Published online Oct 28, 2017. doi: 10.3748/wjg.v23.i40.7232
Peer-review started: August 17, 2017
First decision: August 30, 2017
Revised: September 13, 2017
Accepted: September 20, 2017
Article in press: September 19, 2017
Published online: October 28, 2017
Processing time: 73 Days and 19.1 Hours
To investigate the relationship between autophagy and perineural invasion (PNI), clinical features, and prognosis in patients with pancreatic cancer.
Clinical and pathological data were retrospectively collected from 109 patients with pancreatic ductal adenocarcinoma who underwent radical resection at the First Affiliated Hospital of Zhengzhou University from January 2011 to August 2016. Expression levels of the autophagy-related protein microtubule-associated protein 1A/1B-light chain 3 (LC3) and PNI marker ubiquitin carboxy-terminal hydrolase (UCH) in pancreatic cancer tissues were detected by immunohistochemistry. The correlations among LC3 expression, PNI, and clinical pathological features in pancreatic cancer were analyzed. The patients were followed for further survival analysis.
In 109 cases of pancreatic cancer, 68.8% (75/109) had evidence of PNI and 61.5% (67/109) had high LC3 expression. PNI was associated with lymph node metastasis, pancreatitis, and CA19-9 levels (P < 0.05). LC3 expression was related to lymph node metastasis (P < 0.05) and was positively correlated with neural invasion (P < 0.05, r = 0.227). Multivariate logistic regression analysis indicated that LC3 expression, lymph node metastasis, pancreatitis, and CA19-9 level were factors that influenced neural invasion, whereas only neural invasion itself was an independent factor for high LC3 expression. Univariate analysis showed that LC3 expression, neural invasion, and CA19-9 level were related to the overall survival of pancreatic cancer patients (P < 0.05). Multivariate COX regression analysis indicated that PNI and LC3 expression were independent risk factors for poor prognosis in pancreatic cancer (P < 0.05).
PNI in patients with pancreatic cancer is positively related to autophagy. Neural invasion and LC3 expression are independent risk factors for pancreatic cancer with a poor prognosis.
Core tip:The relationship between autophagy and perineural invasion (PNI) was explored for the first time in pancreatic cancer. Pancreatic cancer PNI is related to microtubule-associated protein 1A/1B-light chain 3 (LC3) expression-determined autophagy. PNI and LC3 expression were independent prognostic factors in pancreatic cancer. There might be a special association between autophagy and PNI, which contributes to pancreatic cancer progression. This study might provide a new insight for the mechanism of PNI in pancreatic cancer.
- Citation: Yang YH, Liu JB, Gui Y, Lei LL, Zhang SJ. Relationship between autophagy and perineural invasion, clinicopathological features, and prognosis in pancreatic cancer. World J Gastroenterol 2017; 23(40): 7232-7241
- URL: https://www.wjgnet.com/1007-9327/full/v23/i40/7232.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i40.7232
Pancreatic cancer, also known as “the king of cancer”, is a malignant tumor with a poor prognosis that has almost equal mortality and morbidity in patients. The incidence of pancreatic cancer is increasing yearly[1]. Surgical resection is the only possible cure of pancreatic cancer, although less than 20% of patients are eligible for radical surgery[2]. At the time of diagnosis, most pancreatic cancer patients have distant metastasis due to early occult symptoms, a lack of effective screening, and perineural growth characteristics. The incidence of perineural invasion (PNI) in pancreatic cancer is up to 80%-100% and is an important factor leading to postoperative pancreatic cancer recurrence. Previous studies have shown a higher recurrence rate after surgery and shorter disease-free and overall survival rates in cases of pancreatic cancer with PNI compared with those of cases without PNI. PNI evaluation of pancreatic cancer can predict disease recurrence and prognosis after surgery[3,4]. However, the pathogenesis of PNI has not yet been defined.
Autophagy has a dual role in promoting and inhibiting tumor growth[5,6]. Autophagy, as a mechanism of avoidance of anoikis in pancreatic cancer, is closely related to the survival of pancreatic cancer cells. Microtubule-associated protein 1A/1B-light chain 3 (LC3) is a typical marker of autophagy. LC3 labeling has been used to evaluate autophagy, and high levels of LC3 expression have been found in pancreatic cancer cells[7]. In addition, a previous study also showed that pancreatic cancer cells with PNI have higher levels of autophagy[7].
No study has examined the relationship between autophagy and PNI in pancreatic cancer cells. However, it can be inferred that only those pancreatic cancer cells that can survive within nerve tissues can eventually develop into a clinically visible form of pancreatic cancer PNI. Autophagy is likely one of the mechanisms involved in cancer cell survival. Therefore, this study focused on the relationship between pancreatic cancer cell autophagy and PNI, clinicopathological features, and prognosis, with an aim to provide a clinical basis for further study of the autophagy mechanisms affecting the pathogenesis of pancreatic cancer PNI.
Retrospective data were collected from 109 cases of pathologically confirmed pancreatic ductal adenocarcinoma patients who underwent radical surgery for pancreatic cancer from January 2011 to August 2016 at the First Affiliated Hospital of Zhengzhou University. The included pancreatic cancer patients were not treated with radiation or chemotherapy prior to surgery, but received postoperative adjuvant gemcitabine- or non-gemcitabine-based chemotherapy, and/or radiotherapy. Tissue specimens were fixed in formalin and paraffin-embedded for histological study. Clinical and pathological data were collected, and all cases were followed. The date of surgical resection was considered as the starting time, and August 2016 was the deadline. The primary endpoint was death due to pancreatic cancer. Eighty cases were followed for more than 12 mo. All patients provided informed consent for the collection of biological samples, and this study was approved by the Institutional Review Board of the First Affiliated Hospital of Zhengzhou University (Scientific Research No. 5, 2015).
Out of the 109 patients studied, 61 were male, and 48 were female. Their ages ranged from 19 to 81 years, and the median age was 59 years. Tumor diameters ranged from 0.7-12 cm with a median value of 4.0 cm. The survival time of 80 patients who were followed for more than 12 mo was 1 to 54 mo, and the median survival time was 20 mo. By the end of the follow-up period, 38 patients had died. The clinical stages were classified according to the AJCC 2011 standard.
Expression levels of LC3 and the nerve fiber marker ubiquitin carboxy-terminal hydrolase (UCH) were detected by immunohistochemistry using a standardized streptavidin-peroxidase (SP) method and the SP immunohistochemical Kit (ZSGB-Bio, Beijing, China) according to the manufacturer’s instructions. The pancreatic cancer tissues were routinely embedded in paraffin and sliced into 4-μm-thick continuous sections. The sections were then warmed in an autoclave to remove residual wax and were hydrated before antigen retrieval. Endogenous peroxidase activity was eliminated by incubating the tissue sections at room temperature with 3% H2O2 for 10 min. The tissue sections were washed three times with PBS continuously and incubated with a small amount of goat serum at room temperature in a closed chamber for 15 min. The goat serum was then poured off (not washed), and anti-LC3 (Proteintech Group, diluted 1:120) and anti-UCH antibodies (Proteintech, diluted 1:100) were added and incubated overnight at 4 °C. The next day, the slides were thoroughly washed with PBS, and a biotinylated secondary antibody in a working liquid was added and incubated at room temperature for 15 min. After the slides were washed carefully with PBS again, horseradish peroxidase was added, and the slides were incubated for 5 min at room temperature. The slides were then rinsed with PBS three times, and a drop of DAB buffer was placed on the tissue slice and rinsed after 1 min to stop the color reaction. Then, the nuclei were stained with hematoxylin, and the samples were dehydrated in gradient alcohol, covered with transparent balata, and observed by microscopy. PBS was used in place of the primary antibody to serve as the negative control, and a known positive slice served as the positive control. The immunohistochemical results were evaluated in a double-blind manner by two pathologists. If the results were inconsistent, a third pathologist reviewed the data to reach a consensus.
LC3-positive cells detected by immunohistochemistry required the following characteristics: (1) clear cell structure; (2) accurate localization of the positive particle; and (3) obviously higher pigmentation than that of the background and clear contrast. Positive LC3 expression was mainly localized in pancreatic cancer cell cytoplasm. Five random power fields (400 × magnification) were observed for each case using an optical microscope. One hundred homogeneous cells were counted, and the staining intensity and the proportion of positive cells were observed. A semi-quantitative analysis was conducted using the product method. Dye intensity was scored as follows: no yellow, 0 points; light yellow, 1 point; yellow or deep yellow, 2 points; and brown or tan, 3 points. The expression range was scored as follows: <10%, 0 points; 10% to 25%, 1 point; 26% to 50%, 2 points; 51% to 75%, 3 points; and >75%, 4 points. The value from dye intensity was then multiplied by the value from the expression range to obtain the overall score: 0 points, negative (-); 1 to 3 points, weakly positive (+); 4 to 6 points, moderately positive (+ +); and 7 to 12 points, strongly positive (+ + +). More than 3 points indicated high expression, and 3 points or fewer indicated low expression.
UCH was expressed in the cytoplasm of all nerve fibers. The location of nerve fibers can be clearly defined by UCH to determine the invasion of cancer cells to the nerve tissue. According to previous reports, positive PNI status was determined as cancer cells being found in the perineural spaces, perineurium or nerve tract[8].
The effects of clinicopathological factors such as LC3 expression and PNI on the overall survival in pancreatic cancer were analyzed in 80 patients with more than 12 mo of follow-up data. The following factors were included in the survival analysis: LC3 expression, age, gender, tumor location, tumor size, histological grade, clinical stage, vascular invasion, lymph node metastasis, pancreatitis, diabetes status, and preoperative CA19-9 level.
Statistical analyses were performed using SPSS 19.0 and GraphPad Prism 5.0 statistical software. Enumeration data were checked by the Chi-square test or the four-grid table Fisher exact probability method. Correlations between clinicopathological factors such as LC3 expression and PNI were analyzed using the Spearman correlation method. LC3 expression and the factors that independently influenced neural infiltration were analyzed using two categories and unconditional logistic regression. Univariate and multivariate analyses were performed on factors that might affect the prognosis according to a COX risk regression model. The survival curve was plotted according to the Kaplan-Meier method. Results were considered significant when P < 0.05.
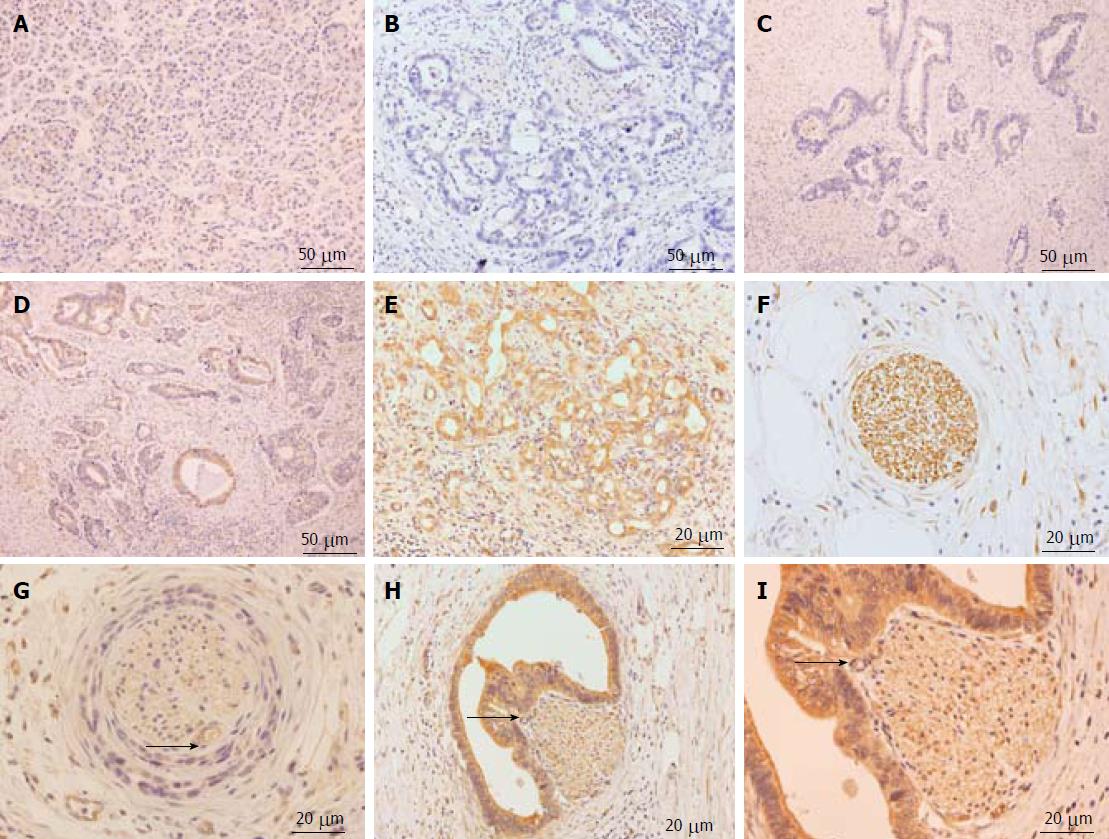
LC3 expression was mainly localized to the cytoplasm. In contrast to normal paraneoplastic pancreatic tissues (Figure 1A), the expression of LC3 in pancreatic cancer tissues ranged from low to high in four grades: negative, weakly positive, moderately positive, and strongly positive (Figure 1B-E). The immunohistochemical results indicated that LC3 protein expression was observed in pancreatic ducts, acinar epithelial cells, islet cells, and pancreatic cancer tissues. There was significantly increased LC3 protein expression in pancreatic cancer tissues and peritumoral tissues. In 109 pancreatic cancer tissues, 42 had low LC3 expression (termed “low autophagy level”), including 6 (5.5%) negative cases and 36 (33%) weakly positive cases (Figure 1B and C); while 67 had high LC3 expression (termed “high autophagy level”), including 50 (45.9%) that were moderately positive and 17 (15.6%) that were strongly positive (Figure 1D and E). PNI by pancreatic cancer cells occurred mainly in the pancreatic cancer stroma. According to a previous study[9], four types of relationships exist between PNI and cancer nests: no PNI, perineurium invasion, perineural space invasion, and invasion to the nerve fiber tracts. In 109 cases of pancreatic cancer, 75 were positive (Figure 1F-I), and 34 were negative for nerve invasion. The positive rate of nerve invasion was 68.8%. High LC3 expression was also found in the nests surrounding the PNI (Figure 1I).
The analysis of LC3 expression and PNI of pancreatic cancer demonstrated a significant positive correlation between these two parameters (P = 0.018, r = 0.227). LC3 expression in pancreatic cancer tissues with PNI was significantly higher than that in pancreatic cancer tissues without PNI (Table 1).
| LC3 | PNI | P value | r | |
| Absent | Present | |||
| Low expression | 18 | 22 | 0.018 | 0.227 |
| High expression | 16 | 53 | ||
LC3 expression was associated with lymph node metastasis (P < 0.05). LC3 expression was not related to sex, gender, tumor location, tumor size, histological grade, clinical stage, vascular invasion, or diabetes mellitus status. PNI was related to lymph node metastasis, pancreatitis, and CA19-9 levels (P < 0.05) and was not related to sex, age, tumor location, tumor size, histological grade, clinical stage, vascular invasion, or diabetes mellitus status (Table 2).
| Parameter | n | PNI | P value | LC3 | P value | ||
| Absent | Present | Low expression | High expression | ||||
| Age (yr) | |||||||
| ≤ 58 | 54 | 19 | 35 | 0.373 | 23 | 31 | 0.206 |
| > 58 | 55 | 15 | 40 | 17 | 38 | ||
| Gender | |||||||
| Male | 61 | 15 | 46 | 0.093 | 26 | 35 | 0.148 |
| Female | 48 | 19 | 29 | 14 | 34 | ||
| Tumor location | |||||||
| Head | 66 | 17 | 49 | 0.129 | 28 | 38 | 0.124 |
| Body/tail | 43 | 17 | 26 | 12 | 31 | ||
| Tumor size (cm) | |||||||
| ≤ 2 | 18 | 6 | 12 | 0.83 | 8 | 10 | 0.455 |
| > 2 | 91 | 28 | 63 | 32 | 59 | ||
| Histologic grade | |||||||
| Well or moderate | 74 | 24 | 50 | 0.685 | 23 | 51 | 0.077 |
| Poor | 35 | 10 | 25 | 17 | 18 | ||
| Vascular invasion | |||||||
| Negative | 83 | 26 | 57 | 0.957 | 31 | 52 | 0.801 |
| Positive | 26 | 8 | 18 | 9 | 17 | ||
| Lymph node metastasis | |||||||
| Negative | 36 | 17 | 19 | 0.011a | 18 | 18 | 0.043a |
| Positive | 73 | 17 | 56 | 22 | 51 | ||
| AJCC stage | |||||||
| I + II | 69 | 22 | 47 | 0.838 | 25 | 44 | 0.895 |
| III + IV | 40 | 12 | 28 | 15 | 25 | ||
| Pancreatitis | |||||||
| Negative | 30 | 15 | 15 | 0.009a | 12 | 18 | 0.659 |
| Positive | 79 | 19 | 60 | 28 | 51 | ||
| Diabetes | |||||||
| Negative | 89 | 27 | 62 | 0.684 | 33 | 56 | 0.862 |
| Positive | 20 | 7 | 13 | 7 | 13 | ||
| CA19-9 level (U/mL) | |||||||
| ≤ 37 | 37 | 19 | 19 | 0.001a | 13 | 24 | 0.808 |
| > 37 | 72 | 15 | 57 | 27 | 45 | ||
The clinicopathological factors possibly associated with LC3 expression and PNI were evaluated by multivariate analysis using a logistic regression model. The clinicopathological factors included LC3 expression and nerve infiltration, age, tumor site, tumor size, histological grade, clinical stage, vascular invasion, lymph node metastasis, diabetes, pancreatitis, and preoperative CA19-9 level. The results showed that LC3 expression, lymph node metastasis, pancreatitis, and CA19-9 level were the factors that influenced the occurrence of PNI, which was an independent factor affecting LC3 expression (Tables 3 and 4).
| Parameter | Estimate, B | Standard error | Wald statistic | P value | Odds ratio | 95%CI |
| Lymph node metastasis (positive vs negative) | 1.068 | 0.499 | 4.581 | 0.032a | 2.911 | 1.094-7.743 |
| CA199 (> 37 U/mL vs ≤ 37 U/mL) | 1.508 | 0.493 | 9.368 | 0.002a | 4.520 | 1.720-11.874 |
| Pancreatitis (present vs absent) | 1.301 | 0.514 | 6.419 | 0.011a | 3.673 | 1.343-10.049 |
| LC3 (high vs low) | 1.032 | 0.491 | 4.406 | 0.036a | 2.806 | 1.071-7.351 |
| Constant | -7.209 | 1.799 | 16.058 | 0 | 0.001 |
| Parameter | Estimate, B | Standard error | Wald statistic | P value | Odds ratio | 95%CI |
| PNI (present vs absent) | 0.997 | 0.427 | 5.451 | 0.02a | 2.71 | 1.174-6.259 |
| Constant | -1.115 | 0.732 | 2.316 | 0.128 | 0.328 |
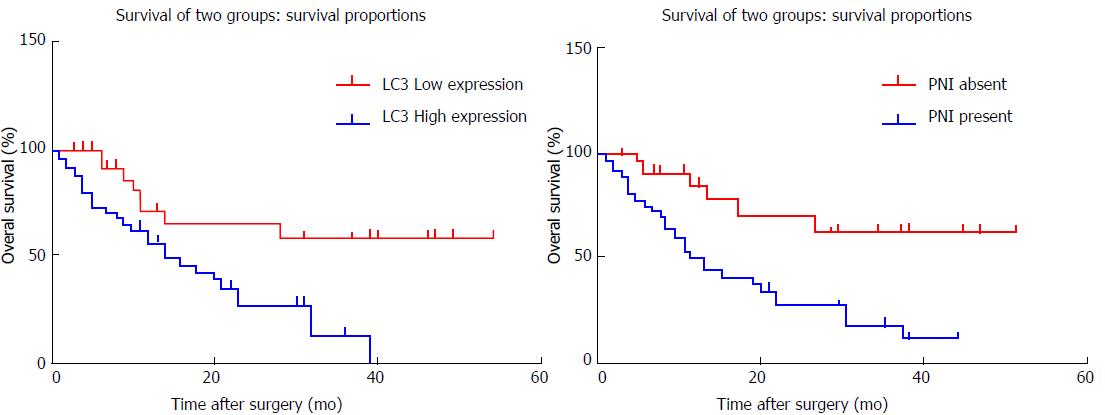
According to LC3 expression, patients were divided into a high-expression or a low-expression group. The overall survival rate of the low-expression group was better than that of the high-expression group, and the risk of death was 2.78-times higher in the LC3 high-expression group than that in the low-expression group. This difference between the two groups was significant (Figure 2A). The patients were also divided into a nerve-invasion group and a no-nerve-invasion group according to whether there was nerve infiltration. The overall survival rate of the patients without nerve invasion was better than that of the nerve-invasion group. The risk of death was 2.93-times greater in the PNI-positive group than in the PNI-negative group, and the difference between the two groups was significant (Figure 2B). Univariate analysis showed that CA19-9 level, PNI, and LC3 expression influenced the prognosis (Table 5). A factor of P < 0.2 was added into the COX risk regression model. Multivariate analysis was performed using the stepwise conditional method. The results showed that PNI and LC3 expression were independent prognostic factors that influenced pancreatic cancer (Table 6).
| Parameter | Hazard ratio | 95%CI | P value |
| PNI (present vs absent) | 3.701 | 1.539-8.903 | 0.003a |
| LC3 (high vs low) | 3.196 | 1.433-7.126 | 0.005a |
| Sex (male vs female) | 1.154 | 0.590-2.260 | 0.676 |
| Age (> 58 yr vs ≤ 58 yr) | 1.176 | 0.621-2.225 | 0.619 |
| Tumor location (body/tail vs head) | 1.102 | 0.570-2.131 | 0.773 |
| Histologic grade (poorly vs well or moderate) | 1.287 | 0.636-2.604 | 0.484 |
| Tumor size (> 2 cm vs ≤ 2 cm) | 0.940 | 0.444-1.991 | 0.871 |
| Vascular invasion (positive vs negative) | 1.821 | 0.883-3.755 | 0.105 |
| Lymph node metastasis (positive vs negative) | 0.871 | 0.449-1.688 | 0.682 |
| AJCC stage (III + IV vs I + II) | 1.473 | 0.752-2.889 | 0.259 |
| Diabetes (present vs absent) | 1.105 | 0.522-2.337 | 0.795 |
| Pancreatitis (present vs absent) | 1.075 | 0.520-2.222 | 0.845 |
| CA19-9 level (> 37 U/mL vs ≤ 37 U/mL) | 2.648 | 1.286-5.454 | 0.008a |
Pancreatic cancer has a poor treatment outcome because of a low resection rate, early invasion and metastasis, and insensitivity to radiotherapy and chemotherapy[10-13]. PNI is common in pancreatic cancer, and also found in breast, prostate, and rectal cancers[14]. Many studies have suggested that PNI is the main cause of abdominal pain in patients and is also one of the important causes of local recurrence of pancreatic cancer[15-19]. PNI is a continuous process involving multiple molecular factors and tumor microenvironment, but it is unclarified how cancer cells maintain their survival and proliferation from pancreatic cancer tissues to the external pancreatic plexus[20]. Autophagy is the process of degrading cytoplasmic proteins or organelles through lysosomes. Under physiological conditions, autophagy plays a major role in maintaining the intracellular environment stability[21,22]. Autophagy is an important mechanism of escaping apoptosis for tumor cells. Moreover, autophagy may mediate resistance to chemotherapy in pancreatic cancer[23-25]. Therefore, this study was designed and completed in a retrospective manner to evaluate the relationship between pancreatic cancer cell autophagy and PNI and patient survival.
This study found that high expression of LC3 was present in the cancer nests around the nerve infiltration, consistent with the discovery of Yang et al[7]. In histology terms, it has been suggested that high LC3 expression is related to PNI. Further analysis of immunohistochemical data showed that there was a significant positive correlation between LC3 expression and PNI in pancreatic cancer tissues. Therefore, we speculated that, in the PNI process, a high autophagy level may assist cancer cells in escaping apoptosis, avoiding the damage of adverse stress and providing energy for the invasion and metastasis of pancreatic cancer.
There is dissidence in the correlation between PNI and lymph node metastasis[26-28]. This study showed that PNI was associated with lymph node metastasis, pancreatitis, and CA19-9 levels (P < 0.05), while it had no association with sex, age, tumor location, tumor size, histological grade, clinical stage, vascular invasion, or diabetes mellitus. LC3 was related to lymph node metastasis but no other clinicopathological features. Multivariate logistic regression analysis also showed that LC3, lymph node metastasis, pancreatitis, and CA19-9 levels were the factors that influenced PNI, while PNI was an independent factor affecting LC3 expression. Tanaka et al[26] and Ozaki et al[28] believe that lymph node metastasis can promote PNI. The cancer cells in lymph node metastases can form a lymphatic satellite around the nerve and break through the nerve membrane; then, nerve invasion occurs. This study is consistent with results suggesting the need for regional lymph node dissection during surgical treatment[27]. Through this study, we can conclude that high LC3 expression, lymph node metastasis, pancreatitis, and CA19-9 levels usually indicate the possibility of nerve invasion. Gender, age, tumor site, tumor size, histological grade, clinical stage, vascular invasion, and diabetes are not effective indicators of neural invasion and autophagy and cannot be used as a determinant of resection of the peripancreatic nerve during surgery.
The overall survival rates of the LC3 high-expression group and the nerve-invasion group were significantly lower than those of the low-expression group and the no-nerve-invasion group. Univariate analysis showed that the level of CA19-9, PNI, and autophagy were associated with prognostic factors. Multivariate analysis showed that PNI and high LC3 expression were independent prognostic factors in pancreatic cancer patients. Autophagy is very complex and often plays an important role in tumor progression[6,29]. Interestingly, this study found that high autophagy level is closely related to PNI, while both of which are independent risk factors for pancreatic cancer with a poor prognosis. The autophagy associated with poor survival in pancreatic cancer could be explained by the properties of autophagy assisting cancer cells to evade stress-induced apoptosis in PNI environment and undoubtedly promote tumor cell survival[30,31]. Therefore, the high autophagy of cancer cells may promote the malignant progression of pancreatic cancer, resulting in PNI and the poor treatment outcome in patients with the disease.
In summary, autophagy and PNI of pancreatic cancer cells are independent risk factors for adverse prognosis. There is a significant correlation between them, and there may be a pathway between them through which they interact with each other to promote the malignant progression of pancreatic cancer. How to control the role of autophagy in PNI of pancreatic cancer and then improve cancer prognosis requires further studies into the molecular mechanisms involved.
Pancreatic cancer is a malignant tumor with a poor prognosis that has almost equal mortality and morbidity in patients. At the time of diagnosis, most pancreatic cancer patients have distant metastasis due to early occult symptoms, a lack of effective screening, and perineural growth characteristics. The incidence of perineural invasion (PNI) in pancreatic cancer is 80%-100% and is an important factor leading to postoperative pancreatic cancer recurrence. PNI evaluation of pancreatic cancer can predict disease recurrence and prognosis after surgery. However, the pathogenesis of PNI has not yet been defined. Autophagy, as a mechanism of avoidance of anoikis in pancreatic cancer, is closely related to the survival of pancreatic cancer cells. Microtubule-associated protein 1A/1B-light chain 3 (LC3) is a typical marker of autophagy. LC3 labeling has been used to evaluate autophagy, and high levels of LC3 expression have been found in pancreatic cancer cells. In addition, a previous study showed that pancreatic cancer cells with PNI have higher levels of autophagy. No study has examined the relationship between autophagy and PNI in pancreatic cancer cells.
The relationship between autophagy and PNI was explored for the first time in pancreatic cancer. Pancreatic cancer PNI is related to LC3 expression-determined autophagy. PNI and LC3 expression were independent prognostic factors in pancreatic cancer. There might be a special association between autophagy and PNI, which contributes to pancreatic cancer progression. This study might provide new insight into the mechanism of PNI in pancreatic cancer.
This study focused on the relationship between pancreatic cancer cell autophagy and PNI, clinicopathological features, and prognosis. The authors found that autophagy and PNI of pancreatic cancer cells are independent risk factors for adverse prognosis. There is a significant correlation between them, and there may be a pathway between them through which they interact with each other to promote the malignant progression of pancreatic cancer. Controlling the role of autophagy in PNI of pancreatic cancer may improve cancer prognosis, which requires further studies into the molecular mechanisms involved.
Clinical and pathological data were retrospectively collected from 109 patients with pancreatic ductal adenocarcinoma who underwent radical resection at the First Affiliated Hospital of Zhengzhou University from January 2011 to August 2016. Expression levels of the autophagy-related protein LC3 and perineural invasion marker ubiquitin carboxy-terminal hydrolase in pancreatic cancer tissues were detected by immunohistochemistry. The correlations among LC3 expression, perineural invasion, and clinical pathological features in pancreatic cancer were analyzed. The patients were followed for further survival analysis.
In this study, they found that LC3, lymph node metastasis, pancreatitis, and CA199 level were factors that influenced neural invasion, whereas only neural invasion itself was an independent factor of high LC3 expression. Perineural invasion and LC3 expression were independent risk factors for poor prognosis in pancreatic cancer. There is a significant correlation between them.
This study focused on the relationship between pancreatic cancer cell autophagy and PNI, clinicopathological features and prognosis. The authors found that autophagy and PNI of pancreatic cancer cells are independent risk factors for adverse prognosis. There is a significant correlation between them, and there must be a pathway between them through which they interact with each other to promote the malignant progression of pancreatic cancer. Controlling the role of autophagy in PNI of pancreatic cancer may improve cancer prognosis, which requires further studies into the molecular mechanisms involved.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 13046] [Article Influence: 1304.6] [Reference Citation Analysis (3)] |
| 2. | Zhang Y, Dang C, Ma Q, Chen W, Nagata K. Predictors of systemic chemotherapy contraindication in pancreatic cancer patients with distant metastasis. Hepatogastroenterology. 2007;54:254-259. [PubMed] |
| 3. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2226] [Article Influence: 139.1] [Reference Citation Analysis (2)] |
| 4. | Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1243] [Cited by in RCA: 1191] [Article Influence: 74.4] [Reference Citation Analysis (3)] |
| 5. | Zhou Y, Zhou Q, Chen R. Pancreatic stellate cells promotes the perineural invasion in pancreatic cancer. Med Hypotheses. 2012;78:811-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Brech A, Ahlquist T, Lothe RA, Stenmark H. Autophagy in tumour suppression and promotion. Mol Oncol. 2009;3:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Yang S, Wang X, Contino G, Liesa M, Sahin E, Ying H, Bause A, Li Y, Stommel JM, Dell’antonio G. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 2011;25:717-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 1212] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 8. | Li J, Ma Q, Liu H, Guo K, Li F, Li W, Han L, Wang F, Wu E. Relationship between neural alteration and perineural invasion in pancreatic cancer patients with hyperglycemia. PLoS One. 2011;6:e17385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Zhu Z, Friess H, diMola FF, Zimmermann A, Graber HU, Korc M, Büchler MW. Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol. 1999;17:2419-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 192] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Moschidis A, Papageorgiou A, Atmatzidis K, Tsalis K, Moschidis E, Livanis J, Chrysogelou E, Mourelatos D, Tsavdaridis D, Harlaftis N. Synergistic antitumor activity of oxaliplatin in combination with gemcitabine in pancreatic tumor-bearing mice. Chemotherapy. 2007;53:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Takahashi H, Akita H, Gotoh K, Kobayashi S, Marubashi S, Miyoshi N, Sugimura K, Motoori M, Kishi K, Noura S. Preoperative gemcitabine-based chemoradiation therapy for pancreatic ductal adenocarcinoma of the body and tail: impact of splenic vessels involvement on operative outcome and pattern of recurrence. Surgery. 2015;157:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1500] [Cited by in RCA: 1744] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 13. | Lee MG, Lee SH, Lee SJ, Lee YS, Hwang JH, Ryu JK, Kim YT, Kim DU, Woo SM. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with advanced pancreatic cancer who have progressed on gemcitabine-based therapy. Chemotherapy. 2013;59:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Agarwal JP, Jain S, Gupta T, Tiwari M, Laskar SG, Dinshaw KA, Chaturvedi P, D’cruz AK, Shrivastava SK. Intraoral adenoid cystic carcinoma: prognostic factors and outcome. Oral Oncol. 2008;44:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 362] [Article Influence: 24.1] [Reference Citation Analysis (1)] |
| 16. | Åkerberg D, Ansari D, Andersson R. Re-evaluation of classical prognostic factors in resectable ductal adenocarcinoma of the pancreas. World J Gastroenterol. 2016;22:6424-6433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1:469-476. [PubMed] |
| 18. | Cavel O, Shomron O, Shabtay A, Vital J, Trejo-Leider L, Weizman N, Krelin Y, Fong Y, Wong RJ, Amit M. Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor. Cancer Res. 2012;72:5733-5743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 19. | Li X, Ma G, Ma Q, Li W, Liu J, Han L, Duan W, Xu Q, Liu H, Wang Z. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol Cancer Res. 2013;11:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Abiatari I, DeOliveira T, Kerkadze V, Schwager C, Esposito I, Giese NA, Huber P, Bergman F, Abdollahi A, Friess H. Consensus transcriptome signature of perineural invasion in pancreatic carcinoma. Mol Cancer Ther. 2009;8:1494-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT. Mitochondrial recycling and aging of cardiac myocytes: the role of autophagocytosis. Exp Gerontol. 2003;38:863-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1271] [Cited by in RCA: 1338] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 23. | Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 1107] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 24. | Noman MZ, Janji B, Kaminska B, Van Moer K, Pierson S, Przanowski P, Buart S, Berchem G, Romero P, Mami-Chouaib F. Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer Res. 2011;71:5976-5986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 25. | Notte A, Leclere L, Michiels C. Autophagy as a mediator of chemotherapy-induced cell death in cancer. Biochem Pharmacol. 2011;82:427-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 26. | Tanaka A, Matsumura E, Yosikawa H, Uchida T, Machidera N, Kubo R, Okuno K, Koh K, Watatani M, Yasutomi M. An evaluation of neural invasion in esophageal cancer. Surg Today. 1998;28:873-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Ayala GE, Dai H, Ittmann M, Li R, Powell M, Frolov A, Wheeler TM, Thompson TC, Rowley D. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64:6082-6090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 189] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 28. | Ozaki H, Hiraoka T, Mizumoto R, Matsuno S, Matsumoto Y, Nakayama T, Tsunoda T, Suzuki T, Monden M, Saitoh Y. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 132] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Schmukler E, Grinboim E, Schokoroy S, Amir A, Wolfson E, Kloog Y, Pinkas-Kramarski R. Ras inhibition enhances autophagy, which partially protects cells from death. Oncotarget. 2013;4:145-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1483] [Cited by in RCA: 1474] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 31. | Fujii S, Mitsunaga S, Yamazaki M, Hasebe T, Ishii G, Kojima M, Kinoshita T, Ueno T, Esumi H, Ochiai A. Autophagy is activated in pancreatic cancer cells and correlates with poor patient outcome. Cancer Sci. 2008;99:1813-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kawakubo K, Kanda T, Rungsakulkij N, Tomizawa M S- Editor: Wei LJ L- Editor: Wang TQ E- Editor: Ma YJ