Published online Nov 28, 2017. doi: 10.3748/wjg.v23.i44.7917
Peer-review started: August 15, 2017
First decision: August 30, 2017
Revised: September 16, 2017
Accepted: September 19, 2017
Article in press: September 19, 2017
Published online: November 28, 2017
Processing time: 104 Days and 11.8 Hours
To compare the clinical outcomes of right hepatectomy for large hepatocellular carcinoma via the anterior and conventional approach.
We comprehensively performed an electronic search of PubMed, EMBASE, and the Cochrane Library for randomized controlled trials (RCTs) or controlled clinical trials (CCTs) published between January 2000 and May 2017 concerning the anterior approach (AA) and the conventional approach (CA) to right hepatectomy. Studies that met the inclusion criteria were included, and their outcome analyses were further assessed using a fixed or random effects model.
This analysis included 2297 patients enrolled in 16 studies (3 RCTs and 13 CTTs). Intraoperative blood loss [weighted mean difference = -255.21; 95% confidence interval (95%CI): -371.3 to -139.12; P < 0.0001], intraoperative blood transfusion [odds ratio (OR) = 0.42; 95%CI: 0.29-0.61; P < 0.0001], mortality (OR = 0.59; 95%CI: 0.38-0.92; P = 0.02), morbidity (OR = 0.77; 95%CI: 0.62-0.95; P = 0.01), and recurrence rate (OR = 0.62; 95%CI: 0.47-0.83; P = 0.001) were significantly reduced in the AA group. Patients in the AA group had better overall survival (hazard ratio [HR] = 0.71; 95%CI: 0.50-1.00; P = 0.05) and disease-free survival (HR = 0.67; 95%CI: 0.58-0.79; P < 0.0001) than those in the CA group.
The AA is safe and effective for right hepatectomy for large hepatocellular carcinoma and could accelerate postoperative recovery and achieve better survival outcomes than the CA.
Core tip: Anterior approach has been suggested as an alternative approach to conventional approach for right hepatectomy. However, comparative studies have shown conflicting results. To evaluate whether right hepatectomy using the anterior approach for large hepatocellular carcinoma results in better clinical outcomes when compared with the conventional approach, we investigated these two techniques in terms of estimated intraoperative blood loss, massive blood loss, intraoperative blood transfusion, operative time, mortality, morbidity, recurrence rate, hospital stay, overall survival and disease-free survival.
- Citation: Tang JX, Li JJ, Weng RH, Liang ZM, Jiang N. Anterior vs conventional approach right hepatic resection for large hepatocellular carcinoma: A systematic review and meta-analysis. World J Gastroenterol 2017; 23(44): 7917-7929
- URL: https://www.wjgnet.com/1007-9327/full/v23/i44/7917.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i44.7917
Conventional right hepatectomy (CRH), which is complete mobilization of the right liver with the right hepatic vein controlled outside the liver before parenchymal transection, is a standard treatment approach[1,2]. However, its use is often difficult and hazardous in cases of large hepatocellular carcinoma (HCC) involving the right liver with extrahepatic organ invasion in the right retrohepatic region. The conventional approach (CA) could result in excessive blood loss, hemodynamic instability, tumor metastasis, tumor rupture, and liver ischemia because of prolonged rotation of the liver remnant during the course of liver mobilization[3]. All of these drawbacks could be ameliorated using the anterior approach (AA) for right hepatectomy, which was first demonstrated by Lai and colleagues in 1996[4]. The AA involves initial vascular inflow control, completion of parenchymal transection, and complete venous outflow control before mobilization of the right liver. Lately, it has been recognized that the AA has some advantages over the CA, including less intraoperative blood loss, fewer requirements for transfusion, shortened operation time, lower hospital mortality, and better disease-free survival (DFS) or overall survival (OS) following right hepatectomy for HCC ≥ 5 cm[5]. However, using the AA, it is difficult to control the branches of the middle hepatic vein at the deeper parenchymal transection, thereby increasing the risk of major vessel injury, especially to the hepatic veins and inferior vena cava[6].
Our initial experience using the AA in a group of patients with large benign or malignant right-lobe liver tumors showed that it was a safe and effective option for selected patients undergoing right hepatectomy. Some prospective randomized controlled trials (RCTs) and retrospective controlled clinical trials (CCTs) documented the clinical outcomes of AA compared to CA for right hepatectomy; however, the clinical significance of the AA over the CA remains unclear.
A recent systematic review evaluated the feasibility, safety, and efficacy of CA vs AA right hepatectomy[7], but data regarding the operative and survival outcomes of patients undergoing surgery are insufficient. A comprehensive systematic review and meta-analysis of the AA over the CA to right hepatectomy in patients with right-lobe large HCC has not been published to date. Many questions on the AA remain unanswered, most notably its clinical and oncologic outcomes and long-term survival. Therefore, the current study aimed to perform a comprehensive systematic review of all available studies to evaluate the safety, feasibility, and effectiveness of AA vs CA right hepatectomy using a meta-analytical method.
Here we acquire evidence through four steps: data sourcing and searches, application of inclusion and exclusion criteria, data extraction, and quality assessment and statistical analysis. We followed the systematic review methods of the Institute of Medicine’s Standards for Systematic Reviews[8] with slight modifications. Our study results are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses[9] and Meta-Analysis of Observational Studies in Epidemiology[10] standards.
An electronic search was performed of and relevant publications from January 2000 to May 2017 that were identified in Pubmed, Embase, and the Cochrane Library. The search was not restricted by language, region, or publication type. The search terms were (“anterior approach right hepatectomy” or “conventional approach right hepatectomy” or “anterior approach right hepatic resection” or “conventional approach right hepatic resection”) and (“liver cancer” or “liver tumor” or “hepatocellular carcinoma” or “liver neoplasms”). Related terms were used to broaden the search, and the computer search was supplemented with manual searches of the reference lists of all retrieved studies. When multiple similar studies describing the same population were identified, the most recent or complete study was included.
The included studies met the following criteria: (1) Comparing AA and CA right hepatectomy; (2) Patients underwent planned selective right-lobe hepatic resection of a large liver tumor; (3) Prospective RCT or retrospective CCT; (4) Adult patients (age ≥ 18 years) who underwent right-lobe hepatic resection; (5) Primary or metastatic large liver tumors; and (6) Reporting at least one of the quantitative outcomes mentioned in these studies.
The excluded studies met the following criteria: (1) Non-comparative or irrelevant to the subject; (2) Lacking a comparison group of CA right hepatectomy; (3) Patients had distant metastases or malignancies in other organs; (4) Left-lobe large hepatocarcinoma resection or minor liver resection; (5) Non-adult patients (age ≤ 18 years) who underwent right hepatectomy; (6) Duplicate publications, editorials, meeting abstracts, letters to the editor, review articles, case reports, and animal experimental studies; and (7) Studies that included no extractable data.
Studies that met all the inclusion criteria were retrieved as full-text articles. Data from the included studies were extracted and summarized independently by two authors (Tang JX and Weng RH). Any disagreement was resolved by the senior author (Jiang N).
The primary outcomes were intraoperative blood loss, massive blood loss, intraoperative blood transfusion, operative time, mortality, morbidity, overall survival, disease-free survival, and recurrence. Recurrence was subdivided into extrahepatic recurrence, intrahepatic recurrence, and extrahepatic plus intrahepatic recurrence. The secondary outcomes were hospital stay, R0 resection rate, bile leakage, and liver failure.
Two review authors (Tang JX and Li JJ) independently assessed the methodological quality of the studies. The methodological quality of the RCTs was assessed using the Jadad score[11], with a cumulative score ≥ 3 indicating high quality. The methodological quality of the retrospective nonrandomized studies was assessed using the modified Newcastle-Ottawa scale[12], which consists of three elements: patient selection, comparability of the study groups, and outcome assessment. A score of 0-9 (allocated as stars) was allocated to all included studies (supplementary Table 1). RCTs and nonrandomized studies achieving six or more stars were considered of high quality.
All included studies were rated at the level of evidence according to criteria provided by the Centre for Evidence-Based Medicine in Oxford, United Kingdom. The meta-analyses were performed using Review Manager 5.0 (Cochrane Collaboration, Oxford, United Kingdom) and Stata 12.0 (StataCorp, College Station, TX, United States). The weighted mean difference (WMD) was used to compare continuous variables, while odds ratio (OR) was used to compare dichotomous variables. We extracted hazard ratio (HR) with 95% CI from the publications as a relevant measure for the effects of overall survival and disease-free survival. We estimated the HR using log-rank χ2 statistics, log-rank P values, the given numbers of events, or Kaplan-Meier curves as described by Parmar et al and Williamson et al[13,14]. We calculated the standard deviations of continuous data presented as means and ranges using the technique described by Hozo et al[15]. The results are reported with 95%CI. Statistical heterogeneity between the included studies was evaluated using the Q measure for statistical significance and the I2 measure for quantifying heterogeneity, with values of P < 0.1 considered statistically significant and I2 > 50% indicating substantial heterogeneity. The random-effects model was used in cases of interstudy heterogeneity; otherwise, the fixed-effects model was used[16]. A sensitivity analysis was performed of the high-quality studies. Funnel plots were used to screen for potential publication bias.
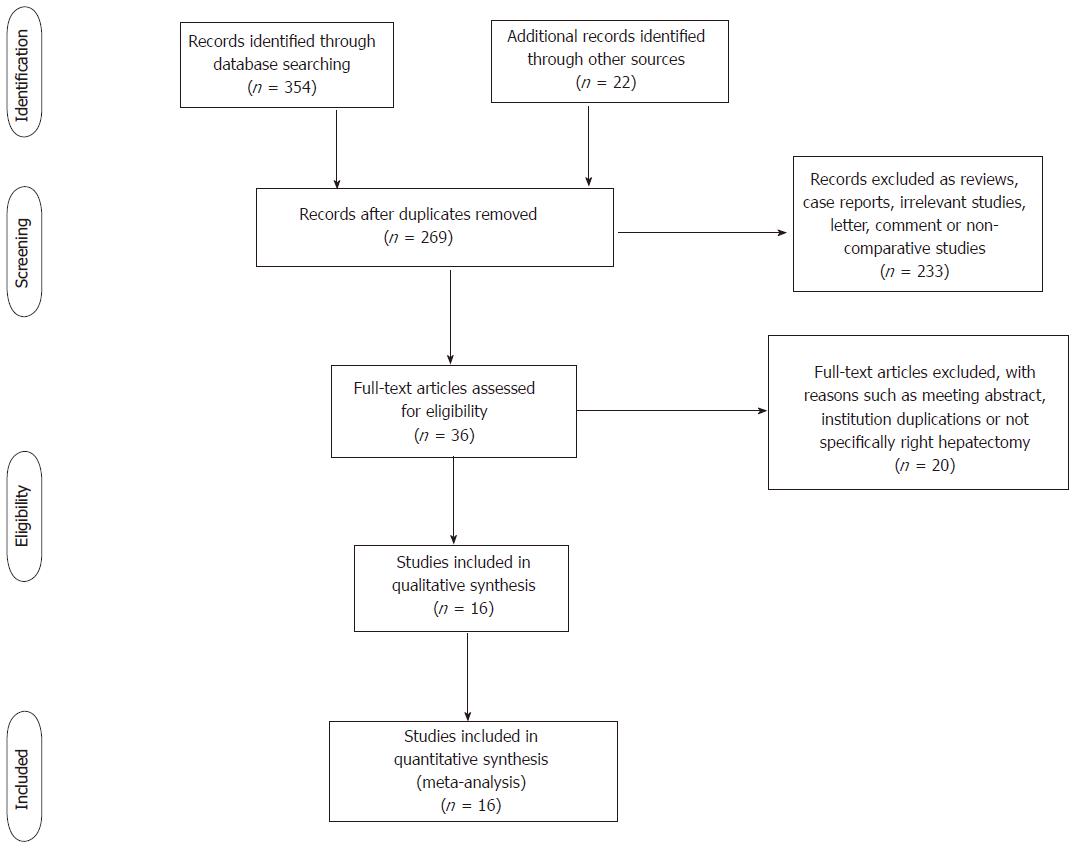
The initial search revealed 376 studies. After the title and abstract screening process, 36 studies were considered potentially useful for inclusion. We then retrieved and reviewed their full text; 20 of these 36 studies were ultimately excluded from the meta-analysis because they were meeting abstracts, not specifically about right hepatectomy, institution duplications, or had no extractable date. Finally, 16 studies fulfilled the predefined inclusion criteria and were included in the final analysis (Figure 1). The included studies were published from 2000 to 2017. All publications were full-text articles; we reviewed the reference list of each to identify additional possible studies for inclusion. Agreement between the two reviewers (Tang JX and Weng RH) was 94% for study selection and quality assessment.
The characteristics of the included studies are shown in Table 1. Among them, there were three RCTs[5,6,17] and thirteen CCTs[3,5,17-29] including a total of 2297 patients (AA = 1076; CA = 1221). All of the patients in the studies underwent right hepatectomy or extended right hepatectomy. Eight studies described simple right hepatectomy[5,6,20,23-26,28], while another eight studies detailed a mixture of right hepatectomy for large liver right-lobe tumors[3,17-19,21,22,27,29].
| Study | Design | Level of evidence | Indication | Indications | Characteristic matching1 | Follow-up, mean or median, ARH/CRH | Quality score | |
| Patients (n) | ||||||||
| ARH | CRH | |||||||
| Beppu et al[18] | R | 3b | MP | 72 | 72 | 1, 2, 5, 6, 7, 8, 9, 10 | 27.2 ± 2.1/18.1 ± 2.8 | ******* |
| Capussotti et al[6] | RCT | 2b | RH | 33 | 32 | 1, 2, 3, 4, 5, 7, 9, 10, 11, 12 | NA | RCT |
| Chan et al[19] | R | 3b | MP | 110 | 169 | 1, 2, 5, 6, 7, 8, 9, 12 | 60/60 | ****** |
| Chen et al[20] | RP | 3b | RH | 11 | 13 | 1, 2, 5, 6, 8, 12 | NA | ***** |
| Cresswell et al[21] | RP | 3b | MP | 62 | 62 | 1, 2, 4, 7, 11 | NA | ***** |
| Habib et al[22] | RP | 3b | MP | 242 | 169 | 1, 2, 4, 7, 10, 11 | 30 ± 20.3 | ****** |
| Hao et al[23] | P | 3b | RH | 107 | 111 | 1, 2, 4, 5, 6, 7, 8, 9, 10 | 49/38 | ******* |
| Higuchi et al[24] | R | 4 | RH | 25 | 44 | 1, 2, 3, 5, 9, 12 | NA | ***** |
| Jabir et al[25] | R | 3b | RH | 40 | 98 | 1, 2, 5, 6, 7, 9, 11 | 36 ± 21.5 | ****** |
| Li et al[26] | R | 3b | RH | 92 | 96 | 1, 2, 5, 6, 7, 8, 9 | 29 ± 7.8 | ******* |
| Liu et al[27] | R | 3b | MP | 54 | 106 | 1, 2, 5, 6, 7, 8, 9, 12 | 59.7/18.6 | ******* |
| Liu et al[27] | RCT | 1b | RH | 60 | 60 | 1, 2, 5, 6, 7, 8, 9, 10, 11, 12 | 21.6 ± 8.0/18.3 ± 5.4 | RCT |
| Llado et al[28] | P | 3b | RH | 33 | 33 | 1, 2, 6, 7, 9, 10 | 24/24 | ****** |
| Takács et al[29] | R | 3b | MP | 52 | 67 | 1, 2, 7, 9, 10, 11 | 32/32 | ***** |
| Wu et al[3] | R | 3b | MP | 33 | 38 | 1, 2, 5, 6, 7, 9, 11, 12 | 19 ± 12.7 | ******* |
| Zhou et al[17] | RCT | 2b | MP | 50 | 51 | 1, 2, 5, 6, 7, 8, 9, 10, 11 | NA | RCT |
The objective quality of the included studies was generally high. True randomization was used in three RCTs[5,6,17]. Most of the retrospective CCTs adopted an appropriate protocol for treatment assignment, and allocation was usually at the physician’s discretion. However, no studies provided information about allocation concealment or blinding method. Matching criteria between the groups were variable (Table 1). Methods for managing missing data and intention to treat right-lobe hepatectomy analyses were generally adequate among the majority of studies. Eleven studies[3,5,18,19,22,23,25-29] mentioned the length of follow-up, and most provided accurate data.
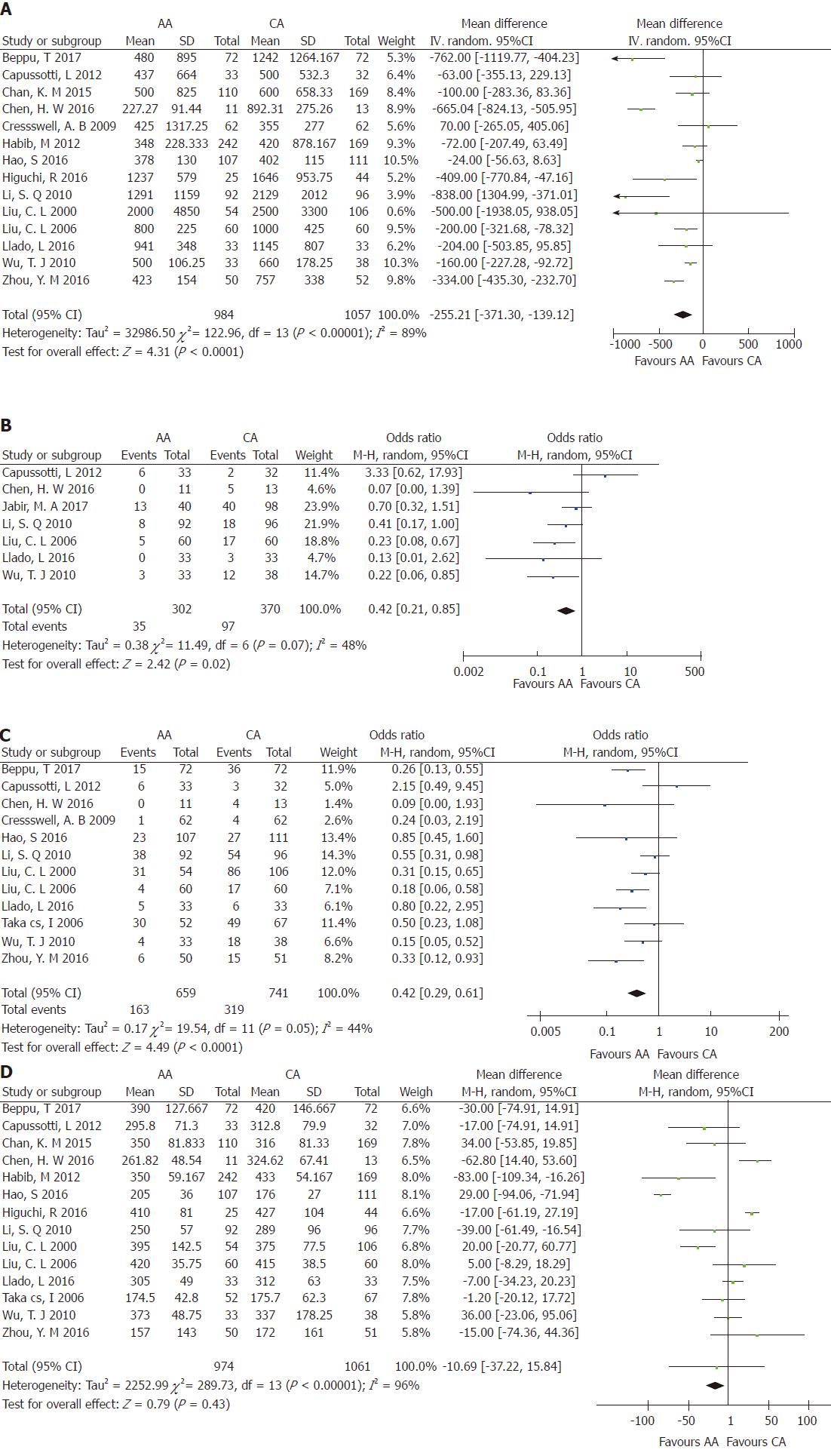
Intraoperative blood loss: Aggregation of the data from 14 studies[3,5,6,17-24,26-28] revealed that 2041 of the patients who underwent right hepatectomy (AA = 984; CA = 1057) experienced intraoperative blood loss. There was significant heterogeneity among the studies (χ2 = 122.96; I2 = 89%; P < 0.00001). The pooled data showed that intraoperative blood loss was significant lower in the AA group than in the CA group (WMD = -255.21; 95%CI: -371.30 to -139.12; P < 0.0001) (Figure 2A).
Massive blood loss: Seven studies[3,5,6,20,25,26,28] including 672 patients reported massive blood loss > 1 L (AA = 302; CA = 370). There was significant heterogeneity among the studies (χ2 = 11.49; I2 = 48%; P = 0.07) (Figure 2B). Meta-analysis using a random-effects model revealed that the OR of massive blood loss differed significantly between the two groups (OR = 0.42; 95%CI: 0.21-0.85; P = 0.02).
Intraoperative blood transfusion: Data concerning intraoperative blood transfusion were available in three RCTs[5,6,17] and nine CCTs[3,18,20,21,23,26-29] including 1400 patients who underwent large right-lobe hepatic cancer resection (AA = 659; CA = 741). Meta-analysis using a random-effects model revealed a significant decrease in blood transfusions in the AA group than in the CA group (OR = 0.42; 95%CI: 0.29-0.61; P < 0.00001) (Figure 2C).
Operative time: Fourteen studies[3,5,6,17-20,22-24,26-29] including 2035 patients who underwent right hepatectomy (AA = 974; CA = 1061) reported operative time. There was significant heterogeneity among the studies (χ2 = 289.73; I2 = 96%; P < 0.00001). A meta-analysis indicated no significant difference in operative time between the two groups (WMD = -10.69; 95%CI: -37.22-15.87; P = 0.43) (Figure 2D).
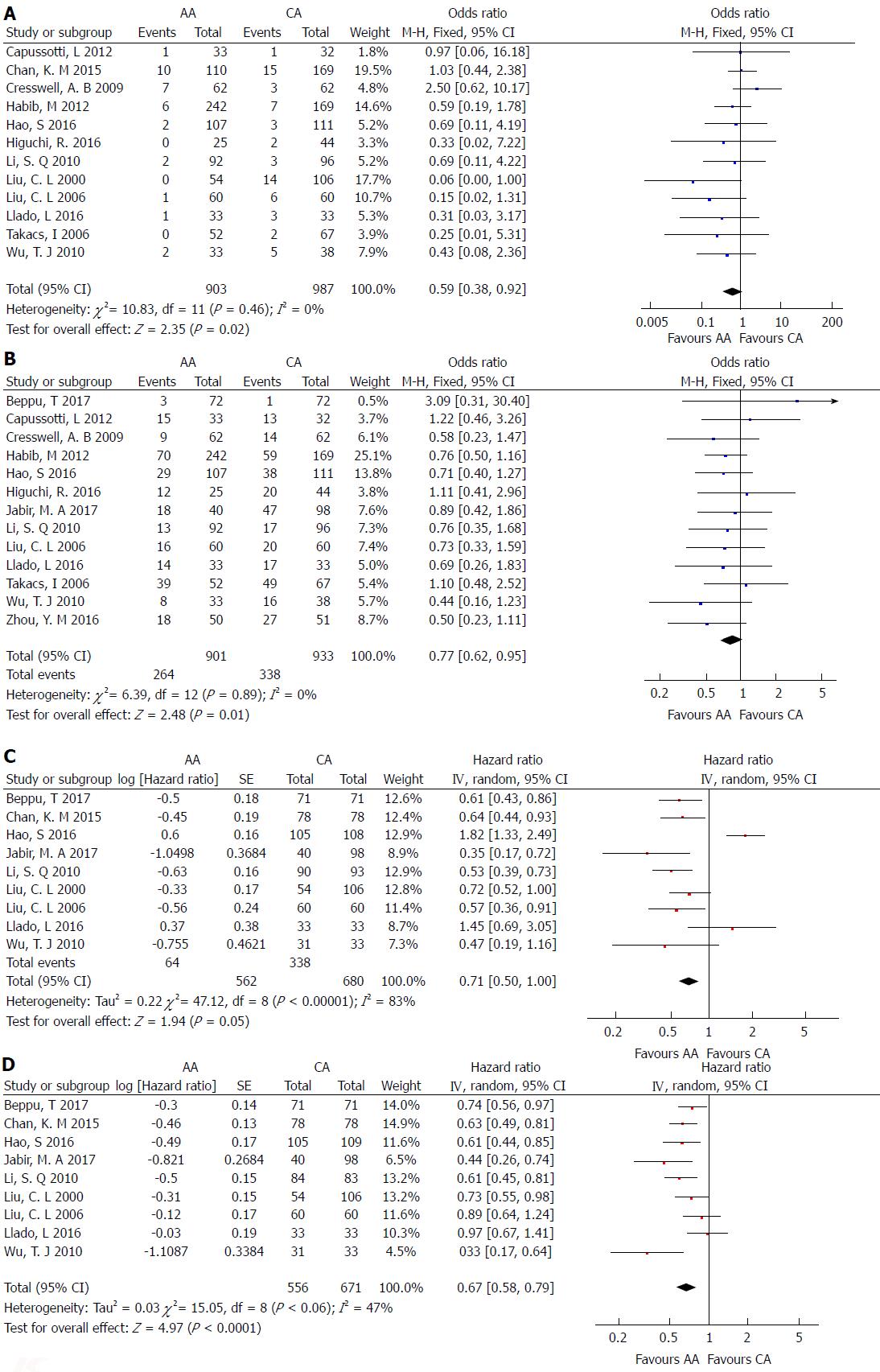
Mortality: Twelve studies[3,5,6,19,21-24,26-29] including 1890 patients who underwent right hepatectomy for large liver tumors evaluated hospital mortality rate. The mortality rate was 3.54% (32/903 patients) in the AA group and 6.48% (64/987 patients) in the CA group. There was no significant heterogeneity among the studies (χ2 = 10.83; I2 = 0%; P = 0.46) (Figure 3A). Using a fixed-effects model, the pooled data showed that mortality rate in the AA group was significantly lower than that in the CA group (OR = 0.59; 95%CI: 0.38-0.92; P = 0.02).
Morbidity: Thirteen studies[3,5,6,17,18,21-26,28,29] including 1834 patients who underwent major right hepatectomy reported operative morbidity events. The overall morbidity rate was 29.30% (264/901 patients) in the AA group and 36.23% (338/933 patients) in the CA group. The operative morbidity rate of the AA group was significantly lower than that of the CA group (OR = 0.77; 95%CI, 0.62-0.95; P = 0.01) (Figure 3B).
Overall survival: One prospective RCT[5] and eight retrospective CCTs[3,18,19,23,25-28] reported overall survival events, including 1242 patients (AA = 562; CA = 680). There was significant heterogeneity among the studies (χ2 = 47.12; I2 = 83%; P < 0.00001). Meta-analysis using a random-effects model revealed that there was a significant increase in overall survival following the AA right hepatectomy in comparison with the CA right hepatectomy (HR = 0.71; 95%CI: 0.50-1.00; P = 0.05) (Figure 3C).
Disease-free survival: One prospective RCT[5] and eight retrospective CCTs[3,18,19,23,25-28] including 1227 patients (AA = 556; CA = 671) reported disease-free survival events. There was little significant heterogeneity among the studies (χ2 = 15.05; I2 = 47%; P = 0.06). Meta-analysis using a random-effects model showed that there was a significant increase in disease-free survival after AA right hepatectomy than after CA right hepatectomy (HR = 0.67; 95%CI: 0.58-0.79; P < 0.00001) (Figure 3D).
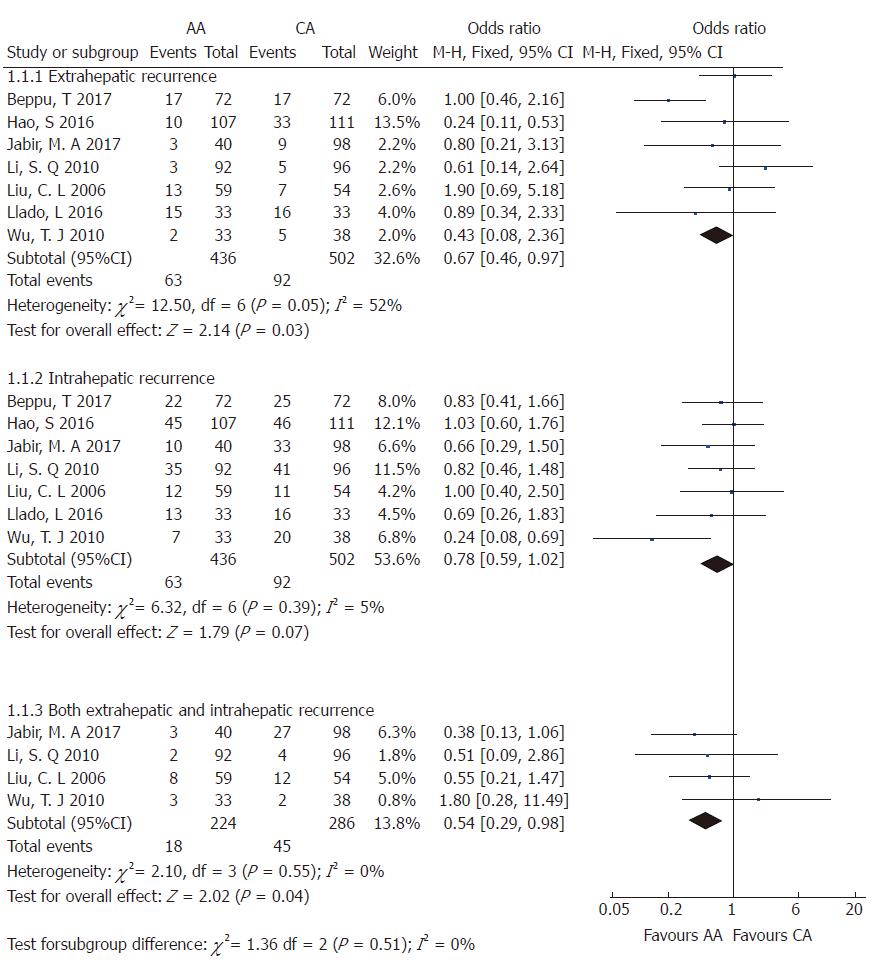
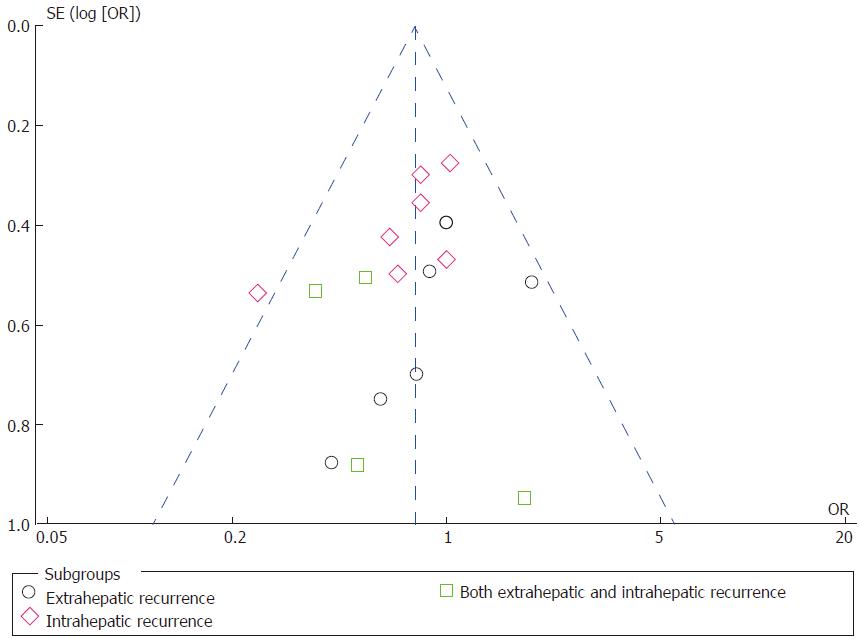
Tumor recurrence: Tumor recurrence rate was available for one prospective RCT[5] and eight retrospective CCTs[3,18,19,22,23,25,26,28] including 1682 patients who underwent right hepatectomy (AA = 788; CA = 840). The total recurrence rate was 47.21% (372/788 patients) in the AA group and 61.19% (514/840 patients) in the CA group. There was no significant heterogeneity among the studies (χ2 = 13.82; I2 = 42%; P = 0.09). Meta-analysis using a random-effects model showed that there was a significant decrease in tumor recurrence rate following the AA right hepatectomy in comparison with the CA right hepatectomy (OR = 0.62; 95%CI: 0.47-0.83; P = 0.001) (Supplementary Figure 1). Then, patients were divided into three subgroups based on the recurrence location, including intrahepatic, extrahepatic, or both intrahepatic and extrahepatic tumor recurrence. There was no significant heterogeneity among the three subgroups (Figure 4). Using a fixed-effects model, the data indicated that the AA group had significantly less extrahepatic or both intrahepatic and extrahepatic recurrence than the CA group (OR = 0.67; 95%CI: 0.46-0.97; P = 0.03; and OR = 0.54; 95%CI: 0.29-0.98; P = 0.04, respectively), but there was no significant intergroup difference in intrahepatic recurrence (OR = 0.78; 95%CI: 0.59-1.02; P = 0.07).
Hospital stay and R0 resection rate: Eleven studies[3,5,6,17,19,20,22,24,26,28,29] including 1513 patients who underwent right hepatectomy reported the length of hospital stay. The length of hospital stay was significantly shorter in the AA group than in the CA group (WMD = -1.13; 95%CI: -1.69 to -0.58; P < 0.0001). Six studies[18,21,22,24,25,27] including 1208 patients who underwent right hepatectomy reported the R0 resection rate. There was little significant heterogeneity among the studies (χ2 = 13.32; I2 = 62%; P = 0.02). Using a random-effects model, a meta-analysis indicated no significant difference in R0 resection rate between the AA and CA groups (OR = 1.10; 95%CI: 0.57-2.14; P = 0.78) (Supplementary Figure 2).
Bile leak and liver failure: Six studies[5,21,22,27-29] with a total of 1000 patients who underwent right hepatectomy for large liver rumor (AA = 503; CA = 497) reported a comparative incidence of bile leak. Using a fixed-effects model, a meta-analysis indicated no significant difference in bile leak after right hepatectomy surgery between the AA and CA groups (OR = 0.48; 95%CI: 0.19-1.19; P = 0.11) (Supplementary Figure 2). Many studies did not provide the postoperative outcomes of liver failure. Therefore, we did a meta-analysis, including only four studies[5,6,26,27], to assess postoperative liver failure in 533 patients who underwent right hepatectomy due to large liver tumor. Analysis using a fixed-effects model revealed a lower rate of liver failure in the AA group than in the CA group, but this difference was not statistically significant (OR = 0.50; 95%CI: 0.21-1.20; P = 0.12).
Three RCTs[5,6,17] and nine CCTs[3,5,17-19,22,23,25-28] that scored six or more stars on the modified Newcastle-Ottawa scale were included in the sensitivity analysis (Supplementary Table 2). There was no change in the significant differences of all outcomes compared with the original outcomes; however, the degree of study heterogeneity was decreased in terms of massive blood loss, mortality, hospital stay, and R0 resection rate.
Funnel plots of the studies included in this meta-analysis reported postoperative outcomes such as perioperative blood transfusion, mortality, disease-free survival, and tumor recurrence (Figure 5). All studies were inside the 95%CI and evenly distributed around the vertical axis, indicating no obvious publication bias.
Hepatectomy for large right HCC is associated with significant operative morbidity and mortality and remains a major surgical challenge, especially when underlying liver cirrhosis is present[30,31]. With regard to the CA, operative complications may arise during the difficult mobilization of the right lobe of the liver, leading to unfavorable surgical outcomes including excessive intraoperative blood loss, tumor rupture, and the spillage of cancer cells into the systemic circulation[5]. The AA was first described by Ozawa et al[32] as a “nonconventional approach” to advanced liver cancer that attempts to avoid prolonged rotation and displacement of the hepatic lobes, which can lead to impairment of the afferent and efferent circulation.
In the current report, the AA, as a “no-touch” technique, was shown to result in favorable surgical and long-term survival outcomes compared to the CA in patients who underwent right hepatectomy for large HCC. The AA runs a risk of massive bleeding from the right or middle hepatic vein at the deeper plane of the parenchymal transection that is often uncontrollable and life-threatening. However, Belghiti et al[33] designed a liver hanging maneuver (LHM) using a tape inserted between the anterior surface of the vena cava and the liver and combined it with AA in 2001. The beneficial effects of this technique have been demonstrated and include better control of bleeding, protection of the inferior vena cava, good exposure during deeper parenchymal dissection, and guidelines for transection direction[34]. Besides, Chen et al reported a five-step stapling technique for right hepatectomy using the AA with the LHM for patients with HCC and liver cirrhosis that resulted in less intraoperative blood loss and significantly shorter parenchymal transection time. Nonetheless, retrograde bleeding is sometimes difficult to control during right hepatectomy for large HCC, especially in patients with liver cirrhosis and portal hypertension.
The long-term outcome after surgery remains unsatisfactory, although hepatectomy is an effective method for treating HCC. The 5-year recurrence rate of HCC after surgery reportedly exceeded 60%-80%[35], which represents a major factor for long-term outcomes. HCC recurrence is a complex process that involves many clinical and pathological factors. Moreover, the no-touch concept is an important principle in surgical oncology[36]. However, it is very difficult to follow it in conventional hepatectomy due to the special anatomical structures of the liver. Moreover, HCC exhibits strong vascular invasion[37]. Liver tumor cells are more easily spread through the portal vein or hepatic vein during right hepatectomy surgery. Hao et al[23] reported that macro- and microvascular invasion, blood transfusion, and the CA of hepatectomy were independent risk factors for HCC recurrence on multivariate analysis. Moreover, excessive blood loss and blood transfusion have been associated with increased morbidity and mortality as well as poorer DFS and OS after right hepatic resection[38,39]. Perioperative transfusion has also been found to promote HCC recurrence after hepatic resection. Technical innovations have mainly focused on minimizing bleeding during hepatic parenchymal transection. Various devices have been developed to promote liver transection and reduce blood loss in right hepatectomy. However, none has proven superiority compared with previous techniques[40]. Therefore, we systematically summarized related studies using a meta-analysis to assess the safety and efficacy of AA and CA for right hepatectomy. In our analysis, the AA was associated with less intraoperative blood loss or massive blood loss, fewer transfusion requirements, lower mortality or morbidity, and less recurrence after right hepatectomy; otherwise, it was associated with longer OS and DFS. Besides, the incidences of extrahepatic or both extrahepatic and intrahepatic tumor recurrence were higher in cases using the CA than those using the AA, and this result seems to support the proposal that excessive blood loss and blood transfusions were associated with increased tumor recurrence as well as poorer DFS and OS after right hepatic resection. Our meta-analysis results indicated that the AA results in favorable surgical and long-term survival outcomes compared to the CA in patients who underwent right hepatectomy for large HCC. The better outcome achieved in the AA group might have been a result of using the no-touch technique, which fulfills the oncological principles of surgical resection.
From the surgical perspective, the AA can prevent complications related to mobilization of the right liver before parenchymal transection. In particular, mobilization of the right liver might be difficult in patients with a large HCC due to limited space, and the surgeon is likely to encounter excessive bleeding or iatrogenic tumor rupture, expansion of liver resection, and a risk of squeezing cancer cells into the blood circulation system[19]. Consequently, the rates of morbidity and operative complications, including bile leak, liver failure, and bleeding, were theoretically low in the AA group. However, data mentioning bile leak or liver failure were available in two RCTs and four CCTs. Our result indicated no significant difference in bile leak or liver failure after right hepatectomy surgery between the AA and CA groups. A few articles have reported on the incidence of intraoperative iatrogenic tumor rupture during mobilization of the right lobe of the liver. Only one clinical study by Liu et al[27] reported that the incidence of tumor rupture appeared to be higher in the CA group (seven patients, 6.6%) than in the AA group (one patient, 1.9%), although the difference was not significant (P = 0.268). However, one RCT and one clinical study[3,5] showed that the rates of tumor rupture were similar between the AA and CA groups. Therefore, additional RCTs with large samples are needed to resolve this conflict.
The AA has a potential advantage of liver function preservation since it does not require twisting the portal pedicle during right liver mobilization as in the CA. This advantage is consistent with the suggestion by Ozawa that the AA could contribute to better preservation of postoperative liver function by avoiding prolonged rotation during right hepatectomy[32]. The results of one CCT study by Capussotti et al[6] showed no difference in postoperative liver function tests such as serum transaminases, bilirubin and prothrombin time, which are considered the barometers of hepatocytic damage[41]. However, it is difficult to conduct an intensive credibility analysis since not all of the included studies reported detailed information about liver function. Whether there is a significant difference between the two approaches should be elucidated in the future.
Safety should be prioritized when selecting a surgical approach. Although the theoretical advantages of the AA over the CA are well established, right hepatectomy for large HCC using AA with or without the LHM remains a technically demanding method, making numerous surgeons reluctant to perform this approach. In addition, others see that the CA has the advantage of preventing critical bleeding during liver transection, while the AA can be an effective alternative when difficulty is encountered during liver mobilization. In our analysis, the AA technique used for right hepatectomy was associated with less intraoperative blood loss, fewer cases of massive blood loss, fewer transfusion requirements, and lower mortality or morbidity. Our meta-analysis results indicated that AA is a safe and effective technique for right hepatectomy for large HCC.
The limitations of this meta-analysis are as follows. The primary limitation is that most of the included studies were retrospective CCTs, evidence for which may be less feasible, except for three RCTs. Therefore, a meta-analysis of several RCTs would be perfect, but the limited number of RCTs prevented us from drawing a definitive conclusion. Besides, inter-study heterogeneity was significant for outcomes including intraoperative blood loss, massive blood loss, intraoperative blood transfusion, operative time, overall survival, disease-free survival, and tumor recurrence. Hence, we processed data using the random-effects model since it might reduce the effect of heterogeneity but does not abolish it. Finally, most studies lacked some available data about intraoperative and postoperative outcomes or insufficient data on factors such as tumor rupture and liver function. Therefore, studies with comprehensive and sufficient data and more RCTs are needed to resolve this limitation.
This meta-analysis was conducted at an appropriate time since sufficient data have accumulated for research, and right hepatectomy for large liver tumor using the AA or the CA is still a hot topic. In our analysis, patients who underwent right hepatectomy in the AA group had less intraoperative blood loss; less frequent massive blood loss; reduced transfusion requirements and hospital stay; lower mortality, morbidity and recurrence, and better OS and DFS than those in the CA group. However, there are no advantages of the AA over the CA regarding operative time, intrahepatic tumor recurrence, R0 resection rate, bile leak, or liver failure. In summary, the AA is a safe, feasible, and effective technique for right hepatectomy for large liver tumor that could accelerate postoperative recovery and achieve better long-term survival outcomes than the CA.
Conventional right hepatectomy (CRH), which is complete mobilization of the right liver with the right hepatic vein controlled outside the liver before parenchymal transection, has been used as the standard procedure. Anterior approach (AA) has been suggested as an alternative approach to conventional approach (CA) for right hepatectomy in recent years. However, comparative studies have shown conflicting results.
Some studies have compared AA and CA to evaluate their safety and efficacy in right hepatectomy for large hepatocellular carcinoma (HCC). Recently, no meta-analysis of the safety, clinical outcome and survival after AA right hepatectomy for HCC compared with the CA was published. Besides, in our article, several conclusions might be used to guide future clinical practice.
To evaluate whether right hepatectomy using the AA for large hepatocellular carcinoma results in better clinical outcomes when compared with the CA, and the safety, efficacy and clinical outcome of the two approaches.
We comprehensively performed an electronic search of PubMed, EMBASE and the Cochrane Library that published between January 2000 and May 2017 for randomized controlled trials (RCTs) or clinical controlled trials (CCTs) concerning using AA and CA in right hepatectomy. Studies that met the inclusion criteria were included, and their outcomes analysis were further assessed using either a fixed or a random effects model.
The analysis included 2297 patients enrolled in 16 studies (3 RCTs and 13 CTTs). Intraoperative blood loss, intraoperative blood transfusion, mortality, morbidity, and recurrence rate were significantly reduced in AA group. Besides, patients in the AA group had better overall survival and disease-free survival than those in the CA group.
The AA is a safe and effective technique for right hepatectomy for large HCC, and it could accelerate postoperative recovery and achieve more advantageous survival over the CA. AA can be an effective alternative when difficulty is encountered during liver mobilization and reduce the risk of bleeding.
| 1. | Fortner JG, Kim DK, Maclean BJ, Barrett MK, Iwatsuki S, Turnbull AD, Howland WS, Beattie EJ Jr. Major hepatic resection for neoplasia: personal experience in 108 patients. Ann Surg. 1978;188:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 132] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Starzl TE, Bell RH, Beart RW, Putnam CW. Hepatic trisegmentectomy and other liver resections. Surg Gynecol Obstet. 1975;141:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 3. | Wu TJ, Wang F, Lin YS, Chan KM, Yu MC, Lee WC. Right hepatectomy by the anterior method with liver hanging versus conventional approach for large hepatocellular carcinomas. Br J Surg. 2010;97:1070-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 4. | Lai EC, Fan ST, Lo CM, Chu KM, Liu CL. Anterior approach for difficult major right hepatectomy. World J Surg. 1996;20:314-317; discussion 318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006;244:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Capussotti L, Ferrero A, Russolillo N, Langella S, Lo Tesoriere R, Viganò L. Routine anterior approach during right hepatectomy: results of a prospective randomised controlled trial. J Gastrointest Surg. 2012;16:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Li L, Wang HQ, Wang Q, Yang J, Yang JY. Anterior vs conventional approach hepatectomy for large liver cancer: a meta-analysis. World J Gastroenterol. 2014;20:17235-17243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 8. | Institute of Medicine (US) Committee on Standards for Systematic Reviews of Comparative Effectiveness Research, Eden J, Levit L, Berg A, Morton S. Washington (DC): National Academies Press (US); 2011. . [PubMed] |
| 9. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9207] [Cited by in RCA: 8357] [Article Influence: 522.3] [Reference Citation Analysis (2)] |
| 10. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 17246] [Article Influence: 663.3] [Reference Citation Analysis (0)] |
| 11. | Jadad AR, Enkin MW. Computers: transcending our limits? BMJ. 2007;334 Suppl 1:s8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (2)] |
| 12. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 13618] [Article Influence: 851.1] [Reference Citation Analysis (1)] |
| 13. | Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815-2834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 14. | Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21:3337-3351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 448] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 15. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 7282] [Article Influence: 346.8] [Reference Citation Analysis (1)] |
| 16. | Higgins JP, Green S. Cochrane Handbook For Systematic Reviews Of Interventions Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie 2009; 2011: S38. . [RCA] [DOI] [Full Text] [Cited by in Crossref: 8311] [Cited by in RCA: 6805] [Article Influence: 141.8] [Reference Citation Analysis (4)] |
| 17. | Zhou YM, Sui CJ, Zhang XF, Li B, Yang JM. Anterior approach combined with infrahepatic inferior vena cava clamping right hepatic resection for large hepatocellular carcinoma: A prospective randomized controlled trial. Medicine (Baltimore). 2016;95:e4159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Beppu T, Imai K, Okuda K, Eguchi S, Kitahara K, Taniai N, Ueno S, Shirabe K, Ohta M, Kondo K. Anterior approach for right hepatectomy with hanging maneuver for hepatocellular carcinoma: a multi-institutional propensity score-matching study. J Hepatobiliary Pancreat Sci. 2017;24:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Chan KM, Wang YC, Wu TH, Lee CF, Wu TJ, Chou HS, Yu MC, Lee WC. The Preference for Anterior Approach Major Hepatectomy: Experience Over 3 Decades and a Propensity Score-Matching Analysis in Right Hepatectomy for Hepatocellular Carcinoma. Medicine. (Baltimore). 2015;94:e1385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Chen HW, Lai EC, Wang FJ, Li JY, Deng FW, Hu JY, Lau WY. Anterior approach for right hepatectomy using the 5-steps stapling technique: A preliminary study. Int J Surg. 2016;32:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Cresswell AB, Welsh FK, John TG, Rees M. Evaluation of intrahepatic, extra-Glissonian stapling of the right porta hepatis vs. classical extrahepatic dissection during right hepatectomy. HPB (Oxford). 2009;11:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Habib M, Cresswell AB, Chandrakumaran K, Welsh FK, John TG, Rees M. Extrahepatic versus intrahepatic hilar control for right hepatectomy: an updated experience. Dig Surg. 2012;29:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Hao S, Fan P, Chen S, Tu C, Wan C. Anterior approach to improve the long-term outcome in patients with large-size hepatocellular carcinoma having liver resection. J Surg Oncol. 2016;114:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Higuchi R, Yazawa T, Uemura S, Yamamoto M. Anterior approach for perihilar cholangiocarcinoma (with video). J Surg Res. 2016;202:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Jabir MA, Hamza HM, Fakhry H, Amira G, Hatano E, Uemoto S. Anterior Versus Conventional Approach for Resection of Large Right Lobe Hepatocellular Carcinoma. J Gastrointest Cancer. 2017;48:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Li SQ, Liang LJ, Peng BG, Yin XY, Lü MD, Kuang M, Li DM, Fu SJ. [A comparative study of anterior versus conventional approach right hepatectomy for large hepatocellular carcinoma]. Zhonghua Yi Xue Za Zhi. 2010;90:1670-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Liu CL, Fan ST, Lo CM, Tung-Ping Poon R, Wong J. Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg. 2000;232:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 28. | Llado L, Muñoz A, Ramos E, Torras J, Fabregat J, Rafecas A. The anterior hanging-approach improves postoperative course after right hepatectomy in patients with colorectal liver metastases. Results of a prospective study with propensity-score matching comparison. Eur J Surg Oncol. 2016;42:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Takács I, Furka A, Kotán R, Boland Mehrdad G, Pósán J, Vágvölgyi A, Hallay J, Sápy P. [Anterior approach for liver resection in the cases of the treatment of large liver tumors]. Magy Seb. 2006;59:362-368. [PubMed] |
| 30. | Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 229] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 31. | Nagasue N, Yukaya H, Kohno H, Chang YC, Nakamura T. Morbidity and mortality after major hepatic resection in cirrhotic patients with hepatocellular carcinoma. HPB Surg. 1988;1:45-56; discussion 56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Ozawa K. Hepatic function and liver resection. J Gastroenterol Hepatol. 1990;5:296-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 33. | Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 360] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 34. | Wang CC, Jawade K, Yap AQ, Concejero AM, Lin CY, Chen CL. Resection of large hepatocellular carcinoma using the combination of liver hanging maneuver and anterior approach. World J Surg. 2010;34:1874-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3630] [Article Influence: 259.3] [Reference Citation Analysis (12)] |
| 36. | Turnbull RB Jr, Kyle K, Watson FR, Spratt J. Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg. 1967;166:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 37. | Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 527] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 38. | Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 471] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 39. | Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Mizuno S, Makuuchi M. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303-309. [PubMed] |
| 40. | Gurusamy KS, Pamecha V, Sharma D, Davidson BR. Techniques for liver parenchymal transection in liver resection. Cochrane Database Syst Rev. 2009;CD006880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Hashimoto N, Niu ZS, Tsoulfas G S- Editor: Wei LJ L- Editor: Wang TQ E- Editor: Ma YJ