endoscopic submucosal dissection (ESD) for the treatment of a patient with adenoid cystic carcinoma of the esophagus (EACC). An 82-year-old woman visited our hospital for evaluation of an esophageal submucosal tumor. Endoscopic examination showed a submucosal tumor in the middle third of the esophagus. The lesion partially stained with Lugol’s solution, and narrow band imaging with magnification showed intrapapillary capillary loops with mild dilatation and a divergence of caliber in the center of the lesion. Endoscopic ultrasound imaging revealed a solid 8 mm × 4.2 mm tumor, primarily involving the second and third layers of the esophagus. A preoperative biopsy was non-diagnostic. ESD was performed to resect the lesion, an 8 mm submucosal tumor. Immunohistologically, tumor cells differentiating into ductal epithelium and myoepithelium were observed, and the tissue type was adenoid cystic carcinoma. There was no evidence of esophageal wall, vertical stump or horizontal margin invasion with pT1b-SM2 staining (1800 μm from the muscularis mucosa). Further studies are needed to assess the use of ESD for the treatment of patients with EACC.
Core tip: Adenoid cystic carcinoma of the esophagus (EACC) is a rare tumor that may be confused with squamous cell carcinoma and basaloid-squamous cell carcinoma. There is limited data regarding the frequency of metastasis, and the prognosis of patients with this tumor is poor. This is the first report of the use of endoscopic submucosal dissection (ESD) for the treatment of a patient with EACC. ESD may represent an additional treatment option for patients with this disease.
- Citation: Yoshikawa K, Kinoshita A, Hirose Y, Shibata K, Akasu T, Hagiwara N, Yokota T, Imai N, Iwaku A, Kobayashi G, Kobayashi H, Fushiya N, Kijima H, Koike K, Kaneyama H, Ikeda K, Saruta M. Endoscopic submucosal dissection in a patient with esophageal adenoid cystic carcinoma. World J Gastroenterol 2017; 23(45): 8097-8103
- URL: https://www.wjgnet.com/1007-9327/full/v23/i45/8097.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i45.8097
Adenoid cystic carcinoma of the esophagus (EACC) is a rare tumor that may be confused with squamous cell carcinoma (SCC) and basaloid-squamous cell carcinoma (BSC). There is limited data regarding the frequency of metastasis and the prognosis in patients with EACC[1]. Many patients have been found to have metastases at the time of initial diagnosis, and the prognosis is thought to be poor[2]. Accurate preoperative diagnosis is difficult because the tumor primarily involves the submucosa and is not easily sampled with endoscopic biopsy[3]. Although treatment is usually surgical resection in principle, the degree of invasion and the frequency of lymph node or other distant metastases are unknown[4].
In this report, we describe the use of endoscopic submucosal dissection (ESD) for the treatment of a patient with EACC. In particular, we describe the image-enhanced endoscopy and endoscopic ultrasound (EUS) findings observed during endoscopy prior to the ESD. This is the first case report describing the use of ESD for the treatment of a patient with EACC; this approach may become a more commonly accepted therapeutic option in the future.
An 82-year-old Japanese woman visited our hospital for evaluation of an esophageal tumor. Her medications included clopidogrel for right internal carotid artery stenosis, and she had a history of a prior cerebral ischemic event. She was undergoing upper gastrointestinal endoscopy as part of her annual health examination. She denied any subjective symptoms, including dysphagia, and laboratory examination revealed no evidence of anemia or abnormalities of liver or renal function. She did not have any family history with esophageal disease, and the values of tumor markers for adenocarcinoma and SCC were within normal limits.
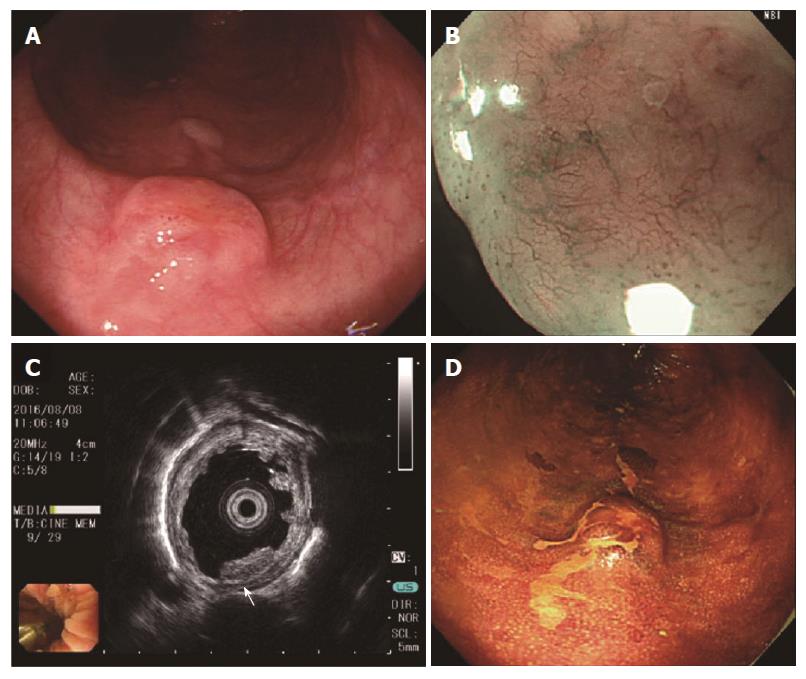
She was referred to our hospital for evaluation of what appeared to be a protruding submucosal lesion in the middle esophagus. The lesion was noted during an upper gastrointestinal endoscopic examination performed a month prior to consultation. Endoscopic examination with normal white light (GIF-H290Z and UM-3R-3-20 MHz; Olympus, Tokyo, Japan) in our hospital revealed a brownish submucosal tumor, located 25 cm from the incisor of the middle esophagus (Figure 1). The tumor surface showed mild reddening with a central planar depression, and was elastic, mobile and hard when compressed with the forceps. Image enhancement with narrow band imaging (NBI) magnification revealed a central brownish area with slightly dilated, non-uniform diameter intrapapillary capillary loops. The central planar depression stained slightly with the application of Lugol’s solution.
EUS revealed a solid 8 mm × 4.2 mm mass, primarily involving the second and third layers of the esophagus; the tumor was hypoechoic and homogeneous with a thickened hyperechoic submucosa, and slight irregularity of the third layer was recognized (Figure 1C, white arrow). The biopsy showed esophagitis and no distinct tumor; enlarged lymph nodes or other lesions suspicious for metastases were not observed with contrast-enhanced computed tomography (CT). With the above endoscopic findings, SCC and gastrointestinal stromal tumor (GIST) were included in the differential diagnosis. In accordance with our treatment protocol, we planned on performing ESD if the lesion could be lifted with a local injection.
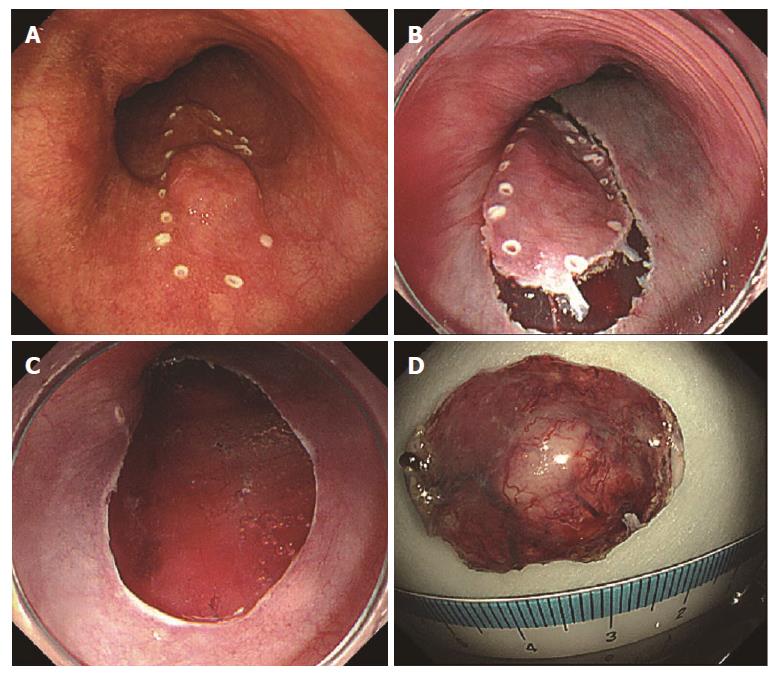
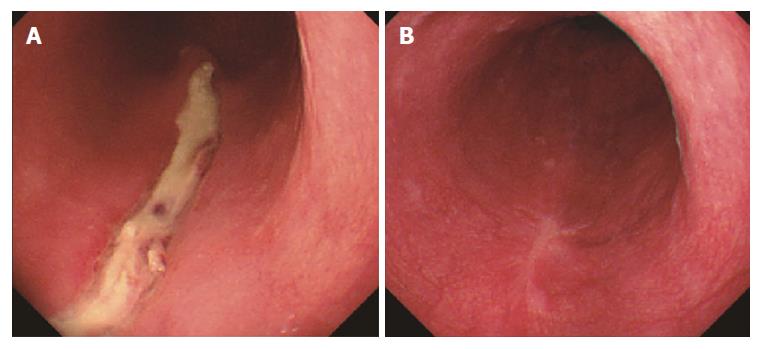
After completing an adequate clopidogrel washout period, the patient was admitted to the hospital for endoscopic treatment (Figure 2). After marking the lesion, saline was locally injected into the submucosal layer on the anal side of the lesion. Next, a mixed solution of glycerol and hyaluronic acid was locally injected, and a perimeter incision was made using a needle knife tip (DualKnifeTM; Olympus, Tokyo, Japan). The en block resection was performed after surrounding trimming and submucosal layer exfoliation were performed. During the procedure, cauterization for bleeding and exposed blood vessels on the resected surface was accomplished using bipolar hemostat forceps. The size of the excised specimen was 24 mm × 16 mm. The postoperative course was uneventful. At follow-up endoscopy 2 d after ESD, the wound was healed and scarred without stenosis (Figure 3). The patient was discharged on the 8th postoperative day and was symptom free at all outpatient visits at 6 mo postoperatively.
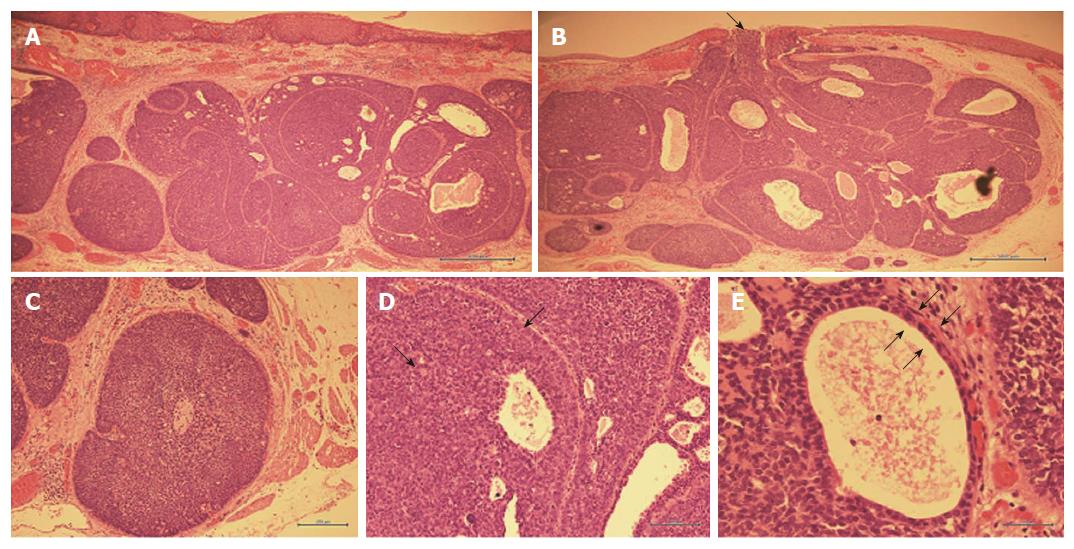
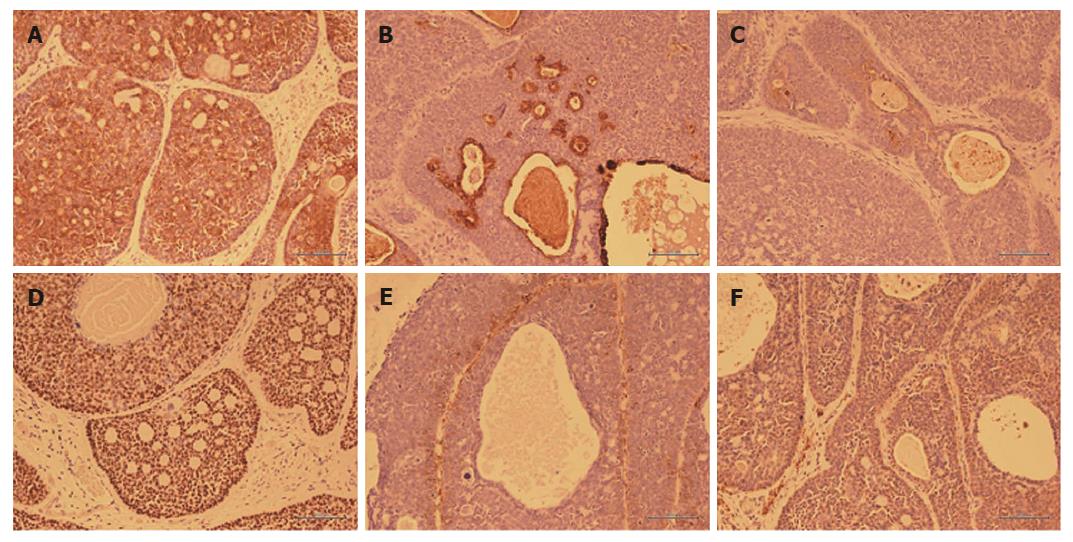
Hematoxylin and eosin staining findings are shown in Figure 4, and immunostaining findings are shown in Figure 5. With low power magnification, the submucosal layer showed proliferative heterotypic cells with cribriform nuclei distributed in an alveolar pattern and having numerous glandular cavities and small cyst-like structures in the alveoli. The upper border of the tumor partially extended to the luminal surface. With higher magnification, the nuclei of the heterotypic cells showed a dark chromatin core and a narrow eosinophilic border. Cells with an eosinophilic cytoplasm lined the wall of the glandular cystic cavity and formed a two-layer structure with small cells and having a high nuclear/cytoplasm ratio forming the outer layer. Immunostaining showed slight staining with cytokeratin CAM 5.2, which stains duct epithelium but not squamous epithelium. The glandular cavities and small cystic structures stained positively with epithelial membrane antigen, which stains glandular epithelium. Carcinoembryonic antigen staining, which stains ductal epithelium, was positive in the intraluminal epithelium and in those areas with differentiation into ductal components. p63 staining, which stains basal cells, was not observed in the intraluminal epithelium, and tumorization of the basal cells was not observed. Alpha-smooth muscle actin staining, which stains smooth muscle cells, was weakly positive in the cells of the outer layers of the cysts, suggesting smooth muscle differentiation. Staining with calponin, which stains smooth muscle cells, was slightly positive in the outer cyst wall cells.
In summary, the lesion was an 8 mm × 8 mm submucosal tumor with ductal epithelial and myoepithelial differentiation on immunohistochemical staining. The histological type was adenoid cystic carcinoma (ACC). The depth of penetration of the wall was pT1b-SM2, 1800 μm from the muscularis mucosa; both the horizontal margins and vertical stump were negative, and lymphovascular invasion was not observed with D2-40 staining. Also, no venous invasion was observed with elastic van Gieson staining.
EACC was first reported by Gregg and Stamler in 1954[5]. ACC is common in the salivary glands and respiratory system, but accounts for only 0.1% of esophageal malignancies[6]. EACC is a highly malignant tumor[1]. Based on previous reports[3,7,8], the average patient age is 60.4-66.4 years and the ratio of males to females is 2.75-5:1. Seven percent of EACCs are in the upper third of the esophagus, 63% are in the middle third, and 30% are in the lower third. Difficulty swallowing is the most common symptom, similar to what is seen in patients with SCC of the esophagus[3].
In our case, the lesion was located in the middle third of the esophagus, but the patient’s sex, age and lack of symptoms were atypical. Although EACC is normally diagnosed by endoscopy, it is frequently misdiagnosed preoperatively[3]. Only 21.6% of EACC cases diagnosed before 1996 were successfully diagnosed by endoscopic biopsy preoperatively[9]. This is thought to occur because components of SCC and BSC are likely to be found in EACC[10]; and, because EACC predominately involves the submucosa, it is impossible to sample the structure of the entire EACC tumor with an endoscopic biopsy alone[11].
When observed endoscopically with white light, an infiltrative growth pattern was reportedly observed in 58.6% of tumors, and 24.1% of tumors were classified as an ulcerative growth[7,8]. With NBI magnification, one observes a mixture of glandular and squamous epithelial components. Tumors with an irregular vascular pattern with large vessels, tumors with an irregular mucosal pattern with mucous, and tumors with no clear pattern have been reported[12].
Chromoendoscopy is useful for discriminating between SCC and adenocarcinoma, but its utility in establishing a diagnosis of ACC has not been determined. The utility of EUS in patients with EACC has only been discussed in a limited number of case reports. In one report, the EUS findings were described as a thickened submucosal layer and a relatively hypoechoic muscular layer[13]. In the present case, submucosal tumor-like lumen formation was observed, and with NBI enhancement some findings common to SCC were observed. Staining with Lugol’s solution was poor in that portion of the tumor protruding into the lumen, and EUS findings were similar to those previously reported. Preoperative endoscopic biopsy did not result in an accurate diagnosis.
Histopathologically, EACC is characterized by tumor differentiation into two types of cells, duct-lining epithelial cells and myoepithelial cells, both of which are commonly seen in salivary gland tumors. Three different cellular patterns have been observed, a cribriform pattern, a lattice-like pattern, and a tubular pattern[14]. With Alcian blue staining, the tumor demonstrates pale blue mucus in the cystic alveolar cavities as well as outside the alveoli. Although it may be necessary to distinguish EACC from BSC because of its location, the finding of myoepithelial cell differentiation with vimentin or S100 staining is considered useful in establishing a diagnosis of EACC[15].
In our case, tumor cell proliferation was primarily submucosal and myoepithelial differentiation was confirmed with immunostaining, and a diagnosis of EACC was made. Characteristics such as the formation of ductal epithelium, the biphasic nature of the tumor, and the presence of an eosinophilic substance in the cell cytoplasm were also typical of ACC.
Compared with ACC of the salivary gland, EACC has a poor prognosis[16], with a 5-year survival rate of 35% and an average life expectancy of 7 mo[2,8]. EACC with a solid growth pattern is also reported to have a poor prognosis[14]. Lymph node metastasis is more frequent than other organ metastasis, and patients with lymph node metastases have poor prognosis[17]. The presence of vascular invasion is associated with a worse prognosis in these patients. However, some cases previously diagnosed as EACC may have been confused with BSC and SCC, and additional larger studies are needed to clarify the data regarding metastasis, prognosis, and the presence of other tumor components in the tumors of patients with EACC.
The primary choice for the treatment of patients with EACC is radical surgery[4,18]; however, the surgical mortality rate was reported to be 15% in previous studies[8]. Adjuvant radiation therapy has been advocated if dysphagia is present or if surgical margins are positive for tumor involvement. A previous case report described the use of chemotherapy, including doxorubicin, mitomycin C and 5-fluorouracil, with local radiation[8], but chemotherapy is generally thought to be ineffective[4]. Overall, there is little data to support the use of chemotherapy, and the effects of adjuvant or primary chemotherapy are unknown[6,19].
One prior report described the endoscopic treatment of EACC with incisional endoscopic enucleation[20]. Ours is the first case to report the use of ESD for the treatment of a patient with EACC. It has been reported that lymph node metastases are rare in patients with esophageal SCC when the tumor is confined to the mucosal epithelial layer and the mucosal lamina propria. The incidence of metastasis is 9.3% when the tumor reaches the muscularis muscle plate and 19.3% when the tumor is within 200 μm of the submucosal layer.
In the present case, the stump of the resected specimen was negative, but invasion to within 1800 μm of the submucosal layer was observed. It is possible that tumor resection in our case will not be curative. The relationship between tumor depth of invasion and the frequency of lymph node metastasis in patients with EACC is not known, and additional studies are necessary. Our patient has been asymptomatic without evidence of recurrence or metastasis at 6 mo after ESD. We believe that continued rigorous monitoring for recurrence or metastasis with contrast-enhanced CT and upper gastrointestinal endoscopy is necessary.
In conclusion, We have described herein the first case of the use of ESD for the treatment of a patient with EACC. The accumulation and analysis of additional cases is needed to clarify the prognosis and most appropriate treatment for these patients.
A Japanese woman was asymptomatic, and the disease was diagnosed as a result of regular upper gastrointestinal endoscopy.
The authors diagnosed adenoid cystic carcinoma of the esophagus (EACC).
The diseases to be considered are squamous cell carcinoma (SCC) and gastrointestinal stromal tumor (GIST), which can be estimated by total biopsy.
The patient had nothing particular changeincluding hemoglobin and tumor marker.
Computed tomography scan showed no morphological changes.
Tumor cell proliferation was primarily submucosal and myoepithelial differentiation was confirmed with immunostaining
The patient received endoscopic submucosal dissection (ESD).
There is no other case report of ESD treatment for EACC. There are reports of cases with incisional enucleation, surgery, chemotherapy, and radiation therapy.
EACC is a rare tumor, that may be confused with SCC and basaloid-squamous cell carcinoma. There is limited data regarding the frequency of metastasis, and the prognosis of patients with this tumor is poor.
This is the first report of the use of ESD for the treatment of a patient with EACC. ESD may represent an additional treatment option for patients with this disease.
Our deepest appreciation goes to Professor Masayuki Saruta, whose comments and suggestions were of inestimable value to our report.
| 1. | Suzuki H, Nagayo T. Primary tumors of the esophagus other than squamous cell carcinoma--histologic classification and statistics in the surgical and autopsied materials in Japan. Int Adv Surg Oncol. 1980;3:73-109. [PubMed] |
| 2. | Perzin KH, Gullane P, Clairmont AC. Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer. 1978;42:265-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Guo XF, Mao T, Gu ZT, Fang WT, Chen WH, Shao JC. Adenoid cystic carcinoma of the esophagus: report of two cases and review of the Chinese literature. Diagn Pathol. 2012;7:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Triantafillidou K, Dimitrakopoulos J, Iordanidis F, Koufogiannis D. Management of adenoid cystic carcinoma of minor salivary glands. J Oral Maxillofac Surg. 2006;64:1114-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | GREGG JB, STAMLER FW. Unusual neoplasms of the esophagus: review of literature and report of a case. AMA Arch Otolaryngol. 1954;59:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Karaoglanoglu N, Eroglu A, Turkyilmaz A, Gursan N. Oesophageal adenoid cystic carcinoma and its management options. Int J Clin Pract. 2005;59:1101-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Sawada G, Moon J, Saito A, Odagiri K, Kimura Y, Takahashi G, Yamashita S, Inoue M, Irei T, Nakahira S. A case of adenoid cystic carcinoma of the esophagus. Surg Case Rep. 2015;1:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Petursson SR. Adenoid cystic carcinoma of the esophagus. Complete response to combination chemotherapy. Cancer. 1986;57:1464-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Morisaki Y, Yoshizumi Y, Hiroyasu S, Shibata H, Terahata S, Tamai S, Sugiura Y, Shima S, Tanaka S. Adenoid cystic carcinoma of the esophagus: report of a case and review of the Japanese literature. Surg Today. 1996;26:1006-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Tsang WY, Chan JK, Lee KC, Leung AK, Fu YT. Basaloid-squamous carcinoma of the upper aerodigestive tract and so-called adenoid cystic carcinoma of the oesophagus: the same tumour type? Histopathology. 1991;19:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Sweeney EC, Cooney T. Adenoid cystic carcinoma of the esophagus: a light and electron microscopic study. Cancer. 1980;45:1516-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Bardou FN, Rivory J, Robert M, Collet Benzaquen D, Fléjou JF, Ponchon T, Pioche M. Endoscopic features of an adenoid cystic carcinoma of the esophagus: narrow-band imaging and dual focus magnification. Endoscopy. 2016;48:E380-E382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Kim JH, Lee MS, Cho SW, Shim CS. Primary adenoid cystic carcinoma of the esophagus: a case report. Endoscopy. 1991;23:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Darling MR, Schneider JW, Phillips VM. Polymorphous low-grade adenocarcinoma and adenoid cystic carcinoma: a review and comparison of immunohistochemical markers. Oral Oncol. 2002;38:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Miyata R, Niihara T, Shimaoka S, Nioh T, Matsuda A, Tashiro K, Torimaru H, Tsukasa K, Nishimata N, Kawabata T. [A retrospective endoscopic case of adenoid cystic carcinoma of the esophagus]. Nihon Shokakibyo Gakkai Zasshi. 2012;109:211-216. [PubMed] |
| 16. | Spiro RH, Huvos AG, Strong EW. Adenoid cystic carcinoma of salivary origin. A clinicopathologic study of 242 cases. Am J Surg. 1974;128:512-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 397] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Bradley PJ. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg. 2004;12:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 18. | Kabuto T, Taniguchi K, Iwanaga T, Terasawa T, Sano M, Tateishi R, Taniguchi H. Primary adenoid cystic carcinoma of the esophagus: report of a case. Cancer. 1979;43:2452-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Matsumoto M, Nomiyama T, Nakae J, Nishikawa H. Combination chemotherapy for a senile patient with adenoid cystic carcinoma of the esophagus: a case report. Jpn J Clin Oncol. 1993;23:258-262. [PubMed] |
| 20. | Na YJ, Shim KN, Kang MJ, Jung JM, Ha CY, Jung HS, Baik SJ, Kim SE, Jung SA, Yoo K. Primary esophageal adenoid cystic carcinoma. Gut Liver. 2007;1:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and Hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chawla S, Huang LY S- Editor: Chen K L- Editor: A E- Editor: Huang Y