Published online Nov 7, 2017. doi: 10.3748/wjg.v23.i41.7450
Peer-review started: July 26, 2017
First decision: August 10, 2017
Revised: August 24, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: November 7, 2017
Processing time: 103 Days and 13.1 Hours
To compare the Glasgow-Blatchford score (GBS), Rockall score (RS) and Baylor bleeding score (BBS) in predicting clinical outcomes and need for interventions in patients with bleeding peptic ulcers.
Between January 2008 and December 2013, 1012 consecutive patients admitted with peptic ulcer bleeding (PUB) were prospectively followed. The pre-endoscopic RS, BBS and GBS, as well as the post-endoscopic diagnostic scores (RS and BBS) were calculated for all patients according to their urgent upper endoscopy findings. Area under the receiver-operating characteristics (AUROC) curves were calculated for the prediction of lethal outcome, rebleeding, needs for blood transfusion and/or surgical intervention, and the optimal cutoff values were evaluated.
PUB accounted for 41.9% of all upper gastrointestinal tract bleeding, 5.2% patients died and 5.4% patients underwent surgery. By comparing the AUROC curves of the aforementioned pre-endoscopic scores, the RS best predicted lethal outcome (AUROC 0.82 vs 0.67 vs 0.63, respectively), but the GBS best predicted need for hospital-based intervention or 30-d mortality (AUROC 0.84 vs 0.57 vs 0.64), rebleeding (AUROC 0.75 vs 0.61 vs 0.53), need for blood transfusion (AUROC 0.83 vs 0.63 vs 0.58) and surgical intervention (0.82 vs 0.63 vs 0.52) The post-endoscopic RS was also better than the post-endoscopic BBS in predicting lethal outcome (AUROC 0.82 vs 0.69, respectively).
The RS is the best predictor of mortality and the GBS is the best predictor of rebleeding, need for blood transfusion and/or surgical intervention in patients with PUB. There is no one 'perfect score' and we suggest that these two tests be used concomitantly.
Core tip: Endoscopic hemostasis represents the cornerstone of upper gastrointestinal bleeding treatment, and several scores have been developed for the prediction of rebleeding. This is a first study on Croatian patients to include over 1000 participants with peptic ulcer bleeding, and the aim was to compare three scores (Glasgow Blatchford score, Rockall score and Baylor bleeding score) in the prediction of peptic ulcer bleeding treatment outcome, including need for hospital-based intervention or 30-d mortality, 30-d rebleeding rate, 30-d mortality rate, and needs for surgical intervention and blood transfusion, and to find optimal cutoff values that indicate high-risk patients.
- Citation: Budimir I, Stojsavljević S, Baršić N, Bišćanin A, Mirošević G, Bohnec S, Kirigin LS, Pavić T, Ljubičić N. Scoring systems for peptic ulcer bleeding: Which one to use? World J Gastroenterol 2017; 23(41): 7450-7458
- URL: https://www.wjgnet.com/1007-9327/full/v23/i41/7450.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i41.7450
Upper gastrointestinal bleeding (UGIB) is a common medical emergency. Incidence rates of UGIB demonstrate variations ranging from 48 to 160 cases per 100000 population[1]. The most common causes of acute UGIB are non-variceal, where 28% to 59% are caused by peptic ulcer bleeding (PUB)[1-3]. Endoscopic hemostasis represents the cornerstone of UGIB treatment, and several scores have been developed for the prediction of clinical intervention (i.e. Rockall score (RS), Glasgow-Blatchford score (GBS), Baylor bleeding score (BBS), Cedars-Sinai Medical Center predictive index, Almela score, AIMS65 score)[4-14]. The recently published American College of Gastroenterology practice guidelines on the management of patients with ulcer bleeding recommend risk assessment in all patients in order to stratify them into high or low risk categories, since it may assist in initial decisions regarding the timing of endoscopy, time of discharge, and level of care[15].
The GBS is a pre-endoscopic score and contains the following parameters: initial hemoglobin levels, urea, blood pressure, pulse, known syncope, melena, and liver or cardiac failure. Each variable has an appointed numeric value and the maximal number of points is 23 (Table 1). The GBS was designed to predict lower risk bleeds, and a GBS value of 1 or lower indicates very low risk category[8,9]. The most commonly used RS consists of a pre-endoscopic evaluation part, which includes age, signs of shock and comorbidities, along with an endoscopic part, which evaluates high-risk endoscopic characteristics as well (known as the post-endoscopic RS) (Table 2). Each variable is appointed a numeric value and every value > 2 indicates a high-risk patient[7]. The maximal pre-endoscopic RS value is 7, and the maximal post-endoscopic value is 11. The post-endoscopic RS can be calculated if bleeding is diagnosed and evaluated with upper endoscopy[7,16,17]. The BBS contains a pre-endoscopic evaluation part, which includes age, severity and duration of associated diseases, along with a post-endoscopic part, which evaluates the position and type of fresh bleeding (Table 3). The maximal pre-endoscopic BBS is 15, and the maximum total (pre-endoscopic and post-endoscopic) BBS is 24[18].
| Assigned score | ||
| Blood urea, mmol/L | 6.5 -7.9 | 2 |
| 8.0-9.9 | 3 | |
| 10.0-24.9 | 4 | |
| ≥ 25 | 6 | |
| Hemoglobin for men, g/dL | 12 -12.9 | 1 |
| 10-11.9 | 3 | |
| < 10 | 6 | |
| Hemoglobin for women, g/dL | 10-11.9 | 1 |
| < 10 | 6 | |
| Systolic blood pressure, mmHg | 100-109 | 1 |
| 90-99 | 2 | |
| < 90 | 3 | |
| Other markers | Pulse ≥ 100 | 1 |
| Melena | 1 | |
| Syncope | 2 | |
| Hepatic disease | 2 | |
| Cardiac failure | 2 | |
| Points | |||||
| Variable | 0 | 1 | 2 | 3 | |
| Pre-endoscopic score | Age, yr | < 60 | 60-79 | ≥ 80 | |
| Shock | Systolic blood pressure ≥ 100 | Systolic blood pressure ≥ 100 mmHg | Systolic blood pressure < 100 | ||
| Pulse ≥ 100/min | |||||
| Pulse < 100/min | |||||
| Comorbidity | No major comorbidity | Cardiac failure, ischemic heart disease, any major comorbidity | Renal failure, liver failure, disseminated malignancy | ||
| Post-endoscopic score | Diagnosis | Mallory-Weiss tear, no lesion identified and no signs of recent hemorrhage | All other diagnosis | Malignancy of upper gastrointestinal tract | |
| Major signs of recent hemorrhage | None or dark spot only | Blood in upper gastrointestinal tract, adherent clot, visible or spurting vessel | |||
| Assigned score | Age, yr | No. of parallel illnesses | Severity of illnesses | Site of bleeding | Stigmata of bleeding |
| 0 | < 30 | 0 | |||
| 1 | 30-49 | 1 or 2 | Clot | ||
| 2 | 50-59 | ||||
| 3 | 60-69 | Visible vessel | |||
| 4 | 3 or 4 | Chronic | Posterior wall bulb | ||
| 5 | ≥ 70 | ≥ 5 | Acute | Active bleeding | |
| Score | Pre-endoscopic | Post-endoscopic | |||
The RS was primarily developed to predict mortality and the GBS to evaluate need for clinical intervention[6-14]. Secondarily, they can be applied to asses rebleeding risk. The BBS was primarily developed to identify patients at high risk for rebleeding after endoscopic hemostasis[6,16]. In previous studies, the GBS has been shown to be better than the pre-endoscopic and post-endoscopic RS in predicting the need for hospital-based intervention in patients with UGIB[6,13,19]. On the other hand, the RS appeared to be better at predicting mortality after rebleeding, contributing to more accurate diagnostics and shorter hospital stay[7,13,14]. Recent studies have shown that early endoscopy (within 24 h of presentation) is performed in only half of patients with UGIB, demonstrating the need for reliable and accurate pre-endoscopic risk assessment[6-15,20-25].
This is the first prospective study in Croatia to include over 1000 patients with PUB, and the aim was to compare the GBS, pre-endoscopic RS and pre-endoscopic BBS, as well as the post-endoscopic RS and post-endoscopic BBS, in the prediction of PUB treatment outcome, need for hospital-based intervention (endoscopic treatment, transfusion, surgery intervention) or 30-d mortality, including 30-d rebleeding rate, 30-d mortality rate, and needs for surgical intervention and blood transfusion, and to find optimal cutoff values that indicate high-risk patients.
This prospective study was conducted in the University Hospital Center “Sestre Milosrdnice” that covers a population of approximately 300000 in the City of Zagreb, Croatia. All patients presenting to the Emergency Unit between January 2008 and December 2013 with hematemesis, melena, hematochezia, or blood admixture upon nasogastric insertion were considered for study enrolment. If initial work-up indicated the need for hospitalization, patients were admitted to the Interventional Gastroenterology Unit.
Upper gastrointestinal endoscopy was performed in all patients within 24 h of admission. Only patients with gastric and/or duodenal ulcers, or an ulcer at the site of gastro-enteric anastomosis found during emergency endoscopy, without any other possible cause of bleeding were included in the study. All patients with high-risk ulcer stigmata and patients selected depending on clinical judgment received high-dose acid suppression therapy (pantoprazole or esomeprazole 80 mg as an intravenous bolus, followed by 40 mg intravenously 2 times daily or 200 mg daily in the form of continuous infusion for at least 48 h followed by 40 mg daily by mouth). The institution’s ethics committee approved the study. Data was prospectively entered into a database, with patient details stored in a depersonalized manner to protect patient confidentiality.
The following data were collected for each patient: demographic data, history of ulcer or liver disease, coexisting and past illnesses, medication use, clinical characteristics of the bleeding episode, laboratory results, endoscopic diagnosis including stigmata of ongoing or recent hemorrhage, endoscopic intervention, medical treatment, rebleeding, surgical therapy, duration of hospitalization and cause of death. The grading of overall health and co-morbidity was performed according to the American Society of Anesthesiology (ASA) classification (grade 1, normal healthy patients; grade 2, mild systemic illness; grade 3, severe but incapacitating systemic illness; grade 4, life-threatening illness). Stigmata of hemorrhage were defined according to the Forrest classification (Forrest Ia, spurting bleeding; Forrest Ib, oozing bleeding; Forrest IIa, non-bleeding visible vessel; Forrest IIb, adherent clot; Forrest IIc, hematin on ulcer base; Forrest III, clean ulcer base).
Shock was defined as syncope or signs of shock at physical examination, including systolic blood pressure less than 100 mmHg and pulse rate more than 100 beats/min.
Post-hemorrhagic anemia was corrected with red blood cell transfusion (2 units, approximately 500 mL) at a hemoglobin threshold of 70-80 g/L.
All patients diagnosed with PUB and high-risk stigmata underwent initial hemostasis (injection of dilute epinephrine into and around the bleeding point, positioning of clips or thermal coagulation, or both, but never epinephrine alone). Two biopsy specimens were obtained from the gastric antrum and body in all patients and the presence of Helicobacter pylori (H. pylori) infection was assessed by histopathological examination of the specimens using hematoxylin-eosin (HE) stain.
All patients with negative histology for H. pylori at index endoscopy had a control endoscopy with repeating biopsy samples, or urea breath test (UBT), performed 2 wk after proton-pump inhibitor treatment was discontinued. Patients in whom the described protocol was not followed were excluded from the study about H. pylori infection.
Rebleeding was defined as one or more signs of recurrent bleeding, including fresh hematemesis or melena, hematochezia, aspiration of fresh blood via nasogastric tube, instability of vital signs, and reduction of hemoglobin levels by 2 g/dL or more, occurring 24-h after the primary bleeding was stopped.
For all patients with gastric ulcer in whom recurrent bleeding was not observed, control endoscopy was performed 4-5 d after initial hemostasis and biopsy specimens were obtained from the margins and base of gastric ulcers to exclude malignancy. Control endoscopy with histology had been planned to be performed in all patients with gastric ulcer.
Documented clinical outcomes were: need for hospital-based intervention or 30-d mortality, 30-d rebleeding, 30-d mortality and interventions (transfer to the Department of Surgery and the need for blood transfusion).
The collected data was used to calculate the GBS score, as well as the pre-endoscopic RS and pre-endoscopic BBS for each patient presenting with UGIB. The post-endoscopic RS and BBS were calculated if bleeding from gastric, duodenal or gastro-enteric ulcers was endoscopically diagnosed. Methods for calculating the GBS, RS and BBS were as previously described. Pre-endoscopic and post-endoscopic scores were separately evaluated.
The Mann-Whitney U-test and Kruskal-Wallis analysis of variance test were used to analyze differences in quantitative data. The discriminative ability of the scoring systems to predict outcomes was evaluated by receiver operating characteristics curves (ROC) with 95%CI. The areas under ROC (AUROC) curves were compared using the method of Delong et al[26] (1988) for the calculation of the standard error of the Area Under the Curve (AUC) and of the difference between two AUCs. The optimal thresholds of the GBS, RS and BBS for the prediction of rebleeding, death, and needs for blood transfusion and/or surgical intervention were identified as the threshold associated with the highest Youden index[27]. A two-tailed significance level of 5% was used in all comparisons. All analyses were performed using a statistical package MedCalc for Windows, version 15.8 (MedCalc Software, Ostend, Belgium).
The analysis included 2643 patients with UGIB, of that 2326 (88%) patients had non-variceal bleeding, 225 (8.5%) had variceal bleeding, and 92 (3.5%) had an unidentified cause of bleeding.
From 2418 patients with non-variceal bleeding, 41.9% (1012) had PUB; specifically, the cause of bleeding in 49% (496) was gastric ulcer, in 47% (476) duodenal ulcer, in 2.4% (24) both gastric and duodenal ulcer, and in 1.6% (16) gastro-enteric anastomosis ulcer. Endoscopic treatment was required in 58% of patients with ulcer bleeding, and in 57.3% hemostasis was achieved with hemoclips or with combination hemoclips/diluted epinephrine. The rate of rebleeding was 9.4%, and in patients that were on anticoagulant therapy the rebleeding rate was 14.8% (P = 0.245), which was not statistically significant. In total, 5.4% of the patients were transferred to the Department of Surgery. The 30-d mortality was 5.2% and the median length of hospitalization was 6 d. Transfusion of red blood cells was performed in 49% of patients. Patients were predominantly men (median age 65.3). In 52% of patients, high-risk ulcers were verified (Forrest Ia-IIb), 11% of the patients presented with shock, and moderate to severe comorbidity was found in 58%. Furthermore, 28.1% patients with peptic ulcer had been taking nonsteroidal anti-inflammatory drugs, 20% acetylsalicylic acid, 3.1% antiplatelet medication and 6% anticoagulant therapy.
H. pylori testing was performed in 760 (75.1%) patients, of which 324 (42.6%) tested positive. Table 4 shows the patient characteristics and clinical outcomes.
| Age | |
| Median, yr | 65.3 (20-100) |
| Sex | |
| Male/Female | 638 (63)/374 (37) |
| Findings at endoscopy | |
| Gastric ulcers | 496 (49) |
| Duodenal ulcers | 476 (47) |
| Gastric and duodenal ulcers | 24 (2.4) |
| Ulcer on gastro-enteric anastomosis | 16 (1.6) |
| High-risk ulcers (Forrest Ia-IIb) | 526 (52) |
| Forrest Ia | 61 (6) |
| Forrest Ib | 111 (11) |
| Forrest IIa | 212 (21) |
| Forrest IIb | 142 (14) |
| Low- risk ulcers (Forrest IIc-III) | 486 (48) |
| Forrest IIc | 172 (17) |
| Forrest III | 314 (31) |
| Hemodynamic shock | 111 (11) |
| Comorbidity | |
| Ischemic and valvular heart disease | 213 (21.5) |
| Liver disease | 172 (17) |
| Renal failure | 111 (11) |
| Any malignancy | 131 (12.9) |
| Comorbidity (ASA class) | |
| ASA I | 142 (14) |
| ASA II | 283 (28) |
| ASA III-IV | 587 (58) |
| H. pylori | |
| Tested | 760 (75.1) |
| H. pylori-positive | 324 (42.6) |
| Drugs | |
| Without previous therapy | 433 (42.8) |
| NSAIDs | 284 (28.1) |
| Acetylsalicylic acid | 203 (20) |
| Antiplatelet therapy | 31 (3.1) |
| Anticoagulant therapy | 41 (4) |
| NOAC | 20 (2) |
| Treatment | |
| Endoscopic therapy | 587 (58) |
| Epinephrine | 213 (36.3) |
| Hemoclips | 156 (26.6) |
| Hemoclips + epinephrine | 180 (30.7) |
| Thermocoagulation | 26 (4.4) |
| Thermocoagulation + epinephrine | 12 (2) |
| Repeated endoscopic therapy | 71 (7) |
| Blood transfusion required | 496 (49) |
| Red blood cell | 406 (40.1) |
| Median (range), unit | 2.5 (1-16) |
| Fresh frozen plasma | 81 (8) |
| Median (range), unit | 2 (1-6) |
| Platelet | 9 (0.9) |
| Median (range), unit | 6 (4-8) |
| Whole blood | 0 (0) |
| Surgery | 55 (5.4) |
| Outcome | |
| Rebleeding | 95 (9.4) |
| Rebleeding (anticoag. and NOAC) | 9 (14.8) |
| 30-d mortality | 53 (5.2) |
| Median hospital stay, d | 6 (0-45) |
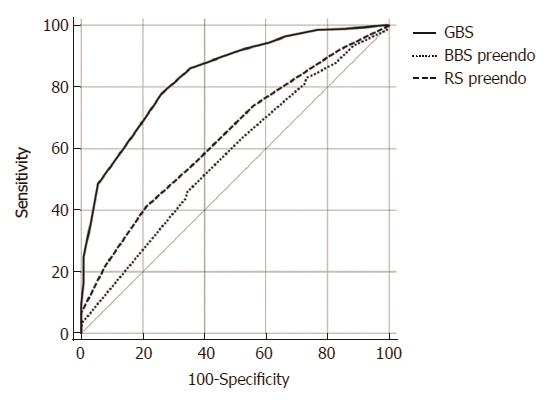
Using ROC curve analysis we found that the GBS was clearly superior to pre-endoscopic RS and pre-endoscopic BBS, in predicting need for hospital-based intervention or 30-d mortality (AUROC 0.84 vs 0.57 vs 0.64 respectively) (Figure 1).
The cutoff value that maximized the sum of the sensitivity and specificity for predicting 30-d mortality for the pre-endoscopic RS was 4 (sensitivity 0.63, specificity 0.85, total 1.48), and 5 for the post-endoscopic RS (sensitivity 0.83, specificity 0.68, total 1.51).
Based on ROC analysis of sensitivity and specificity, the optimal cutoff value of the pre-endoscopic BBS for 30-d mortality was 8 (0.63 sensitivity, 0.58 specificity, total 1.21), and the optimal cutoff post-endoscopic BBS value for 30-d mortality was 9 (0.88 sensitivity, 0.40 specificity, total 1.28).
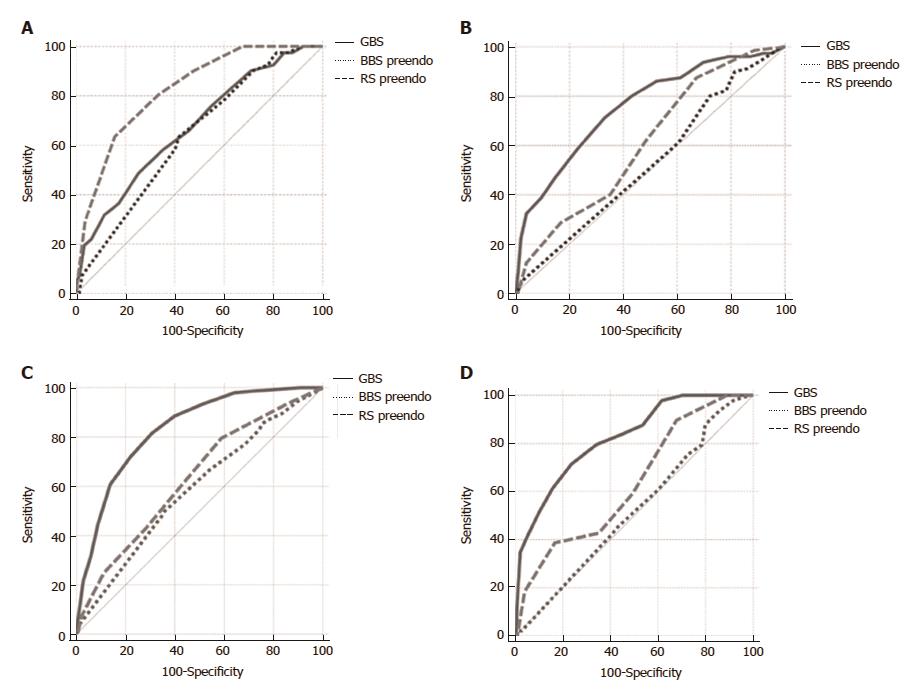
When assessing scores for the prediction of lethal outcome in patients with PUB, the pre-endoscopic RS was superior compared to the GBS and the pre-endoscopic BBS (AUROC 0.82 vs 0.67 vs 0.63, respectively) (Figure 2A).
Based on the ROC analysis of sensitivity and specificity, the optimal cutoff GBS value for 30-d mortality was 12 (0.49 sensitivity, 0.75 specificity, total 1.24), for rebleeding 11 (0.71 sensitivity, 0.67 specificity, total 1.38), for blood transfusion 9 (0.71 sensitivity, 0.67 specificity, total 1.38) and for surgery 12 (0.71 sensitivity, 0.76 specificity, total 1.47).
The GBS score was superior to the pre-endoscopic RS and BBS in the prediction of rebleeding (AUROC 0.75 vs 0.61 vs 0.52) (Figure 2B).
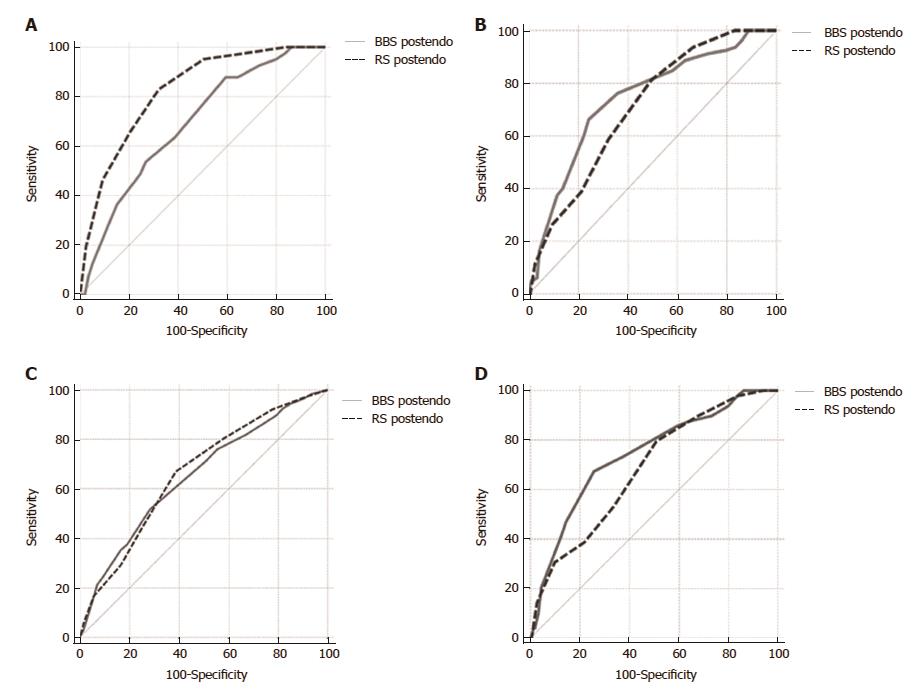
The GBS score was superior to the pre-endoscopic RS and BBS in predicting the need for blood transfusion (AUROC 0.83 vs 0.63 vs 0.59, respectively) (Figure 2C) and transfer to the Department of Surgery (AUROC 0.82 vs 0.63 vs 0.52, respectively) (Figure 2D). Also, the post-endoscopic RS was superior to the post-endoscopic BBS (AUROC 0.82 vs 0.69) in the prediction of lethal outcome (Figure 3A).
There was no significant difference between the post-endoscopic RS and BBS in the prediction of rebleeding (AUROC 0.70 vs 0.73) (Figure 3B).
The rebleeding cutoff point that maximized the sum of the sensitivity and specificity for the pre-endoscopic BBS was 3 (sensitivity 0.90, specificity 0.19, total 1.09), and 11 for the post-endoscopic BBS (sensitivity 0.66, specificity 0.76, total 1.42).
There was no significant difference between the post-endoscopic RS and BBS in predicting the need for blood transfusion (AUROC 0.68 vs 0.71) (Figure 3C) and transfer to the Department of Surgery (AUROC 0.68 vs 0.74) (Figure 3D).
UGIB is the most important cause of emergency gastroenterological admissions and the most frequent condition requiring emergency endoscopy[1]. The most common causes of acute UGIB are non-variceal, of which 30% to 60% are attributed to PUB[28]. In our study, 42% of all non-variceal bleeding was caused by PUB. In order to assess the adequate timing of endoscopy and selection of patients for hospital admission, several scoring systems for risk estimation have been developed. With the array of available scoring systems, it is often difficult to select the ideal scoring system for a particular patient or clinical outcome of interest. Therefore, in this study, we compared the performance of these scoring systems in the risk assessment of various clinical outcomes.
Our study showed that the GBS is superior to the pre-endoscopic RS and BBS in predicting need for hospital-based intervention or 30-d mortality. This is in concordance with the results from a study by Laursen[22] and a study by Bryant et al[19]. Our study also showed that the GBS is superior to the pre-endoscopic RS and BBS in predicting peptic ulcer re-bleeding. An explanation for why the GBS best predicts peptic ulcer rebleeding is that it incorporates hemoglobin and serum urea values. Serum urea is a good biochemical marker for UGIB because it rises rapidly when there is catabolism of isoleucine-poor hemoglobin[8,29]. The maximal level of hemoglobin and urea account for half of the maximal sum of points in the GBS score.
Our study showed that there is no significant difference between the post-endoscopic BBS and post-endoscopic RS in predicting peptic ulcer rebleeding. This is in concordance with the results from a study by Laursen et al[6]. Similar data was published by Italian and Dutch researchers, who also found low values under the ROC curve [(0.59-0.68) and 0.61] and concluded that the RS is not appropriate for prediction of rebleeding[16,30].
Our study showed that the GBS is superior to the pre-endoscopic RS and pre-endoscopic BBS in predicting the needs for blood transfusion and/or transfer to the Department of Surgery. The ROC curve for GBS rebleeding was similar to the GBS ROC curve for blood transfusion requirement and transfer to the Department of Surgery because peptic ulcer rebleeding is the main cause of blood transfusion requirement and need for surgical intervention. Bryant et al[19] published similar data.
Our study showed that the pre-endoscopic RS was superior to the GBS and pre-endoscopic BBS in predicting mortality. The RS best predicted fatal outcome because it incorporated the majority of risk factors (age, shock, moderate to severe co-morbidities and high-risk endoscopic signs for rebleeding), which was valuable in a multivariate analysis of risk for fatal outcome[7,13,30,31]. Our study showed that the post-endoscopic RS is superior to the post-endoscopic BBS in predicting lethal outcome in patients with PUB. Laursen[22] did not find any significant difference in AUROC among post-endoscopic BBS and post-endoscopic RS.
According to studies by Hyett et al[14] and Bryant et al[19], the GBS cutoff points for high-risk of lethal outcome and rebleeding were ≥ 10 and ≥ 12, respectively. In a recent retrospective study, Lim et al[32] suggested urgent endoscopy in the first 13 h after clinical presentation in high-risk patients with GBS > 12, in the first 24 h in patients with GBS > 7 and for patients with GBS values between 4 and 7 urgent endoscopy in the first 24 h is recommended, but not necessary.
Our cutoff points for high-risk of rebleeding and lethal outcome in PUB patients are significantly different in comparison with original research papers (GBS ≥ 2, pre-endoscopic BBS > 5, post-endoscopic BBS ≥ 10, post-endoscopic RS ≥ 4), which all refer to UGIB[6-9,13,14]. An explanation for this could be that the original series included an unselected group of patients with UGIB, with a significant proportion of patients with a low-risk of death, recurrent bleeding, and needs for blood transfusion and/or surgical intervention. These were patients that presented with low-risk bleeding ulcers (Forrest IIc and Forrest III), Mallory-Weiss syndrome, ulcerative esophagitis, angiodysplasia and portal hypertensive gastropathy.
When considering possible limitations of our study, there is always a certain level of subjectivity in the endoscopic classification of ulcers and variation in endoscopic treatment. Furthermore, our study had a relatively short follow-up period of 30 d.
By comparing the ROC curves of the aforementioned pre-endoscopic scores, the RS proved to be the best score for predicting lethal outcome. The post-endoscopic RS was also better than the post-endoscopic BBS in predicting lethal outcome in patients with PUB. On the other hand, among the three pre-endoscopic scores, the GBS best predicted need for hospital-based intervention or 30-d mortality, rebleeding, and needs for blood transfusion and/or surgical intervention.
This paper delivers a prospective single-center study, with a sample size of more than 1000 patients, that compared the scoring systems of the Glasgow-Blatchford score, Rockall score and Baylor bleeding score in predicting clinical outcomes and need for interventions in patients with bleeding peptic ulcers.
Endoscopic hemostasis represents the cornerstone of upper gastrointestinal bleeding treatment, and several scores have been developed for the prediction of rebleeding.
The authors concluded that although there is no ‘perfect score’, the Rockall score is the best predictor of mortality and the Glasgow-Blatchford score is the best predictor of need for hospital-based intervention or 30-d mortality, rebleeding, and needs for blood transfusion and/or surgical intervention in patients with peptic ulcer bleeding.
A detailed description is provided to allow other investigators to reproduce or validate the results. The statistical methods used are appropriate. The results provide sufficient experimental evidence to draw firm scientific conclusions. The discussion is well organized and provides systematic theoretical analysis and valuable conclusions.
| 1. | Holster IL, Kuipers EJ. Management of acute nonvariceal upper gastrointestinal bleeding: current policies and future perspectives. World J Gastroenterol. 2012;18:1202-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 2. | Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 446] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 3. | Bardou M, Benhaberou-Brun D, Le Ray I, Barkun AN. Diagnosis and management of nonvariceal upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2012;9:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Laine L, Jensen DM. Management of patients with ulcer bleeding. Am J Gastroenterol. 2012;107:345-360; quiz 361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 500] [Article Influence: 35.7] [Reference Citation Analysis (1)] |
| 5. | Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, Rotondano G, Hucl T, Dinis-Ribeiro M, Marmo R. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 519] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 6. | Laursen SB, Hansen JM, Schaffalitzky de Muckadell OB. The Glasgow Blatchford score is the most accurate assessment of patients with upper gastrointestinal hemorrhage. Clin Gastroenterol Hepatol. 2012;10:1130-1135.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321. [PubMed] |
| 8. | Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 709] [Article Influence: 27.3] [Reference Citation Analysis (2)] |
| 9. | Blatchford O, Davidson LA, Murray WR, Blatchford M, Pell J. Acute upper gastrointestinal haemorrhage in west of Scotland: case ascertainment study. BMJ. 1997;315:510-514. [PubMed] |
| 10. | Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394-397. [PubMed] |
| 11. | Budimir I, Gradišer M, Nikolić M, Baršić N, Ljubičić N, Kralj D, Budimir I Jr. Glasgow Blatchford, pre-endoscopic Rockall and AIMS65 scores show no difference in predicting rebleeding rate and mortality in variceal bleeding. Scand J Gastroenterol. 2016;51:1375-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Almela P, Benages A, Peiró S, Añón R, Pérez MM, Peña A, Pascual I, Mora F. A risk score system for identification of patients with upper-GI bleeding suitable for outpatient management. Gastrointest Endosc. 2004;59:772-781. [PubMed] |
| 13. | Stanley AJ, Dalton HR, Blatchford O, Ashley D, Mowat C, Cahill A, Gaya DR, Thompson E, Warshow U, Hare N. Multicentre comparison of the Glasgow Blatchford and Rockall Scores in the prediction of clinical end-points after upper gastrointestinal haemorrhage. Aliment Pharmacol Ther. 2011;34:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Hyett BH, Abougergi MS, Charpentier JP, Kumar NL, Brozovic S, Claggett BL, Travis AC, Saltzman JR. The AIMS65 score compared with the Glasgow-Blatchford score in predicting outcomes in upper GI bleeding. Gastrointest Endosc. 2013;77:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P; International Consensus Upper Gastrointestinal Bleeding Conference Group. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 716] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 16. | Vreeburg EM, Terwee CB, Snel P, Rauws EA, Bartelsman JF, Meulen JH, Tytgat GN. Validation of the Rockall risk scoring system in upper gastrointestinal bleeding. Gut. 1999;44:331-335. [PubMed] |
| 17. | Masaoka T, Suzuki H, Hori S, Aikawa N, Hibi T. Blatchford scoring system is a useful scoring system for detecting patients with upper gastrointestinal bleeding who do not need endoscopic intervention. J Gastroenterol Hepatol. 2007;22:1404-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Saeed ZA, Ramirez FC, Hepps KS, Cole RA, Graham DY. Prospective validation of the Baylor bleeding score for predicting the likelihood of rebleeding after endoscopic hemostasis of peptic ulcers. Gastrointest Endosc. 1995;41:561-565. [PubMed] |
| 19. | Bryant RV, Kuo P, Williamson K, Yam C, Schoeman MN, Holloway RH, Nguyen NQ. Performance of the Glasgow-Blatchford score in predicting clinical outcomes and intervention in hospitalized patients with upper GI bleeding. Gastrointest Endosc. 2013;78:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Srirajaskanthan R, Conn R, Bulwer C, Irving P. The Glasgow Blatchford scoring system enables accurate risk stratification of patients with upper gastrointestinal haemorrhage. Int J Clin Pract. 2010;64:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Cameron EA, Pratap JN, Sims TJ, Inman S, Boyd D, Ward M, Middleton SJ. Three-year prospective validation of a pre-endoscopic risk stratification in patients with acute upper-gastrointestinal haemorrhage. Eur J Gastroenterol Hepatol. 2002;14:497-501. [PubMed] |
| 22. | Laursen SB. Treatment and prognosis in peptic ulcer bleeding. Dan Med J. 2014;61:B4797. [PubMed] |
| 23. | Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Use of endoscopy for management of acute upper gastrointestinal bleeding in the UK: results of a nationwide audit. Gut. 2010;59:1022-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 24. | Maggio D, Barkun AN, Martel M, Elouali S, Gralnek IM; Reason Investigators. Predictors of early rebleeding after endoscopic therapy in patients with nonvariceal upper gastrointestinal bleeding secondary to high-risk lesions. Can J Gastroenterol. 2013;27:454-458. [PubMed] |
| 25. | Imperiale TF, Dominitz JA, Provenzale DT, Boes LP, Rose CM, Bowers JC, Musick BS, Azzouz F, Perkins SM. Predicting poor outcome from acute upper gastrointestinal hemorrhage. Arch Intern Med. 2007;167:1291-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] |
| 28. | van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 29. | Olde Damink SW, Dejong CH, Deutz NE, van Berlo CL, Soeters PB. Upper gastrointestinal bleeding: an ammoniagenic and catabolic event due to the total absence of isoleucine in the haemoglobin molecule. Med Hypotheses. 1999;52:515-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Camellini L, Merighi A, Pagnini C, Azzolini F, Guazzetti S, Scarcelli A, Manenti F, Rigo GP. Comparison of three different risk scoring systems in non-variceal upper gastrointestinal bleeding. Dig Liver Dis. 2004;36:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Kim BJ, Park MK, Kim SJ, Kim ER, Min BH, Son HJ, Rhee PL, Kim JJ, Rhee JC, Lee JH. Comparison of scoring systems for the prediction of outcomes in patients with nonvariceal upper gastrointestinal bleeding: a prospective study. Dig Dis Sci. 2009;54:2523-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Lim LG, Ho KY, Chan YH, Teoh PL, Khor CJ, Lim LL, Rajnakova A, Ong TZ, Yeoh KG. Urgent endoscopy is associated with lower mortality in high-risk but not low-risk nonvariceal upper gastrointestinal bleeding. Endoscopy. 2011;43:300-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Beales ILP, Chiu KW, Huang CM, Sugimoto M S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Huang Y