Published online Oct 28, 2017. doi: 10.3748/wjg.v23.i40.7292
Peer-review started: June 8, 2017
First decision: July 10, 2017
Revised: July 27, 2017
Accepted: August 15, 2017
Article in press: August 15, 2017
Published online: October 28, 2017
Processing time: 143 Days and 14.3 Hours
To determine the prevalence of Epstein-Barr virus (EBV)-associated gastric carcinomas in the North Region of Portugal and to study its clinicopathological characteristics.
We have performed a retrospective study including a total of 179 consecutive patients with gastric cancer (GC) submitted to gastrectomy during 2011 at the Portuguese Oncology Institute of Porto. Clinical and pathological data was collected from individual clinical records and inserted on a database with unique codification. Tumour tissues were collected from the institutional tumour bank. EBV was detected by in situ hybridization for the detection of EBV-encoded small RNAs (EBERs) and EBV latent proteins (LMP1 and LMP2A) were detected by immunohistochemistry.
The analysis showed that EBV-associated gastric carcinomas (EBVaGC) represents 8.4% (15/179) of all GC cases, with a significant differential distribution among histological types (P < 0.001): 100% (3/3) of medullary carcinomas, 100% (1/1) of adenosquamous carcinoma, 8.7% (8/92) of tubular adenocarcinomas, 8.0% (2/25) of mixed carcinomas and 2% (1/51) in poorly cohesive carcinomas. The analysis revealed a higher predominance of EBVaGC in the upper third and middle (cardia, fundus and body) of the stomach (P = 0.041), a significant lower number of regional lymph nodes invasion (P = 0.025) and a tendency for better prognosis (P = 0.222). EBV latent protein expression revealed that all EBVaGC cases were LMP1-negative, nevertheless 6 cases (40%) expressed LPM2A, which reveals that these cases show a distinct EBV-Latency profile (latency II-like).
EBVaGC represents 8.4% of all GC in the North Region of Portugal. The EBV-infected patients have specific clinic-pathological features that should be further explored to develop new strategies of management and treatment.
Core tip: This is the first study to report the prevalence of Epstein-Barr virus (EBV)-associated gastric carcinomas (EBVaGC) in Portugal. The EBVaGCs were found in 8.4% of all gastric cancer cases, being more frequent in upper and middle regions of the stomach and among tubular and medullary carcinomas. Patients with EBV-positive tumours also have a significant lower number of regional lymph nodes with metastasis and a tendency for a better prognosis.
- Citation: Ribeiro J, Oliveira A, Malta M, Oliveira C, Silva F, Galaghar A, Afonso LP, Neves MC, Medeiros R, Pimentel-Nunes P, Sousa H. Clinical and pathological characterization of Epstein-Barr virus-associated gastric carcinomas in Portugal. World J Gastroenterol 2017; 23(40): 7292-7302
- URL: https://www.wjgnet.com/1007-9327/full/v23/i40/7292.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i40.7292
Gastric cancer (GC) is the fifth most common malignancy worldwide with nearly one million new cases estimated in 2012 (952000 cases) and despite the significant reduction of its incidence, GC still remains the third leading cause of death by cancer[1,2]. Portugal is one of the European countries with the higher GC incidence and mortality rates, where GC represents the fifth most common cancer with about 3000 new cases per year and with 1387 deaths in men and 898 in women[1,2].
Epstein-Barr virus (EBV) is one of the most common viruses and has been associated with several malignancies including Burkitt’s Lymphoma, Hodgkin Lymphoma, post-transplant lymphoproliferative disease, a subset of T/NK cell lymphomas and nasopharyngeal carcinoma[3]. Recently, EBV has been associated with the development of GC, with several reports pointing that E BV-associated GC (EBVaGC) might account for nearly 10% of all GC cases[4,5]. The mechanisms of EBVaGC carcinogenesis are not well understood although some authors have suggested that EBV infection can represent a late event after Helicobacter pylori (H. pylori) infection[6,7].
The presence of EBV in patients with gastric carcinoma was first reported in gastric lymphoepithelioma-like carcinomas (also classified as medullary carcinomas, gastric carcinomas with lymphoid stroma or uncommon variant), nevertheless it has been detected in different histopathological subtypes of gastric carcinoma[8,9]. The majority of GC are adenocarcinomas and are classified according to the Lauren or World Health Organization (WHO) classification system[10]. These classification systems have a reduced clinical utility and recent studies suggested a new classification based on molecular features of tumours, where EBVaGC arises as a new subtype of gastric cancer[11,12].
Despite the high incidence of both GC and EBV infection prevalence in the Portuguese population, there are no studies reporting the prevalence of EBVaGC in Portugal[2,13]. We aimed to determine the prevalence and characteristics of EBVaGC and analyse the profile of EBV latent proteins expressed in gastric tumours from the North Region of Portugal.
We have developed a retrospective study in a cohort of 179 consecutive patients diagnosed with gastric cancer and submitted to surgery at the Portuguese Institute of Oncology of Porto (IPO Porto FG EPE) in 2011. Inclusion criteria: (1) patients with histologically confirmed gastric cancer; (2) submitted to gastrectomy (total or partial); and (3) with representative tumour blocks for adequate evaluation of EBV presence.
All tumour samples were histologically examined by a senior pathologist and classified according to the WHO classification system[10,14]. All clinical and pathological data was collected from individual clinical records and inserted on a database with unique codification. TNM-staging was performed in accordance to the UICC/AJCC system 7th edition[14]. The clinical outcome of the patients was followed from the date of surgery to either the date of death or 1st August 2016. All procedures were approved by the Ethical Committee of IPO Porto (CES IPO 80/2014).
The presence of EBV infection was investigated in all patients using histological sections (3 µm slides) obtained from formalin-fixed paraffin-embedded (FFPE) tissue blocks. EBV was identified using the in situ hybridization (ISH) for the detection of EBV-encoded small RNAs (EBERs) in FFPE tissue samples. The ISH was performed using Epstein-Barr virus Probe/Antibody ISH Kit (Leica, Newcastle upon Tyne, United Kingdom) in association with Ultra Vision Large Volume Detection System Anti-Polyvalent, HRP (THERMO SCIENTIFIC, Fremont, United States) according to the manufacturer's instructions. Detection of hybrids is achieved by enzymatic reaction using the ImmPACTTM DAB Peroxidase Substrate (VECTOR, Burlingame, CA, United States).
Only gastric tumours with presence of EBV infection in neoplastic cells were considered EBVaGC. For quality control, all EBV-positive cases and 10% of EBV-negative cases were retested and the results were 100% concordant. FFPE samples from a known EBV-positive post-transplant lymphoproliferative disorder (PTLD) tissue were used as positive controls and a sense probe for EBERs were used as the negative controls.
The presence of latent membrane protein 1 (LMP1) and latent membrane protein 2A (LMP2A) were evaluated with specific monoclonal antibodies in EBERs-positive gastric tissues using immunohistochemistry (IHC) (Table 1). IHC was performed using the UltraVision Large Volume Detection System Anti-Polyvalent, HRP kit (THERMO SCIENTIFIC, Fremont, United States) and the signal was detected using the ImmPACTTM DAB Peroxidase Substrate (VECTOR, Burlingame, CA, United States). All procedures were realized according to the manufacturer's instructions. PTLD samples with known positivity for LMP1 and LMP2A were used as positive controls. Gastric tumours were considered positive if 10% or more of the neoplastic cells were stained.
| Protein | Primary Antibody | Dilution | Incubation | Retrieval method | Expression in gastric cells |
| LMP1 | NCL-EBV-CS1-4, Leica, Newcastle upon Tyne, United Kingdom | 1:100 | 3h, RT | 15 min, microwave in Vector® Antigen Unmasking Solution | Cytoplasm |
| LMP2A | 15F9, THERMO SCIENTIFIC, Fremont, United States | 1:250 | On, 4°C | Cytoplasm and membrane |
Statistical analysis was performed using the computer software IBM SPSS statistics for Macintosh, version 20.0 (IBM Corp, Armonk, NY, United States). χ2 or Fisher Exact-test was used to compare categorical variables, with a significance level of 5%. Overall survival was defined as the time between the date of surgery to the date of last follow-up (censored) or the date of patient death (event). Cases lost to follow-up and those ending in death from any cause other than gastric cancer were regarded as censored data during the analysis of survival rates. The differences in survival times between subgroups were calculated using the log-rank test and the Kaplan-Meier method was used to calculate survival probabilities.
The clinicopathological characteristics of all patients are described in (Table 2). A total of 179 patients (108 males and 71 females) with mean age of 64 ± 12 were included in this study; the majority were submitted to gastrectomy (n = 177), wherein 100 were total resections and 77 partial resections, and the remaining 2 patients were submitted to esophagogastrectomy. Regarding the tumour localisation in stomach: 26 (14.5%) were found in the upper third (cardia or fundus); 46 (25.7%) in the middle region (body); and 107 (59.8%) in lower third (antrum or pylorus).
| Characteristics | All cases (n = 179) | EBV status | P value | |
| Negative (n = 164) | Positive (n = 15) | |||
| Gender, n = 179 | ||||
| Male | 108 (60.3) | 96 (88.9) | 12 (11.1) | 0.104 |
| Female | 71 (51.5) | 68 (95.8) | 3 (4.2) | |
| Age, n = 179 (64.38 ± 12 yr of age) | ||||
| < 65 yr of age | 90 (50.3) | 84 (93.3) | 6 (6,7) | 0.433 |
| ≥ 65 yr of age | 89 (49.7) | 80 (89.9) | 9 (10.1) | |
| Surgical procedure type, n = 179 | ||||
| Total gastrectomy | 100 (55.9) | 89 (89.0) | 11 (11.0) | 0.351 |
| Partial gastrectomy | 77 (43.0) | 73 (94.8) | 4 (5.2) | |
| Esophagogastrectomy | 2 (1.1) | 2 (100) | - | |
| Tumour localization, n = 179 | 0.087 | |||
| Upper third | 26 (14.5) | 22 (84.6) | 4 (15.4) | |
| Middle | 46 (25.7) | 40 (87.0) | 6 (13.0) | |
| Lower third | 107 (59.8) | 102 (95.3) | 5 (4.7) | |
| Anatomical site, n = 179 | ||||
| Cardia | 21 (11.7) | 19 (95.7) | 2 (4.3) | 0.041 |
| Fundus | 5 (2.8) | 3 (60.0) | 2 (40.0) | |
| Body | 46 (25.7) | 40 (87.0) | 6 (13.0) | |
| Pylorus | 13 (7.3) | 12 (92.3) | 1 (7.7) | |
| Antrum | 94 (52.5) | 90 (95.7) | 4 (4.3) | |
| Invasion pattern, n = 167 | ||||
| Infiltrative | 88 (52.7) | 83 (94.3) | 5 (5.7) | 0.237 |
| Expansive | 77 (46.1) | 67 (87.0) | 10 (13.0) | |
| Mixed | 2 (1.1) | 2 (100) | - | |
| Differentiation, n = 167 | ||||
| Poor | 91 (54.5) | 84 (92.3) | 7 (7.7) | 0.724 |
| Well+Moderate | 76 (45.5) | 69 (90.8) | 7 (9.2) | |
| Lymphovascular invasion, n = 179 | ||||
| Positive | 103 (57.5) | 94 (91.3) | 9 (8.7) | 0.841 |
| Negative | 76 (42.5) | 70 (92.1) | 6 (7.9) | |
| Histology WHO (2010), n = 179 | ||||
| Papillary adenocarcinoma | 1 (0.6) | 1 (100) | - | <0.001 |
| Tubular adenocarcinoma | 92 (51.4) | 84 (91,3) | 8 (8.7) | |
| Mucinous adenocarcinoma | 5 (2.8) | 5 (100) | - | |
| Poorly cohesive carcinoma | 51 (28.5) | 50 (98.0) | 1 (2.0) | |
| Mixed carcinoma | 25 (14.0) | 23 (92.0) | 2 (8.0) | |
| Medullary carcinoma | 3 (1.7) | - | 3 (100) | |
| Undifferentiated carcinoma | 1 (0.6) | 1 (100) | - | |
| Adenosquamous carcinoma | 1 (0.6) | - | 1 (100) | |
| Primary tumour (T), n = 179 | ||||
| T1a | 22 (12.3) | 22 (100) | - | 0.289 |
| T1b | 18 (10.1) | 15 (83.3) | 3 (16.7) | |
| T2 | 25(14.0) | 24 (96.0) | 1 (4.0) | |
| T3 | 69 (38.5) | 60 (87.0) | 9 (13.0 | |
| T4 | 7 (4.00) | 7 (100) | - | |
| T4a | 37 (20.7) | 35 (94.6) | 2 (5.4) | |
| T4b | 1 (0.56) | 1 (100) | - | |
| Regional lymph nodes (N), n = 179 | ||||
| N0 | 73 (40.8) | 67 (91.8) | 6 (8.2) | 0.024 |
| N1 | 23 (12.9) | 21 (91.3) | 2 (8.7) | |
| N2 | 31 (17.3) | 24 (77.4) | 7 (22.6) | |
| N3 | 8 (4.5) | 8 (100) | - | |
| N3a | 23 (12.8) | 23 (100) | - | |
| N3b | 21 (11.7) | 21 (100) | - | |
| Distant metastasis (M), n = 179 | 0.18 | |||
| M0 | 148 (82.7) | 133 (89.9) | 15 (10.1) | |
| M1 | 12 (6.70) | 12 (100) | - | |
| Mx | 19 (10.6) | 19 (100) | - | |
Tumours were classified according to WHO (2010) classification system and the most common histological type was tubular adenocarcinoma (n = 92, 51.4%) followed by poorly cohesive carcinoma (n = 51, 28.5%), mixed carcinoma (n = 25, 14%) and less frequent were mucinous adenocarcinoma (n = 5, 2.8%), papillary adenocarcinomas (n = 1, 0.6%), undifferentiated carcinoma (n = 1, 0.6%), adenosquamous carcinoma (n = 1, 0.6%) and medullary carcinomas with lymphoid stroma (n = 3, 1.7%) (Table 2). The correlation of histological type with anatomic localisation (data not shown), revealed that tubular type can be found in any stomach region, being more frequent in the lower third (56.5%) followed by upper and middle regions with 20.8% and 20.7%, respectively; poorly cohesive carcinomas were also more frequently located in the lower third (84.3%) and only 11.8% and 3.9% were in the middle and upper third, respectively; mixed adenocarcinomas were also more prevalent in lower third of the stomach with 72% of cases located in this region, with 16% in the middle and 12% in the upper region; and medullary carcinomas showed a similar distribution throughout the stomach with a percentage of 33.3% in all regions.
Regarding the characteristics of tumours, the most frequent invasion pattern was infiltrative (52.7% vs 46.1% expansive); the majority were poorly differentiated (54.5% vs 45.5% well or moderate differentiated); and lymphovascular invasion was extremely frequent (57.5% vs 42.5%) (Table 2). The tumour staging revealed that the majority of cases (63.8%) were at Stage III and IV and in only 6.7% of all cases presented distant metastasis.
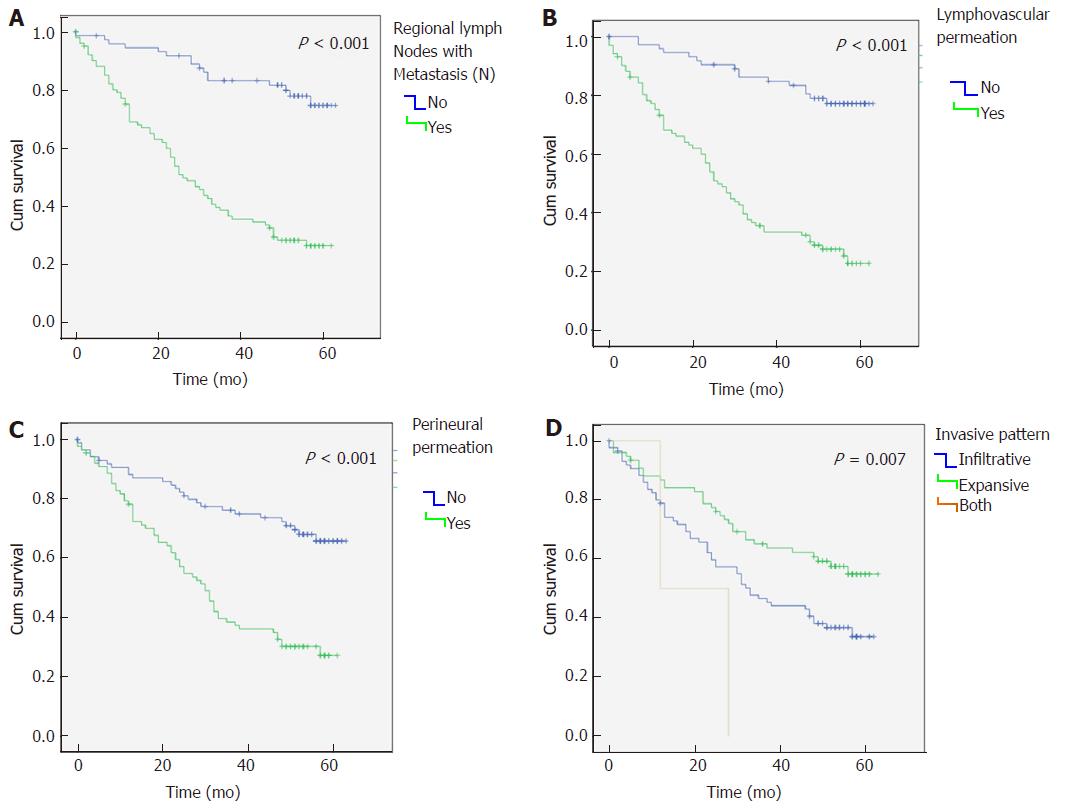
Regarding survival analysis, the median overall survival was of 45 mo (mean 36 ± 20.5; range 1-63). Furthermore, we observed that lymph node metastasis (N), lymphovascular and perineural permeation and infiltrative tumours had a significantly reduced median overall survival (P < 0.001) (Figure 2A-D).
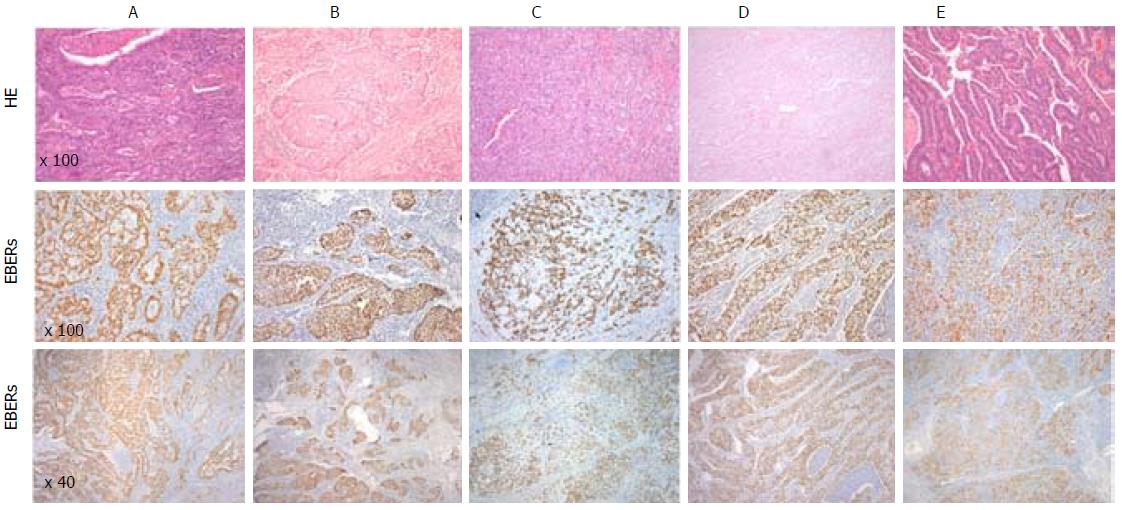
In our series, out of the 179 patients with GC, fifteen patients (8.4%) were positive for EBV - (Table 2). All EBV positive cases showed EBER positivity staining in > 90 of tumour cells while it was not observed in normal mucosa with or without atrophic gastric, intestinal metaplasia or in stromal cells (endothelial cells and fibroblasts) (Figure 1). In addition, one case showed EBER positivity only in the lymphocytic infiltration but not in tumour cells and therefore it was classified as EBV-negative GC.
Despite no statistical differences, it was observed that EBVaGC is more frequent in males (11.1% vs 4.1% in females) and among patients with more than 65 years old (10.1% vs 6.7% in younger patients) (Table 2). EBVaGC were not equally distributed among the stomach localisation (P = 0.087), being more often found in upper third and middle of the stomach than in the lower third; and regarding the anatomical site, it was more frequent (P = 0.041) in the body and fundus regions (40.0% and 13.0%, respectively) (Table 2).
Results have revealed statistical significant differences (P < 0.001) when comparing the distribution of EBV among different histological types according to WHO classification: EBV was detected in 8.7% (8/92) of tubular adenocarcinomas, 8.0% (2/25) of mixed carcinomas and only 2.0% (1/51) of poorly cohesive carcinomas. The results have also shown that all medullary (n = 3) and adenosquamous carcinomas (n = 1) were EBVaGC, while no EBV-positive case was identified in papillary adenocarcinomas and undifferentiated carcinomas (Table 2). The analysis of the invasion pattern of the GC, revealed that tumours with expansive patterns have a higher prevalence of EBV when compared with infiltrative patterns (13.0% vs 5.7%, respectively), despite not statistically significant (P = 0.237) (Table 2). No significant differences were also observed regarding the tumour differentiation and lymphovascular invasion (P > 0.050).
The results show that EBVaGCs were associated with a low number of regional lymph nodes invasion (P = 0.024) and that all cases had no evidence of distant metastasis, nevertheless there was no evidence of association with tumour stage (P = 0.289). Additionally, comparing the number of lymph nodes with metastasis between EBVaGCs and EBV negative cases, it was observed that EBVaGC cases have a significant lower number (mean difference of 3.7; 95%CI: 1.8-1.5; P < 0.001).
Table 3 resumes the individual characteristics of EBVaGC cases. Briefly, of the 15 EBVaGC cases 12 were males and 3 females with a mean age of 66 years old (range 44-82). Anatomically EBVaGCs were found in the body (n = 6), antrum (n = 4), fundus (n = 2), cardia (n = 2) and pylorus (n = 1). Regarding the histological subtypes, 8 were tubular adenocarcinoma, 3 were medullary carcinoma, 2 mixed carcinoma, 1 poorly cohesive carcinoma and 1 adenosquamous carcinoma. The invasion pattern showed that the majority of cases were expansive (n = 10/15) and regarding the differentiation, cases were equally distributed as poorly and moderately differentiated (n = 7 each). Moreover, there was no EBVaGC with distant metastasis.
| ID | Gender | Age | Anatomic Site | WHO Classification | Invasion pattern | Differentiation | n | TNM Classification | LMP-1 | LMP-2A |
| 1 | M | 75 | Body | Tubular Adenocarcinoma | Expansive | Poorly differentiated | 6 | T4aN2M0 | - | - |
| 2 | M | 80 | Antrum | Tubular Adenocarcinoma | Expansive | Moderately differentiated | 0 | T3N0M0 | - | - |
| 3 | M | 56 | Fundus | Tubular Adenocarcinoma | Expansive | Moderately differentiated | 2 | T2N1M0 | - | - |
| 4 | M | 80 | Antrum | Tubular Adenocarcinoma | Expansive | Moderately differentiated | 2 | T3N0M0 | - | - |
| 5 | M | 64 | Body | Tubular Adenocarcinoma | Expansive | Moderately differentiated | 0 | T3N2M0 | - | (+) |
| 6 | M | 56 | Fundus | Tubular Adenocarcinoma | Expansive | Moderately differentiated | 0 | T3N0M0 | - | - |
| 7 | M | 69 | Body | Tubular Adenocarcinoma | Expansive | Moderately differentiated | 3 | T3N2M0 | - | Inc. |
| 8 | M | 82 | Cardia | Tubular Adenocarcinoma | Infiltrative | Moderately differentiated | 4 | T3N2M0 | - | (+) |
| 9 | M | 55 | Body | Mixed Carcinoma | Infiltrative | Poorly differentiated | 3 | T3N2M0 | - | (+) |
| 10 | F | 66 | Antrum | Mixed Carcinoma | NA | Poorly differentiated | 6 | T1bN2M0 | - | (+) |
| 11 | F | 68 | Body | Medullary Carcinoma | Expansive | Poorly differentiated | 1 | T1bN1M0 | - | - |
| 12 | M | 52 | Cardia | Medullary Carcinoma | Expansive | Poorly differentiated | 0 | T1bN0M0 | - | - |
| 13 | M | 44 | Antrum | Medullary Carcinoma | Infiltrative | Poorly differentiated | 0 | T3N0M0 | - | (+) |
| 14 | F | 65 | Pylorus | Poorly cohesive carcinoma | Infiltrative | Poorly differentiated | 0 | T4aN0M0 | - | - |
| 15 | M | 76 | Body | Adenosquamous carcinoma | Expansive | NA | 6 | T3N2M0 | - | (+) |
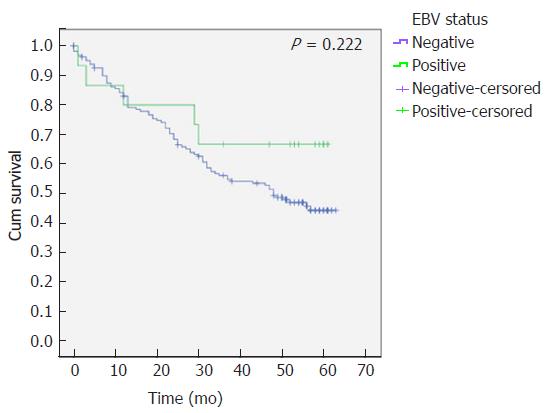
The survival analysis revealed that despite not statistically significant, EBVaGC patients have a higher overall survival (41 ± 1.8 mo vs 46 ± 5.9 mo, P = 0.222) (Figure 3).
Immunohistochemistry results revealed that all of EBVaGC cases were negative for LMP1 while 6 cases (40%) were positive for LMP2A and one case was inconclusive (Table 3). The positive cases for LPM2A expression were randomly distributed among histological subtypes: tubular adenocarcinomas (n = 2), mixed carcinomas (n = 2), medullary carcinomas (n = 1) and adenosquamous carcinoma (n = 1) (Table 3).
Gastric carcinoma is a serious public health problem worldwide with high rates of mortality and Portugal has the highest gastric cancer mortality rates in Western European countries[2,15]. The impact of classification systems in GC clinical decisions has been reduced and more recently two studies suggested a new classification based on molecular features of gastric tumours[11,12]. In this new classification arises four new subtypes of gastric cancer: Epstein-Barr Virus positive tumours (EBVaGC); microsatellite unstable tumours; genomically stable tumours; and tumours with chromosomal instability (CIN) [11,12].
Over the past 30 years, EBV infection has been reported in gastric cancer tumours[16]. Currently, it is accepted that about 10% of all GC represents a specific subset of gastric carcinomas that are associated with EBV carcinogenesis[17]. The gold-standard method for the detection of EBV in tissues is EBER-ISH and the decision to accept one case as EBV-associated should be taken considering the presence of EBERs expression in tumour cells and its absence in the normal surrounding tissue. In our study we found that 8.4% of GC in our population are EBV-associated, based on the EBERs expression in > 90 of tumour cells and its absence in non-malignant or stromal cells. To the best of our knowledge, this is the first study reporting the prevalence of EBVaGC in the North Region of Portugal. The prevalence found in our population is in line with those reported in other studies, which have reported a prevalence ranging 2%-20%[5,8,18]. Similar data was found in other countries from Europe, including Netherlands (7.8%)[18], and Denmark (7.6%)[19] and also from Asiatic countries, such as South Korea (7.8%)[20] and Japan (8%)[21]. Geographic differences have been discussed as a possible reason for the variation of EBVaGC prevalence[8,22] and some studies suggest that EBVaGC prevalence might be inversely correlated with the background incidence of GC[4]. The fact that Portugal has a higher incidence of GC when compared to other European countries seems to indicate that other factors apart from geography may be influencing the distribution of EBVaGC. Indeed, data from different meta-analyses have failed to show this association[8,22].
We found that EBVaGC was more frequent in males than in females, which is in line with several studies and suggests an association with others factors, such as life style or hormonal risks[23-25]. In contrast with other reports, we have found no association between EBV status and patients’ age[8,26]. Regarding the tumour location, our study revealed a higher predominance of EBVaGC in upper third and middle of stomach and considering that H. pylori preferentially colonises the antral region, these results suggest a possible antagonism of EBV and H. pylori in gastric mucosa[27-29]. In fact, Minoura-Etoh et al[30] described in an in vitro study that reactive products from H. pylori seem to induce EBV reactivation from latently in infected gastric epithelial cells, which would avoid EBV transformation of gastric cells in the same areas of H. pylori colonization. Despite this association remains unclear, two recent studies have suggested that H. pylori may contribute for EBV-associated gastric carcinogenesis by recruiting to gastric tissue the inflammatory cells that are already infected by EBV in the majority of adults[7,31]. This mechanism is not well understood and further studies should be made to establish if the recruitment of EBV-infected lymphoid cells might be the explanation for the infection and subsequent transformation of gastric epithelium. In our series, all EBVaGC cases had EBV only in tumour cells and not in normal tissues, nevertheless, we have found one case with EBV only in the lymphocytic infiltration, which we considered negative for EBV, and therefore more studies are required to understand if in such cases tumour cells will be later infected by EBV.
The association between EBV and gastric histological types remains controversial, indeed while one meta-analysis regarding EBV in gastric carcinoma has shown that EBVaGC was associated with diffuse histological type according to Lauren classification[5,8], two other meta-analyses did not find any association between EBV and histology subtypes[22,32]. Several studies demonstrated a strong EBV association with diffuse type while others have reported a similar prevalence between intestinal and diffuse types (classified in our study as poorly cohesive according to the WHO)[9,18,33,34]. Our study revealed that EBVaGC are more frequently associated with specific histologic subtypes. EBVaGC represent about 8% of tubular (intestinal type in the Lauren classification system) and mixed types while it was only found in 2% (n = 1/51) of poorly cohesive carcinomas (diffuse type in the Lauren classification system). These distinct features show that the differences in the GC histological distribution among different populations may impact on the prevalence of EBVaGC subtype, which should gather importance on the detection of EBV in GC. As expected, all medullary carcinomas, also known as gastric carcinoma with lymphoid stroma or lymphoepithelioma-like gastric carcinoma, were EBV positive[35]. In fact, EBV association with gastric cancer was first described in lymphoepithelioma-like gastric subtype and the literature shows that more than 80% of these cases are EBV-positive[5,22,36].
Some studies have revealed that EBVaGC is associated with poor or moderate differentiation of gastric cancer tissues[9,24]. In accordance with these reports, our study showed that 100% of cases were poorly or moderately differentiated and that these differentiation patterns seem to be associated with the histological types. This evidence may be a significant mark of EBVaGC tumours and may provide important information regarding the clinical approach to these tumours.
All these data support the evidence that recent studies have shown by considering EBVaGC as a distinct clinicopathological entity due to its individual features[11,12,18,37]. Interestingly, we found that EBVaGC had a significantly lower number of regional lymph nodes with metastasis and that none have a distant metastasis. Additionally, when analysing the overall survival of EBVaGC compared to all other cases it was observed that EBVaGC seems to have an improved survival, that despite not statistically significant may be explained by the low number of cases in our population. Several studies have reported a better overall survival of EBVaGC, especially in Asiatic populations suggesting that the explanation for this better overall survival could be due to the low frequency of lymph node involvement and distant metastasis[18,23,24,38]. In Portugal, GC remains at high mortality rates due to the late diagnosis of this disease and these patients are frequently only treated with chemo/radiotherapy. In our study we have included only patients with available tissue sample from surgical procedures and therefore we had not included patients that had received only chemo/radiotherapy, therefore our findings should be applied in patients with recommendation for total or partial gastrectomy as according to the ESMO Clinical Practice Guidelines for Diagnosis, Follow-up[39]. Moreover, others factors may be influencing patients survival and this question should be explored with further large-scale studies.
To elucidate the role of EBV in the pathogenesis of gastric carcinoma several studies have focused on the study of EBV latent proteins expression in gastric tumour cells[40-42]. As expect, in our study LMP1 was not found in any of EBVaGC cases and, in fact, literature also shows that LMP1 is generally absent in EBVaGC except for the data reported in a few studies, which have detected low albeit detectable levels of LMP1 mRNA[41-43]. The absence of LMP1 expression in EBV positive cases suggests that LMP1 may not be needed for gastric carcinogenesis or at least not for sustaining the already established malignant tumours. Regarding LMP2A, our results demonstrated that EBV LMP2A is expressed in 40% of EBVaGC cases that is in line with previous studies[41,44]. Recent studies have pointed that LMP2A seems to have an important role contributing to malignant state in epithelial cells by inducing the genome hypermethylation through up-regulation of DNMT1 and phosphorylation of STAT3 and thus contributing for the carcinogenic potential of the cells[45]. A systematic review performed by our group has shown that EBVaGC are usually associated with a distinct latency pattern, characterized by the expression of EBERs, EBNA1, LMP2A and EBV microRNAs. Furthermore, literature also shows that EBV latency I pattern, which is characterized by the expression of EBERs, EBNA 1 and EBV microRNAs[46], has been also frequently found among EBVaGC tumours[47,48]. In line with this, our study demonstrates that the majority of our cases express EBERs with absence of LMP1 and LMP2A, however, 40% of our cases also expressed LMP2A, which does not fit into the standard EBV latency patterns. In fact, literature has described this distinct pattern as latency II-like and has also reported that lytic transcripts such as BARF0 and BARF1 can also be found within EBV-associated gastric neoplasias[44,49]. Our data reinforces that the clarification of which EBV transcripts (including lytic transcripts and EBV microRNAs) are expressed in gastric tumours assumes a great importance to understand the viral carcinogenesis in gastric cancer and may contribute for the identification of new molecular targets for treatment.
In our study, EBVaGC represents 8.4% of our population with gastric cancer and it is more frequent among tubular adenocarcinomas and medullary carcinomas rather than poorly cohesive carcinomas. EBVaGCs were more common in upper and middle regions of the stomach; were correlated with a lower number of regional lymph nodes with metastasis and with no distant metastasis. The results also revealed that EBVaGC patients seem to have an improved survival and better prognosis. These features suggest that EBVaGC is a distinct subtype of gastric cancer and that the mechanisms of carcinogenesis should be further investigated for particular therapeutic targets
Gastric cancer (GC) is the fifth most prevalent cancer and it remains the third most common cause of cancer-related death in the world. Epstein-Barr virus (EBV) is an oncogenic virus that has been associated with several malignancies, including B-cell lymphomas and nasopharyngeal carcinomas (NPC). Recently, EBV has been also associated with GC development with several studies suggesting that EBV-associated gastric carcinomas (EBVaGC) represent approximately 10% of all GC cases.
Gastric carcinomas have been widely classified according to the Lauren or World Health Organization classification systems but these systems have a reduced clinical utility. A recent study has suggested a new classification based on molecular features of the tumours, where EBVaGC arises as a new subtype of gastric cancer. Despite the high incidence of both GC and EBV infection prevalence in the Portuguese population, there are no studies reporting the prevalence of EBVaGC in Portugal. The characterization of the EBVaGC in different populations is crucial to the development of new therapies and classification systems.
This is the first study in Portugal to characterize the EBV infection in gastric cancer. It demonstrated that EBV-associated carcinomas represent 8.4% of all gastric cancer cases and that EBVaGCs are a distinct subgroup of GC. Furthermore, it also showed that EBV displays a new latency profile in some of the EBVaGCs.
The understanding of clinical relevance and carcinogenesis of EBVaGC in different populations worldwide helps the improvement of preventive or management strategies as well as in the identification of more specific therapeutics.
This study showed that 8.4% of GC cases are EBV-associated being more frequent in upper and middle regions of the stomach, among tubular and medullary carcinomas, and having a lower number of regional lymph nodes invasion. Furthermore, this study confirms that EBVaGC may present a new latency-profile which may be useful to study to better characterize this GC subtype.
| 1. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer. Available from: http://globocan.iarc.fr/. |
| 2. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3526] [Cited by in RCA: 3674] [Article Influence: 282.6] [Reference Citation Analysis (2)] |
| 3. | Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1629] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 4. | Sousa H, Pinto-Correia AL, Medeiros R, Dinis-Ribeiro M. Epstein-Barr virus is associated with gastric carcinoma: the question is what is the significance? World J Gastroenterol. 2008;14:4347-4351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2009;24:354-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | de Souza CR, de Oliveira KS, Ferraz JJ, Leal MF, Calcagno DQ, Seabra AD, Khayat AS, Montenegro RC, Alves AP, Assumpção PP. Occurrence of Helicobacter pylori and Epstein-Barr virus infection in endoscopic and gastric cancer patients from Northern Brazil. BMC Gastroenterol. 2014;14:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Camargo MC, Kim KM, Matsuo K, Torres J, Liao LM, Morgan DR, Michel A, Waterboer T, Zabaleta J, Dominguez RL. Anti-Helicobacter pylori Antibody Profiles in Epstein-Barr virus (EBV)-Positive and EBV-Negative Gastric Cancer. Helicobacter. 2016;21:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Camargo MC, Murphy G, Koriyama C, Pfeiffer RM, Kim WH, Herrera-Goepfert R, Corvalan AH, Carrascal E, Abdirad A, Anwar M. Determinants of Epstein-Barr virus-positive gastric cancer: an international pooled analysis. Br J Cancer. 2011;105:38-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Corvalan A, Koriyama C, Akiba S, Eizuru Y, Backhouse C, Palma M, Argandoña J, Tokunaga M. Epstein-Barr virus in gastric carcinoma is associated with location in the cardia and with a diffuse histology: a study in one area of Chile. Int J Cancer. 2001;94:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Lauren P. The Two Histological Main Types Of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt At A Histo-Clinical Classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4389] [Article Influence: 146.3] [Reference Citation Analysis (1)] |
| 11. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 5084] [Article Influence: 423.7] [Reference Citation Analysis (4)] |
| 12. | Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, Siu HC, Deng S, Chu KM, Law S. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 861] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 13. | Sousa H, Silva J, Azevedo L, Pinto-Correia AL, Catarino R, Pinto D, Lopes C, Medeiros R. Epstein-Barr virus in healthy individuals from Portugal. Acta Med Port. 2011;24:707-712. [PubMed] |
| 14. | Waddell T, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; European Society for Medical Oncology (ESMO); European Society of Surgical Oncology (ESSO); European Society of Radiotherapy and Oncology (ESTRO). Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Morais S, Ferro A, Bastos A, Castro C, Lunet N, Peleteiro B. Trends in gastric cancer mortality and in the prevalence of Helicobacter pylori infection in Portugal. Eur J Cancer Prev. 2016;25:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Shibata D, Tokunaga M, Uemura Y, Sato E, Tanaka S, Weiss LM. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol. 1991;139:469-474. [PubMed] |
| 17. | Iizasa H, Nanbo A, Nishikawa J, Jinushi M, Yoshiyama H. Epstein-Barr Virus (EBV)-associated gastric carcinoma. Viruses. 2012;4:3420-3439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 18. | van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, Meijer CJ, Bloemena E. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004;22:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 220] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Boysen T, Friborg J, Stribolt K, Hamilton-Dutoit S, Goertz S, Wohlfahrt J, Melbye M. Epstein-Barr virus-associated gastric carcinoma among patients with pernicious anemia. Int J Cancer. 2011;129:2756-2760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Kim RH, Chang MS, Kim HJ, Song KS, Kim YS, Choi BY, Kim WH. Medical history and lifestyle factors contributing to Epstein-Barr virus-associated gastric carcinoma and conventional gastric carcinoma in Korea. Anticancer Res. 2010;30:2469-2475. [PubMed] |
| 21. | Sukawa Y, Yamamoto H, Nosho K, Kunimoto H, Suzuki H, Adachi Y, Nakazawa M, Nobuoka T, Kawayama M, Mikami M. Alterations in the human epidermal growth factor receptor 2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer. World J Gastroenterol. 2012;18:6577-6586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 22. | Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 392] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 23. | Truong CD, Feng W, Li W, Khoury T, Li Q, Alrawi S, Yu Y, Xie K, Yao J, Tan D. Characteristics of Epstein-Barr virus-associated gastric cancer: a study of 235 cases at a comprehensive cancer center in U.S.A. J Exp Clin Cancer Res. 2009;28:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, Yu J, Sung JJ, Herrera-Goepfert R, Meneses-Gonzalez F. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 25. | Rymbai ML, Ramalingam VV, Samarasan I, Chandran BS, Mathew G, Jerobin J, Abraham AM, Sachithanandham J, Kannangai R. Frequency of Epstein--Barr virus infection as detected by messenger RNA for EBNA 1 in histologically proven gastric adenocarcinoma in patients presenting to a tertiary care center in South India. Indian J Med Microbiol. 2015;33:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Qiu K, Tomita Y, Hashimoto M, Ohsawa M, Kawano K, Wu DM, Aozasa K. Epstein-Barr virus in gastric carcinoma in Suzhou, China and Osaka, Japan: association with clinico-pathologic factors and HLA-subtype. Int J Cancer. 1997;71:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Herrera-Goepfert R, Akiba S, Koriyama C, Ding S, Reyes E, Itoh T, Minakami Y, Eizuru Y. Epstein-Barr virus-associated gastric carcinoma: Evidence of age-dependence among a Mexican population. World J Gastroenterol. 2005;11:6096-6103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Galetsky SA, Tsvetnov VV, Land CE, Afanasieva TA, Petrovichev NN, Gurtsevitch VE, Tokunaga M. Epstein-Barr-virus-associated gastric cancer in Russia. Int J Cancer. 1997;73:786-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Nogueira Tde B, Artigiani R Neto, Herani B Filho, Waisberg J. H. pylori infection, endoscopic, histological aspects and cell proliferation in the gastric mucosa of patients submitted to roux-en-y gastric bypass with contention ring: a cross sectional endoscopic and immunohistochemical study. Arq Gastroenterol. 2016;53:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Minoura-Etoh J, Gotoh K, Sato R, Ogata M, Kaku N, Fujioka T, Nishizono A. Helicobacter pylori-associated oxidant monochloramine induces reactivation of Epstein-Barr virus (EBV) in gastric epithelial cells latently infected with EBV. J Med Microbiol. 2006;55:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Matsusaka K, Funata S, Fukayama M, Kaneda A. DNA methylation in gastric cancer, related to Helicobacter pylori and Epstein-Barr virus. World J Gastroenterol. 2014;20:3916-3926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Li S, Du H, Wang Z, Zhou L, Zhao X, Zeng Y. Meta-analysis of the relationship between Epstein-Barr virus infection and clinicopathological features of patients with gastric carcinoma. Sci China Life Sci. 2010;53:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Chang MS, Lee HS, Kim CW, Kim YI, Kim WH. Clinicopathologic characteristics of Epstein-Barr virus-incorporated gastric cancers in Korea. Pathol Res Pract. 2001;197:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Yoshiwara E, Koriyama C, Akiba S, Itoh T, Minakami Y, Chirinos JL, Watanabe J, Takano J, Miyagui J, Hidalgo H. Epstein-Barr virus-associated gastric carcinoma in Lima, Peru. J Exp Clin Cancer Res. 2005;24:49-54. [PubMed] |
| 35. | Wang ZH, Zhao JJ, Yuan Z. Lymphoepithelioma-like gastric carcinoma: A case report and review of the literature. World J Gastroenterol. 2016;22:3056-3061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990;3:377-380. [PubMed] |
| 37. | Ye XS, Yu C, Aggarwal A, Reinhard C. Genomic alterations and molecular subtypes of gastric cancers in Asians. Chin J Cancer. 2016;35:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Liu X, Liu J, Qiu H, Kong P, Chen S, Li W, Zhan Y, Li Y, Chen Y, Zhou Z. Prognostic significance of Epstein-Barr virus infection in gastric cancer: a meta-analysis. BMC Cancer. 2015;15:782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1150] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 40. | Ryan JL, Morgan DR, Dominguez RL, Thorne LB, Elmore SH, Mino-Kenudson M, Lauwers GY, Booker JK, Gulley ML. High levels of Epstein-Barr virus DNA in latently infected gastric adenocarcinoma. Lab Invest. 2009;89:80-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Cheng N, Hui DY, Liu Y, Zhang NN, Jiang Y, Han J, Li HG, Ding YG, Du H, Chen JN. Is gastric lymphoepithelioma-like carcinoma a special subtype of EBV-associated gastric carcinoma? New insight based on clinicopathological features and EBV genome polymorphisms. Gastric Cancer. 2015;18:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Strong MJ, Xu G, Coco J, Baribault C, Vinay DS, Lacey MR, Strong AL, Lehman TA, Seddon MB, Lin Z. Differences in gastric carcinoma microenvironment stratify according to EBV infection intensity: implications for possible immune adjuvant therapy. PLoS Pathog. 2013;9:e1003341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 43. | Tang W, Morgan DR, Meyers MO, Dominguez RL, Martinez E, Kakudo K, Kuan PF, Banet N, Muallem H, Woodward K. Epstein-barr virus infected gastric adenocarcinoma expresses latent and lytic viral transcripts and has a distinct human gene expression profile. Infect Agent Cancer. 2012;7:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 44. | Zhang YW, Zhao XX, Tan C, Zhang ZG, Jiang Y, Chen JN, Wei HB, Xue L, Li HG, Du H. Epstein-Barr virus latent membrane protein 2A suppresses the expression of HER2 via a pathway involving TWIST and YB-1 in Epstein-Barr virus-associated gastric carcinomas. Oncotarget. 2015;6:207-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Zhao J, Liang Q, Cheung KF, Kang W, Lung RW, Tong JH, To KF, Sung JJ, Yu J. Genome-wide identification of Epstein-Barr virus-driven promoter methylation profiles of human genes in gastric cancer cells. Cancer. 2013;119:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 46. | Ribeiro J, Oliveira C, Malta M, Sousa H. Epstein-Barr Virus Gene Expression And Latency Pattern In Gastric Carcinomas: A Systematic Review. Future Oncology. 2017;13:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Chen JN, Jiang Y, Li HG, Ding YG, Fan XJ, Xiao L, Han J, Du H, Shao CK. Epstein-Barr virus genome polymorphisms of Epstein-Barr virus-associated gastric carcinoma in gastric remnant carcinoma in Guangzhou, southern China, an endemic area of nasopharyngeal carcinoma. Virus Res. 2011;160:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer (review). Int J Oncol. 2015;46:1421-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 49. | zur Hausen A, Brink AA, Craanen ME, Middeldorp JM, Meijer CJ, van den Brule AJ. Unique transcription pattern of Epstein-Barr virus (EBV) in EBV-carrying gastric adenocarcinomas: expression of the transforming BARF1 gene. Cancer Res. 2000;60:2745-2748. [PubMed] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Portugal
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Huang CM, Yuan Y S- Editor: Ma YJ
L- Editor: A E- Editor: Ma YJ