Published online Dec 7, 2017. doi: 10.3748/wjg.v23.i45.8044
Peer-review started: June 14, 2017
First decision: July 17, 2017
Revised: August 30, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: December 7, 2017
Processing time: 173 Days and 23.8 Hours
To explore the relationship of liver and spleen shear wave velocity in patients with liver cirrhosis combined with portal hypertension, and assess the value of liver and spleen shear wave velocity in predicting the prognosis of patients with portal hypertension.
All 67 patients with liver cirrhosis diagnosed as portal hypertension by hepatic venous pressure gradient in our hospital from June 2014 to December 2014 were enrolled into this study. The baseline information of these patients was recorded. Furthermore, 67 patients were followed-up at 20 mo after treatment, and liver and spleen shear wave velocity were measured by acoustic radiation force impulse at the 1st week, 3rd month and 9th month after treatment. Patients with favorable prognosis were assigned into the favorable prognosis group, while patients with unfavorable prognosis were assigned into the unfavorable prognosis group. The variation and difference in liver and spleen shear wave velocity in these two groups were analyzed by repeated measurement analysis of variance. Meanwhile, in order to evaluate the effect of liver and spleen shear wave velocity on the prognosis of patients with portal hypertension, Cox’s proportional hazard regression model analysis was applied. The ability of those factors in predicting the prognosis of patients with portal hypertension was calculated through receiver operating characteristic (ROC) curves.
The liver and spleen shear wave velocity in the favorable prognosis group revealed a clear decline, while those in the unfavorable prognosis group revealed an increasing tendency at different time points. Furthermore, liver and spleen shear wave velocity was higher in the unfavorable prognosis group, compared with the favorable prognosis group; the differences were statistically significant (P < 0.05). The prognosis of patients with portal hypertension was significantly affected by spleen hardness at the 3rd month after treatment [relative risk (RR) = 3.481]. At the 9th month after treatment, the prognosis was affected by liver hardness (RR = 5.241) and spleen hardness (RR = 7.829). The differences between these two groups were statistically significant (P < 0.05). The ROC analysis revealed that the area under the curve (AUC) of spleen hardness at the 3rd month after treatment was 0.644, while the AUCs of liver and spleen hardness at the 9th month were 0.579 and 0.776, respectively. These might predict the prognosis of patients with portal hypertension.
Spleen hardness at the 3rd month and liver and spleen shear wave velocity at the 9th month may be used to assess the prognosis of patients with portal hypertension. This is hoped to be used as an indicator of predicting the prognosis of patients with portal hypertension.
Core tip: Sixty-seven patients with liver cirrhosis with portal hypertension were assessed by acoustic radiation force impulse imaging at different time points after treatment. We found that the portal hypertension was significantly affected by spleen hardness at the 3rd month after treatment [relative risk (RR) = 3.481]. At the 9th month after treatment, the prognosis was affected by liver hardness (RR = 5.241) and spleen hardness (RR = 7.829). ROC analysis revealed that the area under the curve of liver and spleen hardness might be used to predict the prognosis of patients with portal hypertension.
- Citation: Zhang Y, Mao DF, Zhang MW, Fan XX. Clinical value of liver and spleen shear wave velocity in predicting the prognosis of patients with portal hypertension. World J Gastroenterol 2017; 23(45): 8044-8052
- URL: https://www.wjgnet.com/1007-9327/full/v23/i45/8044.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i45.8044
Portal hypertension is a common cause of cirrhosis and presents a series of symptoms[1,2]. In recent years, with the increase in incidence of liver cirrhosis, the number of patients with portal hypertension has rapidly increased[3]. The main clinical manifestations of portal hypertension are hepatosplenomegaly and ascites, which bring great negative impact on patients[4]. Due to its hard texture and obvious symptoms, splenomegaly associated with portal hypertension is significantly different from others, and is regarded as one of the main features of portal hypertension[5-7]. In clinical practice, severe complications of portal hypertension, including gastric fundus, esophageal varices, hepatic encephalopathy and gastrointestinal bleeding, have increased the risk of exacerbation, and even death[8-11]. In order to avoid complications and reduce the mortality of patients, early and effective evaluation indicators should be developed for predicting the prognosis.
As a new technology of ultrasonic elastography, acoustic radiation force impulse (ARFI) imaging can quantitatively reflect the advantages of tissue hardness by detecting the degree of deformation of the organ after compression, in order to assess the elasticity and hardness of tissues[12-15]. These detected results are displayed through imaging[16,17]. Although the clinical value of ARFI in predicting liver fibrosis, tumors and other diseases has been confirmed[18,19], researches on ARFI for detecting portal hypertension caused by cirrhosis have not been studied in detail. Hence, it remains to be determined whether ARFI has the ability to detect and evaluate portal hypertension prognosis.
Therefore, in this study, liver and spleen ARFI shear wave velocity values were determined to evaluate the clinical significance of ARFI for detecting portal hypertension caused by liver cirrhosis in patients, aiming to provide predictive indications for the prognosis of patients and avoid complication and death.
A total of 67 patients with liver cirrhosis, who were diagnosed with portal hypertension by hepatic venous pressure gradient (HVPG) in our hospital from June 2014 to December 2014, were included in this study. Among these patients, 42 were male and 25 were female; age of these patients ranged within 20-70 years old, with an average age of 52.68 ± 7.43 years-old. This research was approved by the Ethics Committee of our hospital, and all patients provided a signed informed consent.
Inclusion criteria: (1) patients diagnosed with liver cirrhosis through clinical symptoms combined with laboratory or image examinations; (2) patients with HVPG ≤ 12 mmHg; and (3) patients classified as grade A or B in the Child-Pugh grading criteria. Exclusion criteria: (1) patients whose shear wave velocity values could not be acquired by ARFI; (2) patients with liver cancer or other complications; (3) patients with acute heart failure, cardiogenic shock, or other vital organs diseases; (4) patients who underwent splenic surgery; (5) patients whose hepatosplenomegaly was caused by acute infection and other reasons; and (6) patients who were receiving propranolol hydrochloride or other drugs that can affect portal pressure.
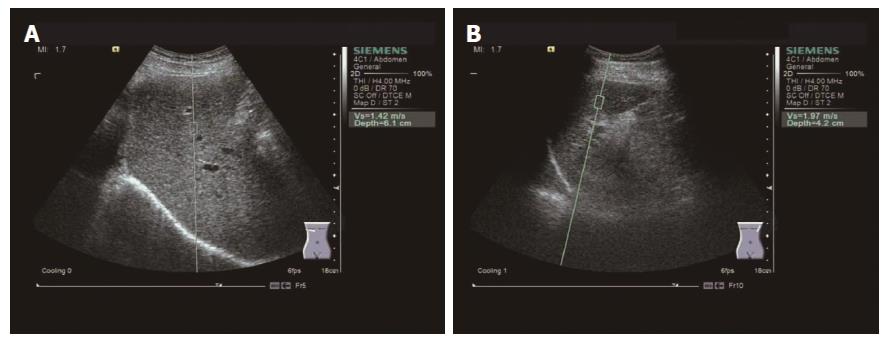
All 67 patients underwent routine clinical examinations at the day of hospitalization, and the serological indicators of liver function and clinical symptoms of these patients were recorded. After treatment, liver and spleen ARFI shear wave velocity was measured continuously for all patients for 1 wk, and the results were recorded. Patients were instructed to fast at least 8 h before the measurement. A Siemens Acuson S2000 ultrasound system was used with a 4C1 convex array probe. During the liver shear wave velocity measurement, patients were asked to completely hold their breath and lie on the right side (Figure 1A). The probe was kept vertically fixed on the intercostal space to avoid larger blood vessels. This was repeated three times, and measurement results averaged. During the spleen shear wave velocity measurement, patients were instructed to hold their breath and lie on their left side (Figure 1B). These steps were repeated and the mean shear wave velocity values were recorded.
All 67 patients were followed-up for 20 mo after treatment. The first follow up was at the 1st month, and subsequent follow ups were performed by telephone every 3 mo. At the 3rd and 9th month, the liver and spleen ARFI shear wave velocity values of patients were measured, and the results were recorded. The endpoint of this study was the unfavorable prognosis of patients during the follow-up period, which include complications or death after treatment. After the follow ups, initial results upon admission and results of the last follow up were collected. In addition, the number of patients with unfavorable prognosis, serological indicators, the liver and spleen shear wave velocity values at the 1st week and at the 3rd and 9th month after treatment, and the diagnosis of the physician were recorded. Patients who were lost, refused visit, quit or died from other causes unrelated to the study were defined as censored.
During the 20-mo follow-up period, patients with favorable prognosis were assigned into the favorable prognosis group, while patients with unfavorable prognosis were as assigned into the unfavorable prognosis group. At the end of the follow-up period, baseline information, prognosis results, serological indicators of liver function, and liver and spleen ARFI shear wave velocity measurements were analyzed. The baseline information of patients included age and sex. The prognosis results were classified as either favorable prognosis or unfavorable prognosis. The serological indicators of liver function included albumin (ALB), alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
SPSS 19.0 was used for statistical analysis. Shear wave velocity values, and the values of serological indicators ALB, AST and ALT and other measurement data were presented as mean ± SD. The unfavorable prognosis rate of patients and follow-up results were expressed via survival curve and pie chart, respectively, to analyze the prognosis of patients with portal hypertension. The variation and difference of shear wave velocity values in these two groups at the 1st week and the 3rd and 9th month after treatment were compared using repeated measures analysis of variance to explore the relationship between shear wave velocity values at different time points and portal hypertension. Age, sex, prognosis results, ALT, ALB and AST, shear wave velocity values and other possible influences were included in the Cox’s proportional hazard regression model analysis. and indicators that affected prognosis were selected. On this basis, the receiver operating characteristic (ROC) curve was used to further compare with the area of all indicators that have statistically significant differences the area under the curve (AUC), and to investigate the ability of indicators that could predict the prognosis of patients with portal hypertension. P < 0.05 was considered statistically significant.
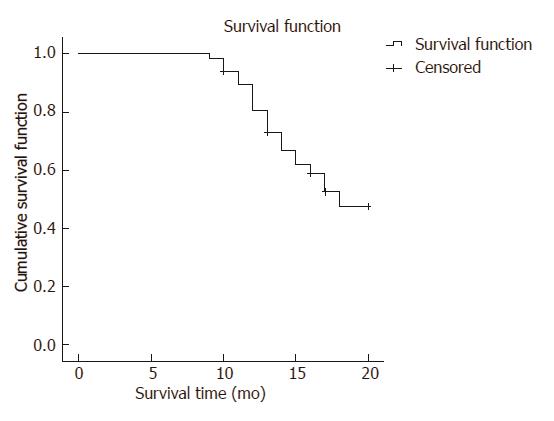
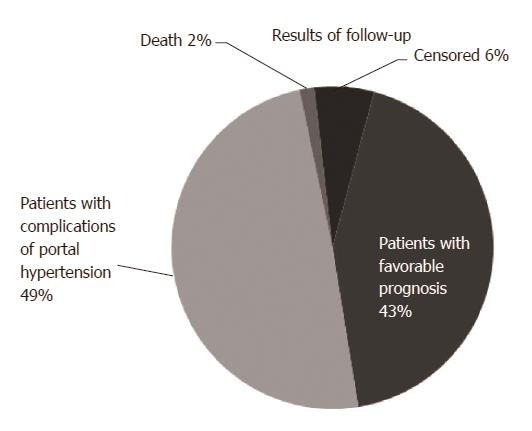
The follow-up results revealed 29 patients in the favorable prognosis group and 34 patients in the unfavorable group. Among these patients, 11 patients died, 60 patients had adverse complications, and 4 patients were lost to follow-up. The Kaplan-Meier survival curve was used to analyze the incidence of portal hypertension complications after treatment. Results revealed that the unfavorable prognosis rate was 58.97%, as shown in Figures 2 and 3.
The analysis results of variations in liver shear wave velocity values revealed that the liver shear wave velocity value in the unfavorable prognosis group exhibited an increasing trend, while a clear decline was observed in the favorable prognosis group (Fwithin group = 2.106, Pwithin group = 0.039). The liver shear wave velocity value was highest at the 1st week after treatment in the favorable prognosis group, which gradually decreased thereafter. This value was lowest at to 9th month after treatment. However, in the unfavorable prognosis group, the liver shear wave velocity value was highest at the 9th month and lowest at the 1st week after treatment.
The liver shear wave velocity values of these two groups were compared among different time points. Although, these values in the favorable prognosis group were slightly higher than those in the unfavorable prognosis group and the difference was not statistically significant (P > 0.05). Furthermore, the difference in liver shear wave velocity values at the 3rd and 9th month was statistically significant (P < 0.05), and these values in the favorable prognosis group were lower. Overall, the liver shear wave velocity values in the unfavorable prognosis group were higher than those in the favorable prognosis group, and the difference was statistically significant (Fbetween groups = 2.193, Pbetween groups = 0.032). Furthermore, these values correlated between the different groups or among different times points (Finteraction = 2.457, Pinteraction = 0.017) (Table 1).
| Group | 1st wk after treatment, m/s | 3rd mo after treatment, m/s | 9th mo after treatment, m/s |
| Favorable prognosis group | 1.88 ± 0.39 | 1.70 ± 0.41 | 1.67 ± 0.38 |
| Unfavorable prognosis group | 1.84 ± 0.43 | 1.92 ± 0.43 | 2.08 ± 0.35 |
| t | 0.384 | 2.068 | 4.455 |
| P value | 0.702 | 0.043 | 0.000 |
In the favorable prognosis group, the spleen shear wave velocity values declined from the 1st week to the 9th month after treatment. The value at the 1st week was highest and the value at the 9th month was lowest. However, spleen shear wave velocity values in the unfavorable prognosis group exhibited an increasing trend. The minimum and maximum values of the liver shear wave velocity were reached at the 1st week and 9th month after treatment, respectively. Values at the different time points of these two groups are presented in Table 2.
| Group | 1st wk after treatment, m/s | 3rd mo after treatment, m/s | 9th mo after treatment, m/s |
| Favorable prognosis group | 3.82 ± 0.44 | 3.71 ± 0.42 | 3.55 ± 0.34 |
| Unfavorable prognosis group | 3.83 ± 0.46 | 4.06 ± 0.44 | 4.29 ± 0.30 |
| t | 0.088 | 3.213 | 9.178 |
| P value | 0.930 | 0.002 | 0.000 |
These results revealed that spleen shear wave velocity values at the 3rd and 9th month in the unfavorable prognosis group were higher, and the difference was statistically significant (P < 0.05). Furthermore, values at the 1st week in these two groups were similar, and the difference was not statistically significant (P > 0.05). The spleen shear wave velocity values were compared between these two groups. These results revealed that values in the unfavorable prognosis group were higher than in the favorable prognosis group, and the difference was statistically significant (Fbetween groups = 8.431, Pbetween groups = 0.000). The values in different groups or at different time points were correlated (Finteraction = 3.422, Pinteraction = 0.001).
A Cox’s proportional hazard regression model was constructed to analyze the effects of all suspicious indicators on portal hypertension prognosis. These results revealed that the age and sex of patients had no effect on prognosis (P > 0.05), and serological indicators including ALB, AST and ALT did not influence the prognosis. At the same time, all liver and spleen shear wave velocity values at different time points were evaluated, and results revealed that liver shear wave velocity values at the 9th month and spleen shear wave velocity values at the 3rd and 9th month could affect the prognosis of patients (P < 0.05); other values had no significant effects (P > 0.05). All indicators that had statistically significant differences were compared. The spleen shear wave velocity value at the 9th month after treatment had the strongest effect on the prognosis of patients (relative risk (RR) = 8.829). The liver hardness value at the 9th month ranked second (RR = 6.271), followed by the spleen hardness value at the 3rd month (RR = 3.481), as shown in Table 3.
| B | SE | Wald | df | P value | RR | 95%CI | ||
| Upper limit | Lower limit | |||||||
| ALB | -0.030 | 0.083 | 0.131 | 1 | 0.718 | 0.970 | 0.824 | 1.143 |
| ALT | -0.014 | 0.021 | 0.415 | 1 | 0.520 | 0.986 | 0.946 | 1.028 |
| AST | -0.007 | 0.028 | 0.068 | 1 | 0.794 | 0.993 | 0.939 | 1.049 |
| Sex | -0.909 | 0.615 | 2.181 | 1 | 0.140 | 0.403 | 0.121 | 1.346 |
| Age | 0.016 | 0.036 | 0.192 | 1 | 0.661 | 1.016 | 0.947 | 1.090 |
| Liver hardness in the 1st week after treatment | 0.175 | 0.698 | 0.063 | 1 | 0.802 | 1.191 | 0.304 | 4.676 |
| Liver hardness in the 3rd mo after treatment | 1.155 | 0.769 | 2.258 | 1 | 0.133 | 3.175 | 0.704 | 14.329 |
| Liver hardness in the 9th mo after treatment | 1.657 | 1.123 | 3.930 | 1 | 0.047 | 5.241 | 1.026 | 83.802 |
| Spleen hardness in the 1st week after treatment | 0.034 | 0.024 | 2.089 | 1 | 0.148 | 1.035 | 0.988 | 1.084 |
| Spleen hardness in the 3rd mo after treatment | 1.247 | 0.583 | 4.576 | 1 | 0.032 | 3.481 | 1.110 | 10.914 |
| Spleen hardness in the 9th mo after treatment | 2.058 | 0.883 | 6.079 | 1 | 0.014 | 7.829 | 1.563 | 49.870 |
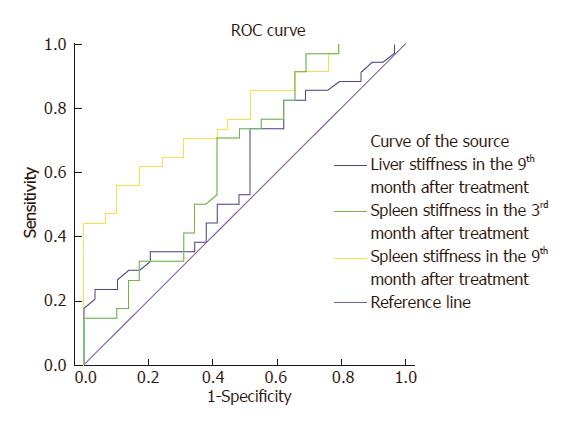
In order to analyze the predictive ability of these three indicators for adverse prognosis, the ROC curve was established. This revealed that the AUC of spleen shear wave velocity values at the 3rd month and the AUC of the liver and spleen shear wave velocity values at the 9th month were 0.644, 0.579 and 0.776, respectively. It was found that the AUC of the spleen shear wave velocity value at the 9th month was highest. The sensitivity was 55.9%, specificity was 89.7% and the best diagnostic value was 0.455. Furthermore, the AUC of the spleen shear wave velocity value at the 3rd month was slightly lower, and the sensitivity, specificity and best diagnostic value was 70.6%, 58.6% and 0.292, respectively. The lowest AUC was the liver shear wave velocity value at the 9th month, and the sensitivity, specificity and best diagnosis value was 73.5%, 48.3% and 0.218, respectively (Figure 4).
Portal hypertension is a clinical syndrome caused by portal venous drainage obstruction. This occurs in middle-age men and develops slowly, and most of these cases are closely associated with cirrhosis[2,20-22]. In China, the number of new patients with cirrhosis increase year after year[23]. At the same time, the incidence of portal hypertension has also rapidly increased[24]. The majority of patients often present with liver dysfunction, bleeding, gastrointestinal vascular disease and other serious diseases, except common clinical symptoms, including hepatosplenomegaly and ascites[25].
At present, the main approaches for the clinical treatment of portal hypertension are surgery and symptomatic treatment[26]. Although these treatment approaches are diverse and effective, the mortality rate of patients who have this disease remains high due to delitescent pathogenetic condition, long disease duration and proneness to complications[27]. Therefore, it is important to establish a simple and effective system for the prognosis of portal hypertension, in order to monitor the disease, adjust the treatment approaches, and improve the survival rate of patients in real time. The varying degrees of hardness of the lesions are usually related to the severity of the disease[28]. Furthermore, the swelling and hardness of the hepatosplenomegaly of portal hypertension are more visible than those of other diseases[29,30]. This shows that there may be relationships between liver and spleen hardness and portal hypertension[31-33].
In the present study, it was shown that studies have investigated the relationship between liver and spleen hardness and liver fibrosis, chronic liver, or other liver diseases[34-36]. However, there is lack of further research on the relationship between liver and spleen hardness and portal hypertension, and the clinical value of liver and spleen hardness in evaluating the prognosis of portal hypertension could not be determined. In recent years, as a mature method of examination, ARFI imaging promotes the implementation of detecting tissue hardness to predict the development of diseases[37,38]. Due to its simple, convenient and good repeatability advantages, ARFI imaging has gained the attention of clinicians. Hence, we detected the liver and spleen ARFI shear wave velocity values of these patients and analyzed their prognosis, in order to evaluate the clinical application value of ARFI in predicting the prognosis of portal hypertension.
The common adverse complications of portal hypertension include esophageal and gastric variceal bleeding, hepatic encephalopathy, hepatorenal syndrome and others; these are the main indications of surgery for treating portal hypertension[39]. Among these complications, esophageal and gastric variceal bleeding were the most dangerous[40,41]. When this occurs, patients will be at risk due to acute upper gastrointestinal bleeding[42-44]. Therefore, it has been considered that the establishment of a prognostic detection system for portal hypertension has clinical value[45-47].
In this study, patients were enrolled according to the Child-Pugh criteria and HVPG results. Patients classified as grade C and having an HVPG ≥ 12 mmHg were excluded due to higher risk of surgery, lower survival rate and poor recovery. The Kaplan-Meier survival curve revealed that the unfavorable prognosis rate in all 67 patients was 58.97% at the end of follow-up, which reflects that it is unsatisfactory of the prognosis of patients with portal hypertension. In order to investigate the variation trend of liver and spleen shear wave velocity values, values at three different time points were collected and analyzed by repeated measures analysis of variance.
Results revealed that the liver and spleen shear wave velocity values in the favorable prognosis group exhibited a decreasing trend, and there were significant differences in these values at three different time points. Values are lowest in the favorable prognosis group at the 9th month, but the variations in these values in the unfavorable prognosis group were the opposite. This suggests that there may be a link between the variation in liver and spleen shear wave velocity values and the development of portal hypertension. When comparing the overall values of liver and spleen shear wave velocity in these two groups, values in the unfavorable prognosis group was significantly higher than in the favorable prognosis group. This demonstrates that there is a potential relationship between liver and spleen shear wave velocity and the prognosis of patients with portal hypertension[9,48,49].
In order to further investigate indicators that affect the prognosis of portal hypertension, Cox’s proportional hazard regression model was performed on liver function serum markers, clinical data and liver and spleen shear wave velocity values at three different time points. As common clinical detection indicators, liver function serum markers were detected to reflect the degree of liver damage. In this study, ALB, ALT and AST were included in the Cox’s regression model to evaluate their effect on the prognosis of patients with portal hypertension.
Results revealed that liver function serum markers ALB, ALT and AST have no significant effect on the prognosis of this disease. The reasons may be that the variation in ALB, ALT, and AST values are also associated with many diseases such as hepatitis, myocarditis and Japanese B encephalitis, except for cirrhosis. Hence, liver function markers have low sensitivity for the diagnosis and prediction of diseases. Therefore, it is unsatisfactory to take serum markers of liver function as a prognostic indicator of portal hypertension.
On the contrary, in analyzing liver and spleen shear wave velocity values at three different time points, it can be found that the spleen shear wave velocity value at the 3rd month and liver and spleen shear wave velocity value at the 9th month can significantly affect the prognosis of portal hypertension. Among these indicators, the effect of the spleen shear wave velocity value is the most significant, while the value at the 3rd month was the lowest. However, liver and spleen hardness at the 1st week after treatment had no significant effect. This may be due to the improvement of the liver and spleen in the short period after treatment. In addition, the liver shear wave velocity value also has no effect on the prognosis of this disease. The possible reasons are that that liver hardness is not a feature of portal hypertension, this value can be affected by many factors, and the variation degree is similar.
The results of this study show that spleen hardness at the 3rd month and liver and spleen hardness at the 9th month have the potential to assess the prognosis of portal hypertension.
Based on the above data, the ROC curve was constructed to further investigate the predictive ability of liver hardness at the 9th month and spleen hardness at the 3rd and 9th month. As a result, the AUC of the spleen shear wave velocity value at the 9th month was highest, which was over 0.7. This revealed that this value had a better predictive ability on the prognosis of portal hypertension, while the AUC of liver hardness at the 9th month and spleen hardness at the 3rd month are lower, and their predictive ability are slightly insufficient. The comprehensive analysis shows that the liver shear wave velocity values at the 9th month and the spleen shear wave velocity values at the 3rd and 9th month are expected to be used as predictive indicators for the prognosis of patients with portal hypertension. Furthermore, this can be combined with other prognosis detection indictors in evaluating the risk of patients.
However, our study still has some deficiency, which includes the small sample data, the inadequate time points for ARFI detection, and the lack of coverage on other types of portal hypertensions. Therefore, future studies with larger samples and adequate detection time points should be conducted to evaluate the other types of portal hypertensions and verify our findings.
In summary, liver and spleen ARFI shear wave velocity values have the potential to monitor the prognosis of portal hypertension, and liver shear wave velocity values at the 9th month and spleen shear wave velocity values at the 3rd and 9th month can reflect the prognosis of patients. It is hoped that this approach could be applied in clinic to reduce complications and improve the survival rate of patients.
Portal hypertension is a common cause of cirrhosis and presents a series of serious symptoms. In recent years, the incidence rate of liver cirrhosis as well as the portal hypertension rate in China are increasing. The main clinical symptoms of portal hypertension are hepatosplenomegaly and ascites, which bring great negative impact on patients. Because of the hard texture and obvious symptoms, splenomegaly associated with portal hypertension is regarded as one of the main features of portal hypertension. In clinical practice, severe complications of portal hypertension, including gastric fundus, esophageal varices, hepatic encephalopathy and gastrointestinal bleeding, have increased the risk of exacerbation, and even death.
Acoustic radiation force impulse (ARFI) imaging can quantitatively reflect the advantages of tissue hardness by detecting the degree of deformation of the organ after compression, in order to assess the elasticity and hardness of tissues. These detected results are displayed through imaging. Although the clinical value of ARFI in predicting liver fibrosis, tumors and other diseases has been confirmed, research on ARFI for detecting portal hypertension caused by cirrhosis has not been carried out in detail.
ARFI could be used to determine liver and spleen hardness by detecting the degree of deformation of the organ after compression. ARFI is convenient, non-invasive and simple. In this study, we used the technology to figure out the relation between portal hypertension and to try to evaluate the predictive value for portal hypertension.
The study illustrated the ability for ARFI to be applied to detecting portal hypertension in clinical practice. The prognosis of patients with portal hypertension was significantly affected by spleen and liver hardness. Spleen hardness at the 3rd month, and liver and spleen shear wave velocity at the 9th month may be used to assess the prognosis of patients with portal hypertension. It is expected to be used as an indicator of predicting the prognosis of patients with portal hypertension.
This study illustrated that ARFI imaging could be used in detecting portal hypertension by detecting liver and spleen harshness. Shear wave velocity is a quantitative indicator that is accurate and objective. The detecting process of ARFI is simple, non-invasive, fast and widely used for detection and prediction in clinical practice. Thus, ARFI imaging is a helpful tool that has a significant clinical value and is worthy of developing.
| 1. | Cucchetti A, Cescon M, Pinna AD. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: A systematic review and meta-analysis. More doubts than clarity. Hepatology. 2015;62:976-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2356] [Article Influence: 214.2] [Reference Citation Analysis (4)] |
| 3. | Park SY, Jung SE, Jeong WK, Kim CK, Park BK, Choi D. Renal function impairment in liver cirrhosis: preliminary results with diffusion-weighted imaging at 3 T. AJR Am J Roentgenol. 2015;204:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Le Roy B, Gelli M, Serji B, Memeo R, Vibert E. Portal biliopathy as a complication of extrahepatic portal hypertension: etiology, presentation and management. J Visc Surg. 2015;152:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 5. | Leoni S, Buonfrate D, Angheben A, Gobbi F, Bisoffi Z. The hyper-reactive malarial splenomegaly: a systematic review of the literature. Malar J. 2015;14:185-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Maazoun F, Deschamps O, Barros-Kogel E, Ngwem E, Fauchet N, Buffet P, Froissart A. [Hyper-reactive malarial splenomegaly]. Rev Med Interne. 2015;36:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Olson AP, Trappey B, Wagner M, Newman M, Nixon LJ, Schnobrich D. Point-of-care ultrasonography improves the diagnosis of splenomegaly in hospitalized patients. Crit Ultrasound J. 2015;7:13-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Campagna F, Montagnese S, Schiff S, Ruzzoli M, Biancardi A, Iannizzi P, Pujatti PL, Angeli P, Gatta A, Merkel C. Confounders in the detection of minimal hepatic encephalopathy: a neuropsychological and quantified EEG study. Liver Int. 2015;35:1524-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Attia D, Schoenemeier B, Rodt T, Negm AA, Lenzen H, Lankisch TO, Manns M, Gebel M, Potthoff A. Evaluation of Liver and Spleen Stiffness with Acoustic Radiation Force Impulse Quantification Elastography for Diagnosing Clinically Significant Portal Hypertension. Ultraschall Med. 2015;36:603-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 11. | Waghray A, Waghray N, Mullen K. Management of covert hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S75-S81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Al-Attar AM, Shawush NA. Influence of olive and rosemary leaves extracts on chemically induced liver cirrhosis in male rats. Saudi J Biol Sci. 2015;22:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Habibi HA, Cicek RY, Kandemirli SG, Ure E, Ucar AK, Aslan M, Caliskan S, Adaletli I. Acoustic radiation force impulse (ARFI) elastography in the evaluation of renal parenchymal stiffness in patients with ureteropelvic junction obstruction. J Med Ultrason (2001). 2017;44:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Sagir A, Ney D, Oh J, Pandey S, Kircheis G, Mayatepek E, Häussinger D. Evaluation of Acoustic Radiation Force Impulse Imaging (ARFI) for the Determination of Liver Stiffness Using Transient Elastography as a Reference in Children. Ultrasound Int Open. 2015;1:E2-E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Karlas T, Dietrich A, Peter V, Wittekind C, Lichtinghagen R, Garnov N, Linder N, Schaudinn A, Busse H, Prettin C. Evaluation of Transient Elastography, Acoustic Radiation Force Impulse Imaging (ARFI), and Enhanced Liver Function (ELF) Score for Detection of Fibrosis in Morbidly Obese Patients. PLoS One. 2015;10:e0141649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Liu A, Perumpail RB, Kumari R, Younossi ZM, Wong RJ, Ahmed A. Advances in cirrhosis: Optimizing the management of hepatic encephalopathy. World J Hepatol. 2015;7:2871-2879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Zhou J, Yang Z, Zhan W, Zhang J, Hu N, Dong Y, Wang Y. Breast Lesions Evaluated by Color-Coded Acoustic Radiation Force Impulse (ARFI) Imaging. Ultrasound Med Biol. 2016;42:1464-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Ogul H, Pirimoglu B, Kantarci M. Unusual MRI findings in a girl with acute hepatic encephalopathy: leptomeningeal enhancement and cortical laminar necrosis. Acta Neurol Belg. 2015;115:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Shih CC, Lai TY, Huang CC. Evaluating the intensity of the acoustic radiation force impulse (ARFI) in intravascular ultrasound (IVUS) imaging: Preliminary in vitro results. Ultrasonics. 2016;70:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Gracia-Sancho J, Maeso-Díaz R, Fernández-Iglesias A, Navarro-Zornoza M, Bosch J. New cellular and molecular targets for the treatment of portal hypertension. Hepatol Int. 2015;9:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Vorobioff JD, Groszmann RJ. Prevention of portal hypertension: from variceal development to clinical decompensation. Hepatology. 2015;61:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Dai J, Qi X, Li H, Guo X. Role of D-dimer in the Development of Portal Vein Thrombosis in Liver Cirrhosis: A Meta-analysis. Saudi J Gastroenterol. 2015;21:165-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Lim YL, Choi E, Jang YO, Cho YZ, Kang YS, Baik SK, Kwon SO, Kim MY. Clinical Implications of the Serum Apelin Level on Portal Hypertension and Prognosis of Liver Cirrhosis. Gut Liver. 2016;10:109-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Qin J, Tang S, Jiang M, He Q, Zhou J, Yao X, Zeng W, Liang Y, Gu M. Portal hypertension caused by right common iliac artery-superior mesenteric vein fistula. Jpn J Radiol. 2015;33:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Yu H, Guo S, Wang L, Dong Y, Tian G, Mu S, Zhang H, Li D, Zhao S. Laparoscopic Splenectomy and Esophagogastric Devascularization for Liver Cirrhosis and Portal Hypertension Is a Safe, Effective, and Minimally Invasive Operation. J Laparoendosc Adv Surg Tech A. 2016;26:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Belli A, Cioffi L, Russo G, Belli G. Liver resection for hepatocellular carcinoma in patients with portal hypertension: the role of laparoscopy. Hepatobiliary Surg Nutr. 2015;4:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 27. | Yan M, Geyer H, Mesa R, Atallah E, Callum J, Bartoszko J, Yee K, Maganti M, Wong F, Gupta V. Clinical features of patients with Philadelphia-negative myeloproliferative neoplasms complicated by portal hypertension. Clin Lymphoma Myeloma Leuk. 2015;15:e1-e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Colecchia A, Marasco G, Taddia M, Montrone L, Eusebi LH, Mandolesi D, Schiumerini R, Di Biase AR, Festi D. Liver and spleen stiffness and other noninvasive methods to assess portal hypertension in cirrhotic patients: a review of the literature. Eur J Gastroenterol Hepatol. 2015;27:992-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Grgurevic I, Puljiz Z, Brnic D, Bokun T, Heinzl R, Lukic A, Luksic B, Kujundzic M, Brkljacic B. Liver and spleen stiffness and their ratio assessed by real-time two dimensional-shear wave elastography in patients with liver fibrosis and cirrhosis due to chronic viral hepatitis. Eur Radiol. 2015;25:3214-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Balakrishnan M, Souza F, Muñoz C, Augustin S, Loo N, Deng Y, Ciarleglio M, Garcia-Tsao G. Liver and Spleen Stiffness Measurements by Point Shear Wave Elastography via Acoustic Radiation Force Impulse: Intraobserver and Interobserver Variability and Predictors of Variability in a US Population. J Ultrasound Med. 2016;35:2373-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Yasar TK, Wagner M, Bane O, Besa C, Babb JS, Kannengiesser S, Fung M, Ehman RL, Taouli B. Interplatform reproducibility of liver and spleen stiffness measured with MR elastography. J Magn Reson Imaging. 2016;43:1064-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (9)] |
| 32. | Licinio R, Losurdo G, Carparelli S, Iannone A, Giorgio F, Barone M, Principi M, Ierardi E, Di Leo A. Helicobacter pylori, liver cirrhosis, and portal hypertension: an updated appraisal. Immunopharmacol Immunotoxicol. 2016;38:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Takabatake H, Matsueda K, Yamamoto H. Portal Hypertension in Patients with Liver Cirrhosis: Diagnostic Accuracy of Spleen Stiffness. Radiology. 2016;279:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Watson GA, Abu-Shanab A, O’Donohoe RL, Iqbal M. Enteroscopic Management of Ectopic Varices in a Patient with Liver Cirrhosis and Portal Hypertension. Case Reports Hepatol. 2016;2016:1-4 2018642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Garbuzenko DV. Contemporary concepts of the medical therapy of portal hypertension under liver cirrhosis. World J Gastroenterol. 2015;21:6117-6126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 36. | Steib CJ, Schewe J, Gerbes AL. Infection as a Trigger for Portal Hypertension. Dig Dis. 2015;33:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Chang S, Kim MJ, Kim J, Lee MJ. Variability of shear wave velocity using different frequencies in acoustic radiation force impulse (ARFI) elastography: a phantom and normal liver study. Ultraschall Med. 2013;34:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Garcia PH, Feliciano MA, Carvalho CF, Crivellenti LZ, Maronezi MC, Almeida VT, Uscategui RR, Vicente WR. Acoustic radiation force impulse (ARFI) elastography of kidneys in healthy adult cats: preliminary results. J Small Anim Pract. 2015;56:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Jiang M, Wang Z, Tsauo J, Li X. Basal ganglia hyperintensity may be a marker of hepatic encephalopathy secondary to portosystemic shunting. Clin Res Hepatol Gastroenterol. 2015;39:e5-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Duan YF, Li XD, Sun DL, Chen XM, An Y, Zhu F. A preliminary study on surgery for hepatocellular carcinoma patients with portal hypertension. Am J Surg. 2015;210:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Veiga ZST, Villela-Nogueira CA, Fernandes FF, Cavalcanti MG, Figueiredo FA, Pereira JL, Pereira GH, Moraes Coelho HS, Peralta JM, Marques CE. Transient elastography evaluation of hepatic and spleen stiffness in patients with hepatosplenic schistosomiasis. Eur J Gastroenterol Hepatol. 2017;29:730-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Lee BH, Kim YM, Kim JH, Kim GH, Kim JM, Kim JH, Woo KH, Yang SH, Kim CJ, Choi IH. Atypical manifestation of carnitine palmitoyltransferase 1A deficiency: hepatosplenomegaly and nephromegaly. J Pediatr Gastroenterol Nutr. 2015;60:e19-e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Sarper N, Ipek IO, Ceran O, Karaman S, Bozaykut A, Inan S. A rare syndrome in the differential diagnosis of hepatosplenomegaly and pancytopenia: report of identical twins with Griscelli disease. Ann Trop Paediatr. 2003;23:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 44. | Ho HL, Huang HC. Molecular mechanisms of circulatory dysfunction in cirrhotic portal hypertension. J Chin Med Assoc. 2015;78:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Lauridsen MM, Bajaj JS. Hepatic encephalopathy treatment and its effect on driving abilities: A continental divide. J Hepatol. 2015;63:287-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Takata T, Koda M, Sugihara T, Sugihara S, Okamoto T, Miyoshi K, Matono T, Hosho K, Mae Y, Iyama T. Renal shear wave velocity by acoustic radiation force impulse did not reflect advanced renal impairment. Nephrology (Carlton). 2016;21:1056-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Su YY, Yang GF, Lu GM, Wu S, Zhang LJ. PET and MR imaging of neuroinflammation in hepatic encephalopathy. Metab Brain Dis. 2015;30:31-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Kassym L, Nounou MA, Zhumadilova Z, Dajani AI, Barkibayeva N, Myssayev A, Rakhypbekov T, Abuhammour AM. New combined parameter of liver and splenic stiffness as determined by elastography in healthy volunteers. Saudi J Gastroenterol. 2016;22:324-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Qiu B, Zhao MF, Yue ZD, Zhao HW, Wang L, Fan ZH, He FL, Dai S, Yao JN, Liu FQ. Combined transjugular intrahepatic portosystemic shunt and other interventions for hepatocellular carcinoma with portal hypertension. World J Gastroenterol. 2015;21:12439-12447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Noboru H, Jeong PS S- Editor: Chen K L- Editor: Filipodia E- Editor: Lu YJ