Published online Nov 21, 2017. doi: 10.3748/wjg.v23.i43.7735
Peer-review started: August 18, 2017
First decision: August 30, 2017
Revised: September 18, 2017
Accepted: October 17, 2017
Article in press: October 17, 2017
Published online: November 21, 2017
Processing time: 94 Days and 16.6 Hours
To evaluate the safety and efficacy of combined endovascular brachytherapy (EVBT), transarterial chemoembolization (TACE), and sorafenib to treat hepatocellular carcinoma (HCC) patients with main portal vein tumor thrombus (MPVTT).
This single-center retrospective study involved 68 patients with unresectable HCC or those who were unfit for liver transplantation and percutaneous frequency ablation according to the BCLC classification. All patients had Child-Pugh classification grade A or B, Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and MPVTT. The patients received either EVBT with stent placement, TACE, and sorafenib (group A, n = 37), or TACE with sorafenib (group B, n = 31). The time to progression (TTP) and overall survival (OS) were evaluated by propensity score analysis.
In the entire cohort, the 6-, 12-, and 24-mo survival rates were 88.9%, 54.3%, and 14.1% in group A, and 45.8%, 0%, and 0% in group B, respectively (P < 0.001). The median TTP and OS were significantly longer in group A than group B (TTP: 9.0 mo vs 3.4 mo, P < 0.001; OS: 12.3 mo vs 5.2 mo, P < 0.001). In the propensity score-matched cohort, the median OS was longer in group A than in group B (10.3 mo vs 6.0 mo, P < 0.001). Similarly, the median TTP was longer in group A than in group B (9.0 mo vs 3.4 mo, P < 0.001). Multivariate Cox analysis revealed that the EVBT combined with stent placement, TACE, and sorafenib strategy was an independent predictor of favorable OS (HR = 0.18, P < 0.001).
EVBT combined with stent placement, TACE, and sorafenib might be a safe and effective palliative treatment option for MPVTT.
Core tip: As portal vein tumor thrombus occurs in a high proportion of hepatocellular carcinoma patients and no standard treatment has been established, we aimed to evaluate the effect of endovascular brachytherapy (EVBT) combined with stent placement, transarterial chemoembolization (TACE), and sorafenib and compared this strategy with TACE plus sorafenib alone. The results of our study revealed that EVBT along with stent placement, TACE, and sorafenib is a safe and effective palliative treatment option for main portal vein tumor thrombus.
- Citation: Zhang ZH, Liu QX, Zhang W, Ma JQ, Wang JH, Luo JJ, Liu LX, Yan ZP. Combined endovascular brachytherapy, sorafenib, and transarterial chemobolization therapy for hepatocellular carcinoma patients with portal vein tumor thrombus. World J Gastroenterol 2017; 23(43): 7735-7745
- URL: https://www.wjgnet.com/1007-9327/full/v23/i43/7735.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i43.7735
Portal vein tumor thrombus (PVTT) occurs in a substantial proportion of patients with hepatocellular carcinoma (HCC), with 44% of the patients presenting at the time of death and about 10%-40% at the time of diagnosis[1]. In particular, HCC with tumor thrombus in the main portal trunk or the opposite side portal branch represents an end-stage condition with poor prognosis due to malignant hepatic tumor cells occluding the blood flow and deteriorating the portal hypertension[2], with a perioperative mortality rate of 0%-28% and a 5-year overall survival (OS) rate of 0%-26.4%[3]. As main PVTT (MPVTT) is contraindicated to surgical resection and transplantation due to a high tumor recurrence rate, no standard treatment has been established[4]. Three-dimensional conformal radiotherapy (3-DCRT) has shown survival benefits in HCC patients with PVTT[5]. However, blood flow to the obstructed main portal vein (MPV) cannot be restored immediately with radiotherapy alone. Further, PVTT is generally considered a contraindication for TACE due to the interruption of hepatic arterial flow which could result in a large segment of hepatic necrosis in patients whose blood supply is already compromised[6]. Furthermore, tumor thrombus in the MPV could not be effectively controlled by TACE combined with intra-portal stent, leading to a shorter stent patency rate along with increased risk of liver necrosis and treatment-related death[7]. Thus, the use of TACE is limited to only selected group of patients with good hepatic function and adequate collateral circulation around the occluded portal vein[8]. Given the limitation of TACE, transarterial radioembolization (brachytherapy) has emerged as a safer and more effective treatment for HCC with PVTT than TACE[9,10]. Endovascular brachytherapy (EVBT) by interstitial implantation of iodine-125 (125I) seeds has been studied extensively[11-13]. Combined endovascular implantation of 125I seed strand with stent placement and TACE provided long-term survival benefits and increased patency rates of the stent[14,15]. Although sorafenib has been recommended as the first-line treatment for advanced-stage disease (i.e., Barcelona Clinic Liver Cancer (BCLC) stage C with PVTT), the survival outcomes obtained were also only modest[16]. Recent studies have reported survival benefits in patients with PVTT who underwent combination treatments of TACE with sorafenib, radiotherapy with sorafenib, and hepatic arterial infusion chemotherapy with sorafenib[17-19]. A recent study by Huang et al[20] reported survival benefit of chemoembolization plus 125I seed implantation in unresectable hepatitis B-related HCC with PVTT. However, reports of such combined therapeutic strategies aiming at MPVTT are obscure. Endovascular implantation of 125I seeds strand and portal vein stenting followed by TACE combined with sorafenib could improve the progression free survival (PFS) of HCC patients with MPVTT[21]. In the present retrospective study, we aimed to evaluate the safety and efficacy of EVBT combined with stent placement, TACE, and sorafenib compared with TACE with sorafenib in the treatment of HCC patients with MPVTT.
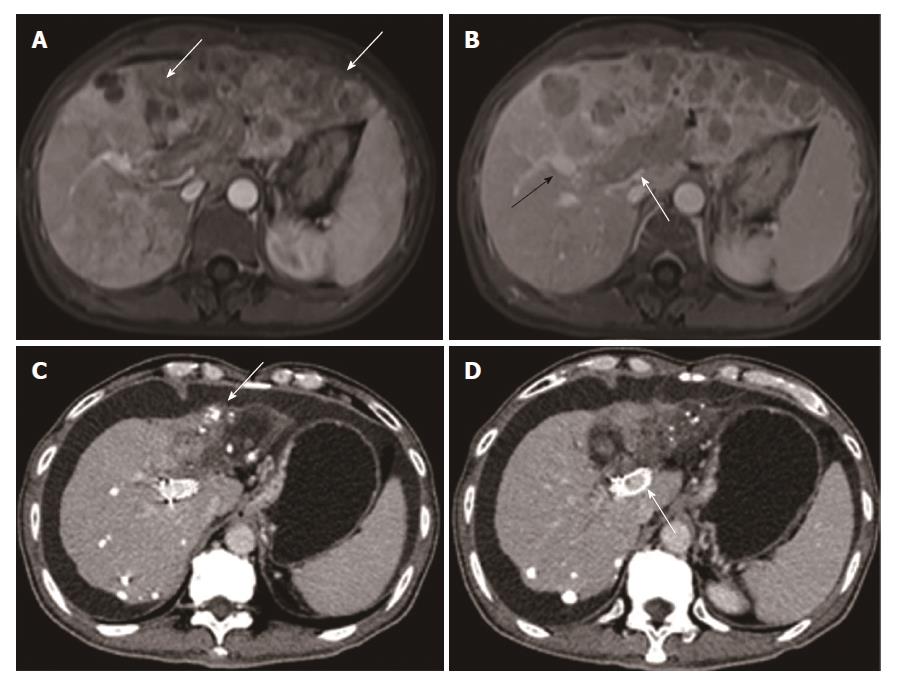
This was a single-center, retrospective study conducted on advanced HCC patients with MPVTT from January 2009 and December 2015. The study protocol was approved by the institutional ethics committee of the respective hospital involved. MPVTT was detected based on the presence of low-attenuation intraluminal mass expanding the main portal vein, and/or filling defects in the main portal vein, as determined by three-phase dynamic computed tomography (Figure 1).
Patients aged between 18-75 years with unresectable HCC or unfit for liver transplantation and percutaneous frequency ablation according to the BCLC classification were included. All patients had Child-Pugh classification grade A or B, Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and MPVTT confirmed by demonstration of tumor thrombus in MPV and HCC in three-phase dynamic CT images 7 d before treatment.
Patients who had undergone surgery, local-regional therapies (radiofrequency ablation, percutaneous ethanol injection, or -125I seed implantation), liver transplantation, previous sorafenib therapy, systemic chemotherapy, intra-arterial chemoinfusion, or TACE; patients with serious medical comorbidities such as encephalopathy and uncorrectable bleeding diathesis; patients who currently had or had a history of malignant tumors in addition to HCC; and those with intrahepatic portal vein completely occluded by HCC, tumor thrombus extending into the superior mesenteric vein (SMV) or splenic vein (SV), advanced liver disease, or contraindication for chemoembolization (HCC burden > 70% of total liver volume or high flow intrahepatic arterial venous shunt) were excluded from the study.
Written informed consent was obtained from all eligible patients who were recommended to choose either combined EVBT-stenting-TACE-sorafenib (group A) or TACE-sorafenib (group B) treatment. TACE-sorafenib was recommended for patients who refused EVBT-stenting-TACE-sorafenib treatment.
Sorafenib treatment: Sorafenib (Nexavar; Bayer, Leverkusen, Germany) was taken for 3 d after the first TACE procedure at a recommended dose of 400 mg twice daily in all patients and with a 3-d interruption after subsequent TACE cycles (30 d).
Stent and iodine-125 seed: Nitinol self-expandable stent (Luminxx III; Bard, Covington, Georgia; diameter: 12-14 mm; length: 60-100 mm) was used. Brachytherapy source was titanium encapsulated model 6711 125I seed (XinKe; Shanghai, China; active length: 3.25 mm), with radioactivity and half-life of each 125I seed of 25.9 MBq and 59.4 d, respectively. The principal photon emissions of X-ray and gamma ray and incipient dose rate were 31.4 keV, 35.5 keV, and 7 cGy/h, respectively. The 240-d accumulated dose at 10 mm from the axis of the 125I seed strand source (Z = 0, r = 10 mm) was calculated with radiation field distribution calculation software (version 0.1, Institute of Radiation Medicine, Fudan University, Shanghai, China) based on the American Association of Physicists in Medicine TG43U1 brachytherapy formalism.
Intra-MPV stent and 125I seed strand implantation: In group A patients, the patent second-order branch of the intrahepatic portal vein in the unaffected side with hepatopetal flow was punctured with a 22-gauge Chiba needle (Cook, Inc., Bloomington, Indiana) under ultrasound guidance, followed by insertion of a 0.018-inch wire (Cook Inc.) into the portal vein. A 5-F calibrated pigtail catheter (Cook Inc.) was used to gauge the pressure in filling splenic mesenteric veins and portography was performed to measure the diameter and length of the obstructed MPV (stenosis). The number of 125I seeds to be implanted was calculated by the following formula: N = Length of obstructed MPV (mm)/4.5 + 4.
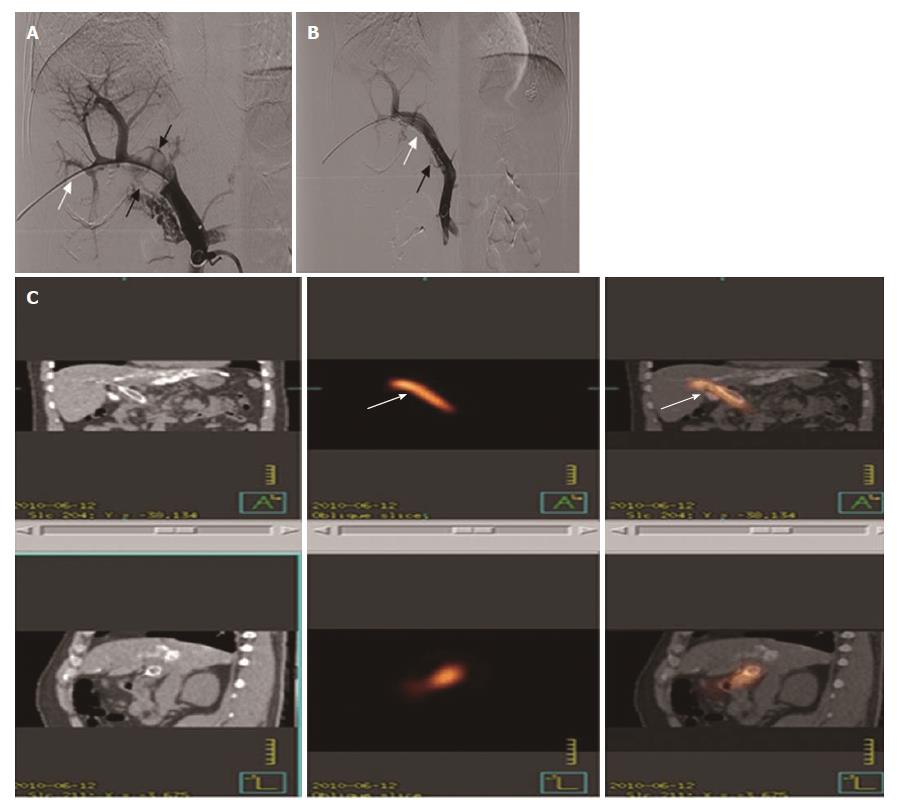
These seeds were arranged linearly and sealed into a 4-F catheter continuously to construct a 125I seed strand. After 50 U/kg heparin (XinYi, Shanghai, China) was administered intravenously, two 0.035-inch, 260-cm-long stiff wires (Terumo) were inserted into the superior mesenteric vein through the 7-F sheath. After the sheath had been removed, the outer cannula of the NEFF set and a self-expendable stent of appropriate size were introduced to the MPV over one of the stiff wires, respectively. The stent was deployed from the distal MPV into the proximal patent intrahepatic portal vein. The 125I seed strand was delivered to the target position via outer cannula of the NEFF set and released between the stent and MPV (Figure 2A and B). Portography and pressure measurement were repeated.
Segmental TACE was performed by experienced interventional radiologists immediately after stent and 125I seed implantation. Regardless of the type of HCC (unilobar or bilobar), all feeding arteries of tumor identified by angiography of the celiac, hepatic, superior mesenteric, left gastric, and bilateral inferior phrenic arteries were chemoembolized using a 5-F RH catheter (Cook). The target artery was catheterized with a 2.7-F microcatheter (Renegade, Boston Scientific, Natick, MA). Under fluorescence imaging, a mixture of 10-50 mg/m2 of epirubicin (Pharmorubicin, Pfizer, NY) and 5-20 mL of iodized oil (Lipiodol Ultrafluide, Laboratoire Guerbet, Aul-nay-sous-Bois, France) was infused at a rate of 0.5-1 mL/min through the microcatheter until stasis flow in the tumor vascularity was achieved. Finally, gelatin sponge (Jingling, Jiangsu, China) was used to embolize the feeding artery of the tumor.
Single photon emission computed tomography (SPECT) combined with CT (SPECT/CT) scan was performed on day 1 of the therapy to evaluate the distribution of radiation by the 125I seed strand implanted in group A patients (Figure 2C).
The total number of hospitalization days was 5-7 d, which were prolonged if grade 3-4 adverse events occurred. All the patients were followed every 30 d until death or till March 1, 2016. Repeat TACE with the same protocol was performed upon detection of residual tumors or new lesions in all patients.
Efficacy was evaluated as per the Modified Response Evaluation Criteria in Solid Tumor (mRECIST)[22]. The primary endpoints were OS and time to progression (TTP). OS was defined as the period from the day of the procedure to patients’ death or to their last follow-up. TTP was defined as the period from the day of the procedure until the radiologic confirmation of tumor progression in liver parenchyma. For HCC in liver parenchyma, disease control rate (DCR) was defined as the percentage of patients with complete response (CR), partial response (PR), or stable disease (SD). Tolerance and AEs were measured as secondary endpoints. Sorafenib- and TACE-related AEs were monitored using the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0[23].
As MPVTT would increase the incidence of tumor dissemination, elevate portal vein pressure, and impair the liver functional reserve, occurrence of events like intrahepatic HCC spread, variceal bleeding, and liver function decompensation was also compared between the two groups.
SPSS version 22.0 (SPSS, Chicago, Illinois) was used for all the analyses. Continuous variables are presented as mean ± SD and were compared by t test. Categorical variables are expressed as frequencies and were compared by χ2 test. OS and disease-free survival were analyzed using the Kaplan-Meier curves and log-rank test. A P-value of less than 0.05 was considered statistically significant. Variables with P < 0.05 were chosen for multivariate analysis using the Cox proportional hazards model. Regression analysis was used to determine independent predictors of survival. Sex, age, type of tumor, HCC maximum diameter, degree of MPVTT, Child-Pug class, ECOG performance status, etiology of liver disease, serum alfa-fetoprotein level, and extrahepatic metastasis were considered within the propensity model. Propensity score matching analysis was performed, with a matching ratio of 1:1 for two groups, using the nearest-neighbor matching method with a caliper distance of 0.2 without replacement.
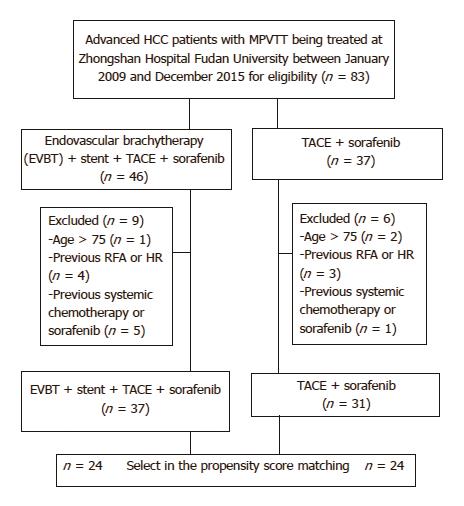
A total of 83 unresectable HCC patients were identified. Of them, 15 patients were excluded due to reasons listed in Figure 3. Finally, 68 patients were included in this study (group A, n = 37; group B, n = 31). Baseline characteristics before score matching are shown in Table 1. After propensity score matching, we created 24 matched pairs of patients in Table 2.
| Characteristic | Group A | Group B |
| (n = 37) | (n = 31) | |
| Sex | ||
| Male | 34 (91.9) | 26 (83.9) |
| Female | 3 (8.1) | 5 (16.1) |
| Age, yr (%) | ||
| ≥ 55 yr | 18 (48.9) | 14 (45.2) |
| < 55 yr | 19 (51.4) | 17 (54.8) |
| Type of tumor | ||
| Nodular | 30 (81.1) | 29 (93.5) |
| Infiltrative | 7 (18.9) | 2 (6.5) |
| HCC maximum diameter | ||
| ≥ 5 cm | 26 (70.3) | 19 (61.3) |
| < 5 cm | 11 (29.7) | 12 (38.7) |
| Degree of MPVTT | ||
| Stenosis | 31 (83.8) | 24 (77.4) |
| Occlusive | 6 (16.2) | 7 (22.6) |
| Child-pugh class | ||
| A | 33 (89.2) | 24 (77.4) |
| B | 4 (10.8) | 7 (22.6) |
| ECOG performance status | ||
| 0/1 | 33 (89.2) | 26 (83.9) |
| 2 | 4 (10.8) | 5 (16.1) |
| Etiology of liver disease | ||
| HBV | 35 (94.6) | 29 (93.5) |
| Other | 2 (5.4) | 2 (6.5) |
| Serum AFP level (in ng/mL) | ||
| ≥ 400 | 20 (54.1) | 17 (54.8) |
| < 400 | 17 (45.9) | 14 (45.2) |
| Extrahepatic metastasis | 3 (8.1) | 3 (9.7) |
| Characteristic | Group A | Group B |
| (n = 24) | (n = 24) | |
| Sex | ||
| Male | 22 (91.7) | 23 (95.8) |
| Female | 2 (8.3) | 1 (4.2) |
| Age | ||
| ≥ 55 yr | 14 (58.3) | 9 (37.5) |
| < 55 yr | 10 (41.7) | 15 (62.5) |
| Type of tumor | ||
| Nodular | 21 (87.5) | 22 (91.7) |
| Infiltrative | 3 (12.5) | 2 (8.3) |
| HCC maximum diameter | ||
| ≥ 5 cm | 16 (66.7) | 17 (70.8) |
| < 5 cm | 8 (33.3) | 7 (29.2) |
| Degree of MPVTT | ||
| Stenosis | 21 (87.5) | 19 (79.2) |
| Occlusive | 3 (12.5) | 5 (20.8) |
| Child-pugh class | ||
| A | 20 (83.3) | 21 (87.5) |
| B | 4 (16.7) | 3 (12.5) |
| ECOG performance status | ||
| 0/1 | 20(83.3) | 22(91.7) |
| 2 | 4(16.7) | 2(8.3) |
| Etiology of liver disease | ||
| HBV | 22 (91.7) | 23 (87.5) |
| Other | 2 (8.3) | 1 (12.5) |
| Serum AFP level | ||
| ≥ 400 | 12 (50.0) | 10 (41.7) |
| < 400 | 12 (50.0) | 14 (58.3) |
| Extrahepatic metastasis | 1 (3.8) | 3 (11.5) |
The 125I seeds strand and stent placement procedure was completed in all patients in group A (technical success rate, 100%) and the TACE procedure was completed in all patients in both groups.
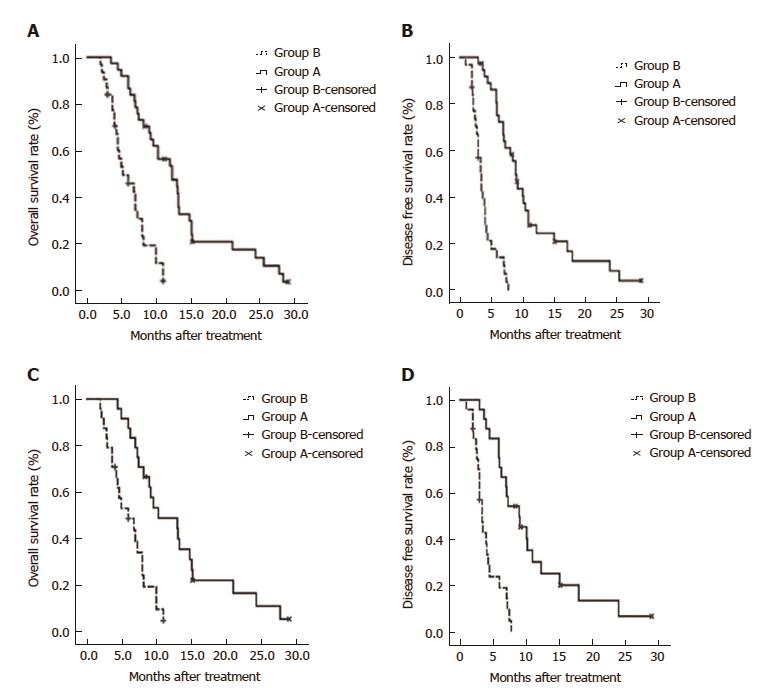
The median follow-up durations were 12.7 mo and 5.9 mo for the prematched groups A and B, respectively. The 6-, 12-, and 24-mo survival rates were 88.9%, 54.3%, and 14.1% in group A, and 45.8%, 0%, and 0% in group B, respectively (P < 0.001). However, there were no procedure-related deaths. Kaplan-Meier curves for survival outcomes in the two groups showed significantly higher OS in group A compared to group B (12.3 mo vs 5.2 mo; P < 0.001; Figure 4A).
The median TTP was longer (9.0 mo) in group A, compared to group B (3.4 mo) (P < 0.001; Figure 4B).
In the propensity score-matched cohorts, the median OS was longer in group A than in group B (10.3 mo vs 6.0 mo; P < 0.001; Figure 4C). Similarly, the median TTP was significantly longer in group A than in group B (9.0 mo vs 3.4 mo; P < 0.001; Figure 4D).
In multivariate Cox analysis, treatment regimen (HR = 0.18, P < 0.001) was identified as an independent predictor of OS for group A vs group B (Table 3).
| Group A vs Group B | ||||
| Log-rank test | Cox regression | |||
| Factors | n | P | HR (95%CI) | P |
| Treatment regimen | - | < 0.001 | - | - |
| EVBT-stenting-TACE-sorafenib | 37 | 0.18 (0.09-0.35) | < 0.001 | |
| TACE-sorafenib | 31 | 1 | - | |
| Type of tumor | 0.466 | - | - | |
| Nodular type | 59 | - | - | |
| Diffuse type | 9 | - | - | |
| HCC maximum diameter | - | 0.320 | - | - |
| ≥ 5 cm | 45 | - | - | |
| < 5 cm | 23 | - | - | |
| Child-pugh class | 0.298 | - | - | |
| A | 57 | - | - | |
| B | 11 | - | - | |
| ECOG performance status | - | 0.125 | - | - |
| 0 and 1 | 59 | - | - | |
| 2 | 9 | - | - | |
| Extrahepatic metastasis | - | 0.742 | - | - |
| Yes | 62 | - | - | |
| No | 6 | - | - | |
| Serum AFP level (ng/mL) | - | 0.586 | - | - |
| ≥ 400 | 37 | - | - | |
| < 400 | 31 | - | - | |
After stent placement in group A patients who were implanted with 17.2 ± 4.9 (range, 10-31) 125I seeds, the mean pressure of MPV dropped from 38.1 ± 5.8 cm H2O (range, 23-46 cm H2O) to 32.0 ± 5.6 cm H2O (range, 16-37 cm H2O) (P = 0.002). The estimated mean accumulated dose (R = 10 mm, z = 0, 240 d) was 62.9 ± 2.3 Gy (range, 57.4-65.3 Gy). Stent occlusion was observed in 9 (24.3%) patients and the median stent patency period was 22.1 ± 6.1 mo (95%CI: 9.5-34.7 mo).
HCC response was assessed using the mRECIST criteria. During the course of the study, 4.4 ± 3.2 and 2.9 ± 1.2 TACE procedures were performed in groups A and B, respectively. The ORR and DCR in group A were significantly higher than the rates observed in group B (ORR: 45.9% vs 16.1%, P = 0.009; DCR: 67.6% vs 29.0%, P = 0.002).
During the course of the study, the occurrence rate of complications related to MPVTT, such as intrahepatic metastasis, variceal bleeding, and liver function decompensation, were observed in 15 (40.5%), 6 (16.2%), and 11 (29.7%) patients in group A and 22 (71.0%), 18 (58.1%), and 25 (80.6%) patients in group B, respectively (P = 0.012, P < 0.001, and P < 0.001, respectively).
A total of 49 TACE-related AEs occurred in the two groups. The percentages of patients who experienced new ascites, liver dysfunction, and gastrointestinal hemorrhage were significantly higher in group B than in group A.
A total of 140 sorafenib related AEs occurred in 91.2% of patients. Seven patients required a sorafenib dose reduction to 400 mg once daily for grade 3 hand-foot skin reactions (4.2%) and grade 3 diarrhea (5.3%) and resumed a regular dose after the AEs subsided. One patient with grade 4 hypertension was subjected to a drug interruption period of 20 d until the AEs subsided (Table 4).
| Adverse event | Group A | Group A | P |
| (n = 37) | (n = 31) | ||
| Sorafenib related AEs | |||
| Hand-foot skin reaction | |||
| Grade 1-2 | 26 (92.9) | 27 (96.4) | 0.143 |
| Grade 3-4 | 2 (7.1) | 1 (3.6) | 0.593 |
| Diarrhea | |||
| Grade 1-2 | 23 (88.5) | 18 (94.7) | 0.994 |
| Grade 3-4 | 3 (11.5) | 1 (3.6) | 0.620 |
| Hypertension | |||
| Grade 1-2 | 8 (21.6) | 6 (19.4) | 0.818 |
| Grade 3-4 | 1 (3.2) | 0 | 1.000 |
| Alopecia | |||
| Grade 1-2 | 2 (7.1) | 4 (12.9) | 0.400 |
| Grade 3-4 | 0 | 0 | |
| Fatigue | |||
| Grade 1-2 | 9 (24.3) | 4 (12.9) | 0.354 |
| Grade 3-4 | 0 | 0 | |
| Voice change | |||
| Grade 1-2 | 0 | 1 (3.6) | 464 |
| Grade 3-4 | 0 | 0 | |
| Epistaxis | |||
| Grade 1-2 | 2 (7.1) | 2 (6.5) | 1.000 |
| Grade 3-4 | 0 | 0 | |
| TACE related AEs | |||
| New ascites | 4 (10.8) | 11 (35.5) | 0.020 |
| Liver dysfunction | 2 (16.2) | 15 (48.4) | < 0.000 |
| Gastrointestinal hemorrhage | 0 | 8 (25.8) | 0.001 |
| Hepatorenal syndrome | 0 | 2 (6.5) | 0.204 |
| Liver abscess | 0 | 0 | - |
| Spontaneous bacterial peritonitis | 0 | 0 | - |
| Inguinal hematoma | 0 | 0 | - |
| Pulmonary/cerebral oil embolization | 0 | 0 | - |
MPVTT is the most important independent predictive factor for poor prognosis of patients with HCC[24]. Although TACE is effective and safe for intrahepatic primary HCC lesions including few cases of HCC-cholangiocellular carcinoma, its benefit in PVTT has less importance, especially in type III PVTT or MPVTT. A combined treatment of TACE with novel drugs or other therapies might be a better alternative strategy for HCC with PVTT[22-25]. Huang et al[20] reported better survival outcomes with TACE plus 125I-seed implantation than with TACE alone in patients with type I and II PVTT. Currently, sorafenib is the recommended standard treatment for advanced HCC with PVTT[26]. Novi et al[27] reported that 15 wk of sorafenib monotherapy played a key role in PVTT revascularization. TACE combined with sorafenib could be a feasible alternative treatment option in patients with HCC and PVTT in the first-order or lower order portal vein branches but not in MPVTT[12]. However, a propensity-score analysis reported a significantly shorter OS in the sorafenib group than in the radiotherapy group[28].
According to these reports, the benefits of OS and TTP were lower in patients with MPVTT than in patients with PVTT of first-order or lower order portal vein branches. The main reason is that the occlusion of MPV is associated with an increased risk of tumor spread, elevated portal venous pressure causing variceal hemorrhage, and decreased portal flow resulting in ascites, jaundice, hepatic encephalopathy, and liver failure[1]. Restoring the flow of obstructed MPV and effectively inhibiting tumor thrombus progression might confer further survival benefit to patients with advanced HCC and MPVTT[13,21].
The strategy of implantation of 125I seed strand combined with stent placement and TACE has been reported to treat MPVTT[14,15]. This method of treatment has two advantages. On one hand, the blood flow to the portal vein is increased immediately and the portal vein pressure elevated by MPV obstruction is reduced effectively after stent deployment. One the other hand, the half-life of the gamma ray emitted by 125I seeds is 59.4 d. Sustained radiation can inhibit tumor cell growth by inducing apoptosis. Therefore, it is rational to hypothesize that placing a stent to restore the blood flow of obstructed MPV and implantation of 125I seeds might inhibit the progression of tumor thrombus. This increases the safety of subsequent TACE because of previous concerns that hepatic arterial flow interruption in TACE procedure would result in serious liver necrosis in patients whose hepatic blood supply has been already compromised[29].
Previously, combined brachytherapy with TACE and sorafenib showed greater OS compared to combined brachytherapy with TACE alone in HCC patients with MPVTT[30]. The success rate of TACE with stent placement and 125I implantation was 88.5%, with a mOS and mTTP of 8.9 mo and 7.9 mo, respectively, both of which were higher than mOS (5.7 mo) and mTTP (5.3 mo) associated with TACE with portal vein stenting alone[6]. Further, in our study addition of sorafenib to the TACE plus portal vein stenting and 125I implantation increased the OS and TTP to 10.3 mo and 9.0 mo, respectively. This study reported encouraging efficacy for the combination of sorafenib, EVBT, stent placement, and TACE in advanced HCC patients with MPVTT. The OS and TTP were longer in the EVBT-stenting-TACE-sorafenib group than in the TACE-sorafenib group. Although our results show moderate sorafenib-related side effects, they were mostly manageable after TACE and were comparable among the groups. However, TACE-related toxicities were lower in the combination group compared to the sorafenib plus TACE group. The data also demonstrated that the combination therapy has significant benefit in term of ORR and DCR compared with sorafenib-TACE. Zhang et al[31] reported that sorafenib monotherapy is a better treatment strategy over sorafenib plus TACE therapy for MPVTT due to the adverse events related to TACE. However, the results of our study suggest that combining EVBT with the sorafenib-TACE combination offers added benefits to sorafenib and decreases the toxicities of TACE. After overall analysis of all the side effects observed in the sorafenib-TACE group, we believe that combined EVBT with sorafenib and TACE may be a better approach for managing this specific subgroup of patients with advanced HCC and MPVTT.
The major limitations of this study are the single-center retrospective design, which may affect the generalization of results, and small sample size. Further, cost-benefit analysis was not performed for the expensive procedures in this study, which may be a topic of interest to be covered in our future studies.
In conclusion, EVBT combined with sorafenib and TACE might be a safe and effective palliative treatment option for MPVTT.
Despite the beneficial outcomes of individual therapies, studies pertaining to the clinical outcome of endovascular brachytherapy (EVBT) combined with stent placement, transarterial chemoembolization (TACE), and sorafenib to treat hepatocellular carcinoma (HCC) with main portal vein tumor thrombus (MPVTT) are scarce.
Recent studies have reported survival benefits in patients with PVTT who underwent combined treatments of TACE with sorafenib, radiotherapy with sorafenib, hepatic arterial infusion chemotherapy with sorafenib, and iodine-125 seed implantation with TACE. However, reports of such combined therapeutic strategies aiming at MPVTT are obscure. According to these previous studies, we aimed to find an effective therapy for HCC patients with MPVTT.
To evaluate the safety and efficacy of combined EVBT, stent placement, TACE, and sorafenib to treat HCC with MPVTT.
We conducted this retrospective study involving 68 patients with unresectable HCC. The patients received either EVBT with stent placement, TACE, and sorafenib or TACE with sorafenib. The time to progression (TTP) and overall survival (OS) were evaluated by propensity score analysis.
In the EVBT with stent placement, TACE, and sorafenib group, the 6-, 12-, and 24-mo survival rates were 88.9%, 54.3%, and 14.1%, respectively, and in the TACE with sorafenib group, they were 45.8%, 0%, and 0%, respectively. The median TTP and OS were significantly longer in the EVBT with stent placement, TACE, and sorafenib group (P < 0.001). In the propensity score-matched cohort, the median OS was longer in the EVBT with stent placement, TACE, and sorafenib group (P < 0.001).
EVBT combined with stent placement, TACE, and sorafenib might be a safe and effective palliative treatment option for MPVTT.
| 1. | Pirisi M, Avellini C, Fabris C, Scott C, Bardus P, Soardo G, Beltrami CA, Bartoli E. Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J Cancer Res Clin Oncol. 1998;124:397-400. [PubMed] |
| 2. | Ikai I, Hatano E, Hasegawa S, Fujii H, Taura K, Uyama N, Shimahara Y. Prognostic index for patients with hepatocellular carcinoma combined with tumor thrombosis in the major portal vein. J Am Coll Surg. 2006;202:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Wu CC, Hsieh SR, Chen JT, Ho WL, Lin MC, Yeh DC, Liu TJ, P’eng FK. An appraisal of liver and portal vein resection for hepatocellular carcinoma with tumor thrombi extending to portal bifurcation. Arch Surg. 2000;135:1273-1279. [PubMed] |
| 4. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6631] [Article Influence: 442.1] [Reference Citation Analysis (1)] |
| 5. | Lee DS, Seong J. Radiotherapeutic options for hepatocellular carcinoma with portal vein tumor thrombosis. Liver Cancer. 2014;3:18-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Jelic S, Sotiropoulos GC; ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v59-v64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 7. | Zhang XB, Wang JH, Yan ZP, Qian S, Liu R. Hepatocellular carcinoma invading the main portal vein: treatment with transcatheter arterial chemoembolization and portal vein stenting. Cardiovasc Intervent Radiol. 2009;32:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Lau WY, Sangro B, Chen PJ, Cheng SQ, Chow P, Lee RC, Leung T, Han KH, Poon RT. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology. 2013;84:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Salem R, Thurston KG. Radioembolization with yttrium-90 microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies: part 3: comprehensive literature review and future direction. J Vasc Interv Radiol. 2006;17:1571-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Cho YY, Lee M, Kim HC, Chung JW, Kim YH, Gwak GY, Bae SH, Kim do Y, Heo J, Kim YJ. Radioembolization Is a Safe and Effective Treatment for Hepatocellular Carcinoma with Portal Vein Thrombosis: A Propensity Score Analysis. PLoS One. 2016;11:e0154986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Zhang FJ, Li CX, Jiao DC, Zhang NH, Wu PH, Duan GF, Wu YX. CT guided 125iodine seed implantation for portal vein tumor thrombus in primary hepatocellular carcinoma. Chin Med J (Engl). 2008;121:2410-2414. [PubMed] |
| 12. | Nag S, DeHaan M, Scruggs G, N . Mayr, E.W.Martin. Long-term follow up of patients of intrahepatic malignancies treated with iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys. 2006;64:736-744. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 13. | Kim DY, Park W, Lim DH, Lee JH, Yoo BC, Paik SW, Kho KC, Kim TH, Ahn YC, Huh SJ. Three-dimensional conformal radiotherapy for portal vein thrombosis of hepatocellular carcinoma. Cancer. 2005;103:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Luo J, Yan Z, Liu Q, Qu X, Wang J. Endovascular placement of iodine-125 seed strand and stent combined with chemoembolization for treatment of hepatocellular carcinoma with tumor thrombus in main portal vein. J Vasc Interv Radiol. 2011;22:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Luo JJ, Zhang ZH, Liu QX, Zhang W, Wang JH, Yan ZP. Endovascular brachytherapy combined with stent placement and TACE for treatment of HCC with main portal vein tumor thrombus. Hepatol Int. 2016;10:185-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 844] [Article Influence: 52.8] [Reference Citation Analysis (2)] |
| 17. | Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, Cai M, Shan H. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology. 2014;272:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, Ryoo BY, Kang YK, Lee D, Kim KM. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015;26:320-329.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Lai YC, Shih CY, Jeng CM, Yang SS, Hu JT, Sung YC, Liu HT, Hou SM, Wu CH, Chen TK. Hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2003;9:2666-2670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Huang M, Lin Q, Wang H, Chen J, Bai M, Wang L, Zhu K, Jiang Z, Guan S, Li Z. Survival benefit of chemoembolization plus Iodine125 seed implantation in unresectable hepatitis B-related hepatocellular carcinoma with PVTT: a retrospective matched cohort study. Eur Radiol. 2016;26:3428-3436. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Li WW, Dai ZY, Wan HG, Yao LZ, Zhu J, Li CL, Wang XJ, Pan J, Chen LZ. [Endovascular implantation of iodine-125 seeds strand and portal vein stenting followed by transcatheter arterial chemoembolization combined therapy with sorafenib for hepatocellular carcinoma with main portal vein tumor thrombus]. Zhonghua YiXue ZaZhi. 2016;96:1838-1842. [PubMed] |
| 22. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3439] [Article Influence: 214.9] [Reference Citation Analysis (43)] |
| 23. | National Cancer Institute C. Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Available from: http://ctep.cancer.gov. |
| 24. | Matono R, Yoshiya S, Motomura T, Toshima T, Kayashima H, Masuda T, Yoshizumi T, Taketomi A, Shirabe K, Maehara Y. Factors linked to longterm survival of patients with hepatocellular carcinoma accompanied by tumour thrombus in the major portal vein after surgical resection. HPB (Oxford): The Official Journal of the International Hepato Pancreato Biliary Association 2012; 247-253. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Zhang YF, Guo RP, Zou RH, Shen JX, Wei W, Li SH, OuYang HY, Zhu HB, Xu L, Lao XM. Efficacy and safety of preoperative chemoembolization for resectable hepatocellular carcinoma with portal vein invasion: a prospective comparative study. Eur Radiol. 2016;26:2078-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4740] [Article Influence: 263.3] [Reference Citation Analysis (0)] |
| 27. | Novi M, Lauritano EC, Piscaglia AC, Barbaro B, Zocco MA, Pompili M, Gasbarrini A. Portal vein tumor thrombosis revascularization during sorafenib treatment for hepatocellular carcinoma. Am J Gastroenterol. 2009;104:1852-1854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Nakazawa T, Hidaka H, Shibuya A, Okuwaki Y, Tanaka Y, Takada J, Minamino T, Watanabe M, Kokubu S, Koizumi W. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol. 2014;14:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 29. | Chuan-Xing L, Xu H, Bao-Shan H, Yong L, Pei-Jian S, Xian-Yi Y, Xiao-Ning L, Li-Gong Lu. Efficacy of therapy for hepatocellular carcinoma with portal vein tumor thrombus: Chemoembolization and stent combined with iodine-125 seed. Cancer Biology & Therapy. 2011;12:865-871. [RCA] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Hu H, Duan Z, Long X, Hertzanu Y, Shi H, Liu S, Yang Z. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. PLoS One. 2014;9:e96620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Fan W, Wang Y, Lu L, Fu S, Yang J, Huang Y, Yao W, Li J. Sorafenib With and Without Transarterial Chemoembolization for Advanced Hepatocellular Carcinoma With Main Portal Vein Tumor Thrombosis: A Retrospective Analysis. Oncologist. 2015;20:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: El-Bendary MM, Mizuguchi T, Ohkohchi N, Sergi CM S- Editor: Wei LJ L- Editor: Wang TQ E- Editor: Huang Y