Published online Oct 28, 2017. doi: 10.3748/wjg.v23.i40.7221
Peer-review started: August 19, 2017
First decision: August 29, 2017
Revised: September 10, 2017
Accepted: September 20, 2017
Article in press: September 19, 2017
Published online: October 28, 2017
Processing time: 71 Days and 14.7 Hours
To compare the effect of University of Wisconsin (UW) solution with or without metformin, an AMP-activated protein kinase (AMPK) activator, for preserving standard and marginal liver grafts of young and aged rats ex vivo by hypothermic machine perfusion (HMP).
Eighteen young (4 mo old) and 18 aged (17 mo old) healthy male SD rats were selected and randomly divided into three groups: control group, UW solution perfusion group (UWP), and UW solution with metformin perfusion group (MUWP). Aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), interleukin-18 (IL-18), and tumor necrosis factor-alpha (TNF-α) in the perfused liquid were tested. The expression levels of AMPK and endothelial nitric oxide synthase (eNOS) in liver sinusoidal endothelial cells were also examined. Additionally, microscopic evaluation of the harvested perfused liver tissue samples was done.
AST, ALT, LDH, IL-18 and TNF-α levels in the young and aged liver-perfused liquid were, respectively, significantly lower in the MUWP group than in the UWP group (P < 0.05), but no significant differences were found between the young and aged MUWP groups. Metformin increased the expression of AMPK and eNOS protein levels, and promoted the extracellular release of nitric oxide through activation of the AMPK-eNOS mediated pathway. Histological examination revealed that in the MUWP group, the extent of liver cells and tissue damage was significantly reduced compared with the UWP group.
The addition of metformin to the UW preservative solution for ex vivo HMP can reduce rat liver injury during cold ischemia, with significant protective effects on livers, especially of aged rats.
Core tip: Metformin can activate the AMP-activated protein kinase pathway that could enhance the activity of endothelial nitric oxide synthase and finally increase the generation of nitric oxide, which plays an important role in the protection of liver sinusoidal endothelial cells. Hence, our study was designed to evaluate the protective effect of University of Wisconsin storage solution with metformin for preserving standard and marginal liver grafts of young and aged rats ex vivo by hypothermic machine perfusion (HMP). According to the results, HMP with metformin plays a significant protective role for liver grafts during cold ischemia, with significant effects especially for aged-marginal donors.
- Citation: Chai YC, Dang GX, He HQ, Shi JH, Zhang HK, Zhang RT, Wang B, Hu LS, Lv Y. Hypothermic machine perfusion with metformin-University of Wisconsin solution for ex vivo preservation of standard and marginal liver grafts in a rat model. World J Gastroenterol 2017; 23(40): 7221-7231
- URL: https://www.wjgnet.com/1007-9327/full/v23/i40/7221.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i40.7221
Currently, liver transplantation is the only effective therapy for end-stage liver disease[1]. Both preservation of donor organs and post-transplant ischemic reperfusion injury (IRI) are important factors affecting the prognosis of transplantation[2]. At present, due to the shortage of liver donation, marginal donation, which includes aged donation, adipo-hepatic donation, and donation after cardiac death (DCD), increases the risk for more severe IRI because of suboptimal function and long-term warm and cold ischemia[3-5]. Cold ischemia injury plays an important role in the IRI mechanism after revascularization of transplants. In this period, the liver sinusoidal endothelial cells are the first to be injured in a donor liver, causing damage of the stable hepatic microenvironment, hepatic microcirculation disturbance, and exacerbation of IRI[6]. Therefore, there is a current pressing need to explore and improve methods of organ preservation and minimize IRI of donor livers during transplantation[7,8].
In recent years, machine perfusion (MP) has been explored as an alternate method of organ preservation to static cold storage. Clinically, hypothermic machine perfusion (HMP, 4-6 °C) has been effective for kidney transplantation, but MP methods have not been widely used in liver transplantation. According to the latest research, MP has been meaningful for the preservation and repair of marginal liver donation[9], but this still needs further clinical verification[10,11].
Another important direction of research on donor liver cold preservation is the auxiliary protective intervention of donor livers against IRI factors of microcirculation[12] and hepatocyte metabolism[13] through drugs. Activation of adenosine 5’-monophosphate-activated protein kinase (AMPK) signaling pathways increases the activity of endothelial nitric oxide synthase (eNOS) to generate nitric oxide. This provides a cytoprotective effect to the hepatic sinusoidal endothelium of the donor liver and has been considered for preconditioning of the donor liver to reduce IRI. In addition, AMPK signaling is also known to regulate glucose metabolism and prevent cell death, thus extending the cytoprotective effect on hepatocytes[14]. Therefore, it plays an important role in protecting hepatic sinusoidal endothelium and reducing injury of donor livers[15]. As an agonist of AMPK, metformin additionally lowers the blood glucose by reducing hepatic gluconeogenesis and strengthening glucose uptake of peripheral tissue[16].
Hence, we hypothesized that liver sinusoidal endothelial cells can be protected from injury by activating AMPK signaling pathways with the addition of metformin perfused in vitro, which could ultimately lead to an improvement of liver donor organ preservation. Consequently, in this study, we added metformin to University of Wisconsin (UW) solution, ex vivo, in HMP models of livers of young and aged rats and investigated the effects on biochemical indicators and sinusoidal cell morphology.
A total of 18 young healthy male SD rats (4 mo old, weighing 250–300 g) and 18 aged (17 mo old, weighing 600–630 g) SD rats were randomly selected for the study. The experimental animals were provided by Animal Experiment Center of Xi’an Jiaotong University.
LongerPump DG-2-B/D Precise Miniature Peristaltic Pump (Longer Precision Pump Co., Ltd., Baoding, China) with a rotation speed of 0-100 rpm and a flow rate of 0-48 mL/min; SPS-1 Static Preservation Solution (UW solution, Organ Recovery Systems, Inc.); and metformin (1,1-dimethylbiguanide hydrochloride, CAS # 1115-70-4, Biomol GmbH) were used. A 165 mg/L stock solution of metformin was prepared by adding 2.5 mL of metformin solution at a concentration of 1 g/10 mL to 1000 mL of sterile water, stirring until dissolved, and then adding this (0.66 mL) to 1000 mL of UW solution. A final metformin concentration of 0.165 mg/L or 1 mmol/L was used. Serum interleukin-18 (IL-18) and tumor necrosis-alpha (TNF-α) levels were measured by enzyme-linked immunosorbent assay (ELISA) using commercial kits (Rat Interleukin 18 ELISA kit, CLOUD-CLONE CORP., TX, United States; Rat TNF-alpha ELISA kit, MultiSciences Biotech Co., Ltd, Shanghai, China).
Young and aged rats were respectively randomly divided into three groups, with six rats in each group. The groups were: control groups A (young rats) and D (aged rats), UW solution perfusion (UWP) groups B (young rats) and E (aged rats), and experimental groups C (young rats) and F (aged rats) that were perfused with metformin-UW solution (MUWP).
Rats were fasted for 8 h and general anesthesia was induced in all rats by intraperitoneal injection of 5% pentobarbital sodium at 20 mg/kg. Fixation, skin preparation, and disinfection were completed, and then a large median and transverse abdominal incision for laparotomy was made. The liver was isolated, heparinized, perfused in situ with cold UW solution until the liver turned into a khaki color, and rapidly harvested at room temperature. The ex vivo liver was then placed into a basin filled with cold UW solution and made to lie in the basin on an ice pad. All ex vivo livers were grouped and underwent HMP with circulating UW solution at 4 °C at a flow rate of 4 mL/min maintained at 80 mL of the total circulation volume with the help of a peristaltic pump. Groups A and D did not require extended period of HMP (only 2 h); groups B and E were perfused with UW solution for 12 h; and groups C and F were perfused mechanically with UW solution with 0.165 mg/L of metformin for 12 h. After HMP, 6 mL of the perfused liquid was collected from every group and stored at -20 °C.
Extraction of liver sinusoidal endothelial cells from young rats: After step one, the ex vivo livers of every group were perfused with Gey’s balanced salt solution (150 mL free of Ca2+ and Mg2+, mixed with pronase 400 mg and collagenase 40 mg) for 7 min at 37 °C at a flow rate of 20 mL/min. Then, the resulting mixture was centrifuged at 2500 rpm with Krebs-Henseleit solution (with 10% fetal calf serum and 0.002% DNase I) perfused at a flow rate of 10 mL/min. Cell pellet was then resuspended in DMEM supplemented with 20% fetal calf serum and 10 mL of 100 U/L penicillin G and loaded carefully in the centrifuge for 3 min at a flow perfusion rate of 15 mL/min, and accelerated at 18 mL/min for 1 min. The input flow rate was increased again by 20 mL/min, while 50 mL of the cell suspension of the effluent was collected. Once more, a 50 mL cell suspension was collected by 25 mL/min. The 100 mL homogenate was centrifuged at 2000 rpm for 5 min. The supernatant liquid was extracted out, and the liver sinusoidal endothelial cell-debris pellet was collected in the bottom of collection tube.
Extraction of total protein and examination of the expression levels of target proteins: The cell debris was lysed in Cell Lysis Buffer (Cell Signaling Technology; Beverly, MA, United States; carefully chilling 1 × stock buffer on ice and add 1 mmol/L PMSF immediately just prior to use) for 30 min on ice, and then centrifuged at 12000 rpm for 5 min at 4 °C. The supernatant with total cellular proteins was collected from every group, determined, transferred to a sterile tube (1.5 mL), and stored at -20 °C. Then, the two-dimensional electrophoresis (2-DE) maps of target proteins were obtained by Western blot to examine the expression levels, and the amounts of expression of AMPK and eNOS were analyzed with gray scale from the maps.
Tests included: (1) Biochemical indicator tests: the levels of aspartate transaminase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH), IL-8, and TNF-α in the perfused liquid were examined; (2) Histo-morphological by light microscopy: the liver tissues after perfusion were fixed with 10% formalin and immersed in the wax for sections and hematoxylin-eosin (HE) dye. A scoring system was used to grade the degree of histological damage quantitatively (by blinding) at the Department of Pathology, based on the following histological features: hydropic degeneration of hepatocytes and liver sinusoidal endothelial cells, stenosis of the hepatic sinusoid, and number of Kupffer cells. Each feature was graded as absent, mild, moderate, or massive, with a score of 0-3, respectively; and (3) Electron microscopic examination: The specimens of perfused hepatic tissue were fixed to observe by transmission electron microscopy.
All calculations were performed with SPSS 22.0 software. The grayscale of 2-DE maps of target proteins was analyzed with ImageProPlus 6.0 software. Quantitative data are expressed as mean ± SD and assessed by one- or two-way analysis of variance, and multiple comparisons between groups were performed using Student-Newman-Keuls method. Scoring data were analyzed by Kruskal-Wallis one-way analysis of variance on ranks and compared between any two groups using Mann-Whitney U test. P < 0.05 was considered statistically significant.
The control group (groups A and D) was subject to HMP for only 2 h, while the UWP (groups B and E) and MUWP groups (groups C and F) underwent HMP for 12 h.
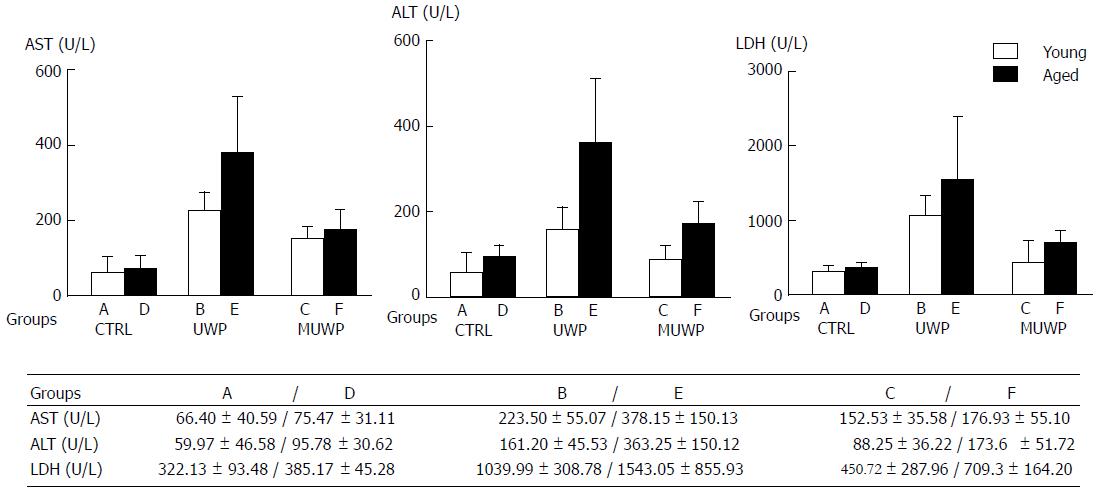
After completing the perfusion of each group, AST, ALT, and LDH levels in the perfused liquid were determined (Figure 1). There were no significant differences in AST, ALT, or LDH between the young rat control group (group A) and the aged rat control group (group D). The AST, ALT, and LDH in the UWP group (groups B and E) were significantly higher than those in groups A and D (P < 0.05), Besides, the AST and ALT in the aged group E were significantly higher than those in the young group B (P < 0.05). The AST, ALT, and LDH in the MUWP group, which consisted of the young group C and aged group F, were, respectively, significantly lower than those in the young group B and aged group E of UWP (P < 0.05), although no significant differences between the young and the aged group were found.
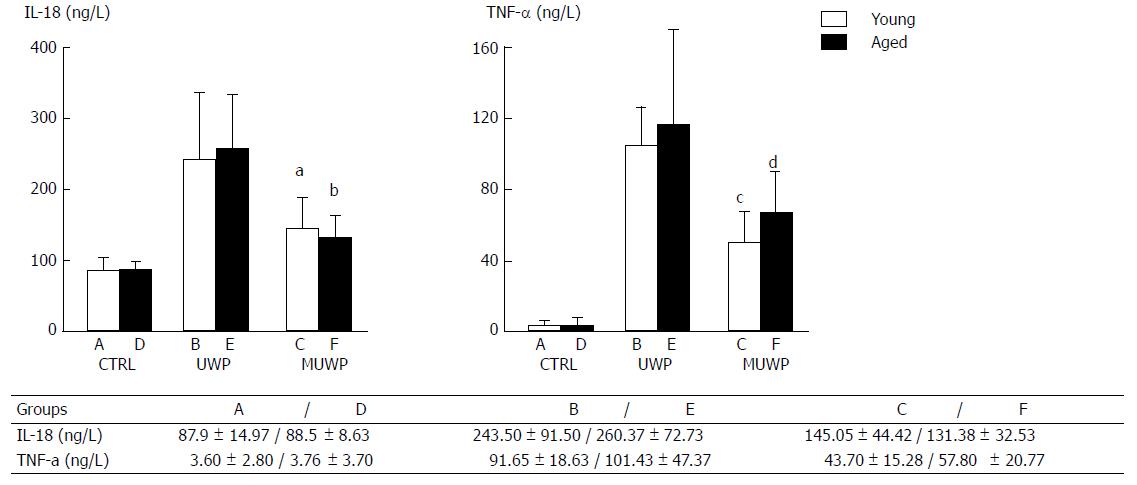
IL-18 and TNF-α levels in the perfused liquid were measured by ELISA (Figure 2). There were no significant differences in the levels of IL-18 or TNF-α between the young rat control group (group A) and the aged rat control group (group D). IL-18 and TNF-α levels in the UWP group were significantly higher than those in the control group (P < 0.05). Nevertheless, there were no significant differences between the young group B and the aged group E of UWP. IL-18 and TNF-α levels in the young group C and the aged group F of MUWP were, respectively, appreciably lower than those in the young group B and the aged group E of UWP (P < 0.05), but no significant differences between the young group C and the aged group F of MUWP were found.
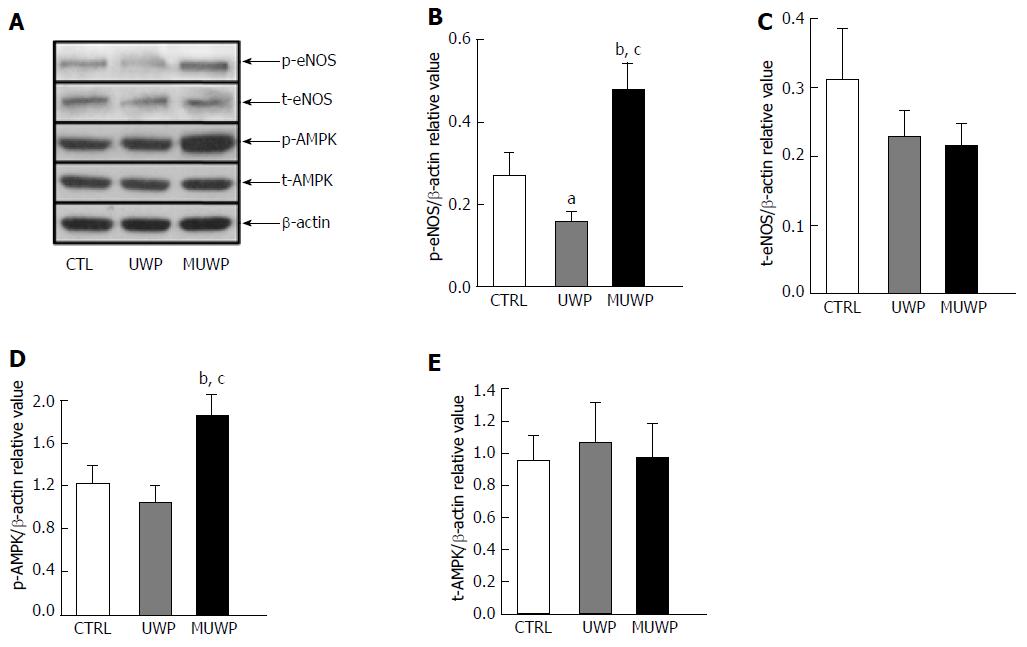
Discriminant analysis of relative gray values was used to determine the expression levels of p-eNOS, t-eNOS, p-AMPK, and t-AMPK in liver sinusoidal endothelial cells of young rats (Figure 3). The expression level of p-eNOS in the MUWP group was significantly higher (80.1%) than that in the control group (P = 0.036) and 205.88% higher than that in the UWP group (P = 0.008), which was significantly (41.1%) lower than that in the control group (P = 0.045) (Figure 3B). The expression level of t-eNOS in the control group exhibited no significant difference compared with the UWP and MUWP groups (P = 0.251) (Figure 3C). The expression level of p-AMPK in the MUWP group was significantly higher (51.7%) than that in the control group (P = 0.038) and 78.5% higher than that in the UWP group (P = 0.018), even though there were no significant differences between the control and UWP groups (P = 0.217) (Figure 3D). The expression level of t-AMPK in the control group demonstrated no significant differences compared with the UWP and the MUWP groups (P = 0.868) (Figure 3E).
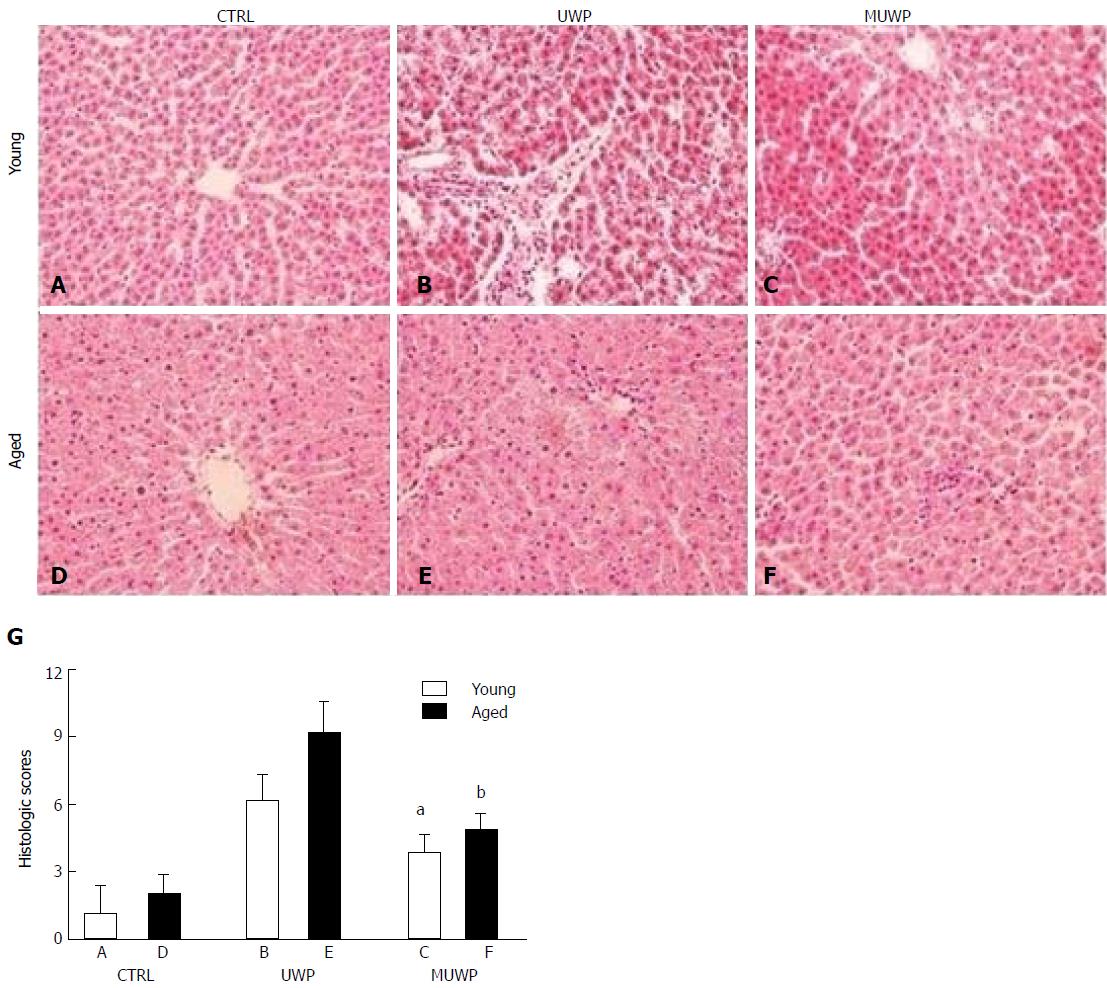
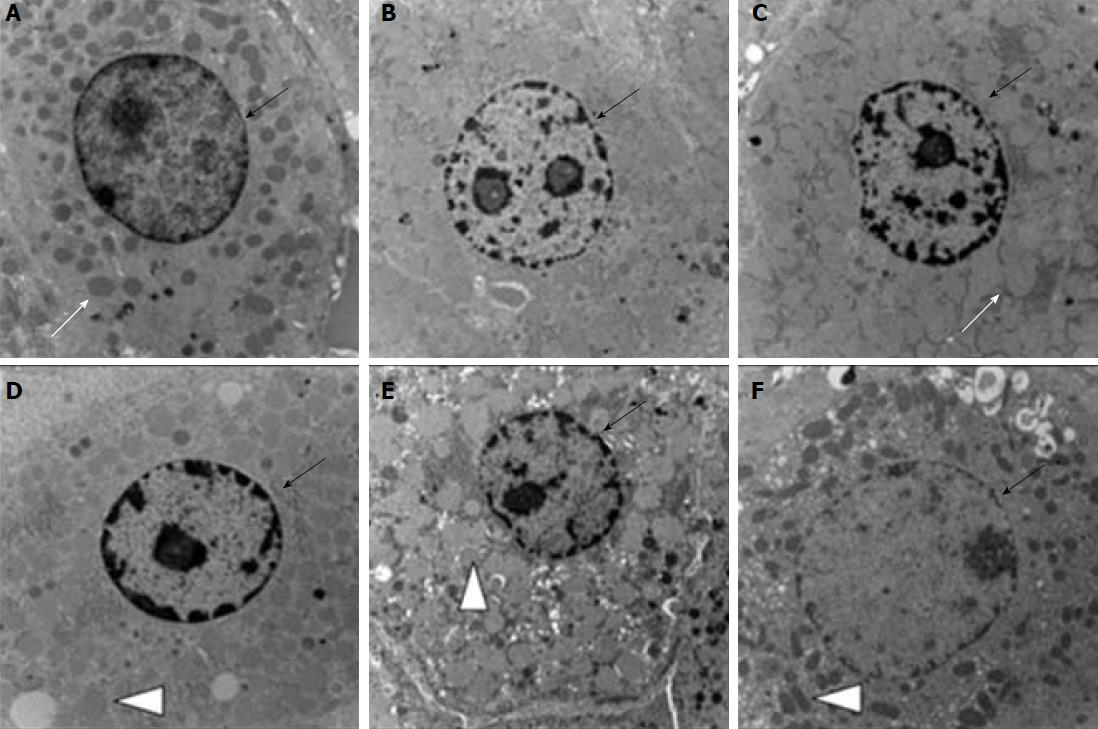
The HE-stained sections were observed under a microscope. The hepatocytes in the young rat control group (group A) and the aged rat control group (group D) were shaped normally, with no stenosis observed in the hepatic sinusoid and no swelling in the sinusoidal endothelial cells. The size of hepatocytes in group D was slightly larger than that in group A (Figure 4A-D). Cellular swelling was obvious, accompanied by narrowing of the hepatic sinusoid, in the UWP group (groups B and E), particularly, more severe in the aged group E. The swelling of the sinusoidal endothelial cells, as well as Kupffer cells was observed in both groups B and E (Figure 4B-E). Hepatocytes in the MUWP group showed mild edema, which was slightly more severe in group F than in group C, although there were no obvious differences in the hepatic sinusoid between them. Swelling of sinusoidal endothelial cells was inconspicuous in both groups; however, a small number of Kupffer cells were observed (Figure 4C-F). There were no significant differences in the histologic scores between the young rat control group (group A) and the aged rat control group (group D) (P < 0.05). The histologic scores of the young rat group and the aged rat group of UWP and MUWP were, respectively, appreciably higher than those of the young rat control group (group A) and the aged rat control group (group D), and that histologic scores of the young group C and the aged group F of MUWP were, respectively, appreciably lower than those of the young group B and the aged group E of UWP (P < 0.05). Besides, the histologic scores of aged group E were significantly higher than those of young group B of UWP (P < 0.05), but no significant differences were found between the young group C and the aged group F of MUWP (Figure 4G).
The ultrastructural changes of hepatocytes were observed under an electron microscope. Structure of hepatocytes in the young control group (group A) and aged control group (group D) was generally normal with a round and clear nucleus located in the center of the cells, while lipid droplets in the cytoplasm increased in the aged control group (group D), and fat-storing cells were seen in the Disse’s space with irregular shapes, and the cytoplasm in it contained a large amount of lipid droplets. In contrast, lipid droplets and fat-storing cells were not found in the young control group (group A), but the cells contained rich cytoplasmic organelles (Figure 5A-D). Hepatocyte swelling, nuclear chromatin condensation, extensive mitochondrial swelling, and crista fragmentation were observed in groups B and E of UWP, more severe in the aged group E. Likewise, fat-storing cells in the Disse’s space increased in the aged group E, but not found in the young group E (Figure 5B-E). In contrast, hepatocytes of the MUWP group showed mild edema with round nucleus, slightly more evident in the young group C than in the aged group F, and also the mitochondrial swelling degree was slightly higher in the young group C. In addition, the increase in lipid droplets in the cytoplasm and the number of fat-storing cells, dilation of the smooth endoplasmic reticulum, and intercellular collagenous fibrosis were only seen in the aged group F (Figure 5C-F).
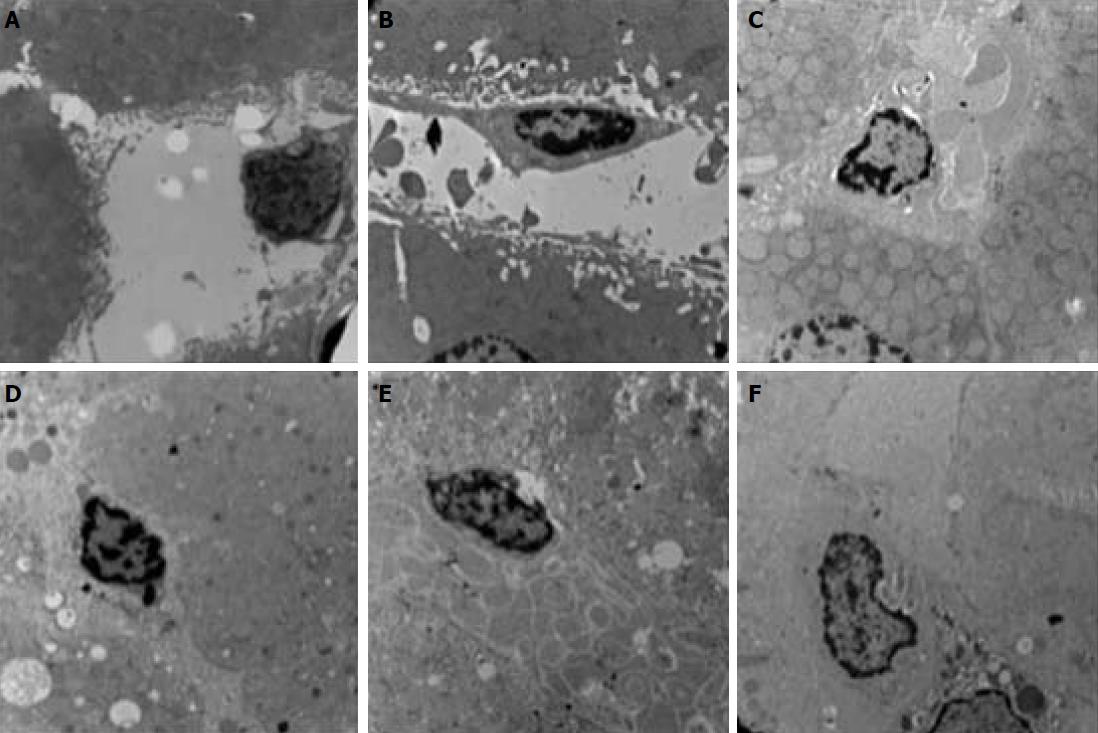
The ultrastructural changes of the hepatic sinusoid were also observed under an electron microscope. Structure of the hepatic sinusoidal endothelial cells in the young control group A and the aged control group D remained generally intact with protrusion into the sinusoid, being clear in the young group A. Kupffer cells were seen in the aged group D, though (Figure 6A-D). Sinusoidal endothelial cells were kept meristematic and flat-shaped, with chromatin showing mild margination, and cell debris-like structure together with Kupffer cells in the sinusoids was increased in groups B and E of UWP, particularly, more severely in aged group E. Likewise, fat-storing cells increased in the aged group E, while they were not found in the young group E (Figure 6B-E). The structure of the hepatic sinusoidal endothelial cells in the MUWP group remained generally normal, while a slightly larger cell volume was seen in the aged group F, and cubic-shaped sinusoidal endothelial cells with protrusion into the sinusoid were seen in the young group C. In addition, the low-electron-density protein substance could be seen in the sinusoid in the aged group F.
An increasing number of patients with liver disease await liver transplantation; the acute shortage of donor livers is only likely to continue. It was believed that aged livers from donors above 60 years old were characterized by small sizes, atherosclerosis, steatosis, and decreased function of metabolism, and were not suitable for donation[17]. However, to cope with the demand for donor livers, marginal liver donation has been applied clinically after following strict screening protocols with good transplantation outcomes and breaking the myth for the age limit of liver donors[18,19]. Unlike hearts, lungs, and kidneys, livers are much less influenced by age with respect of pathophysiological changes. However, aged livers also show morphologic changes, such as hepatic fibrosis, hepatocyte swelling, trend for multinucleation, increase of lipofuscin, and reduction in the size of the hepatocyte. Compared with young donors, aged donors show reduced and shrunken fenestra of liver sinusoidal endothelial cells, thickened hepatic sinusoidal endothelium, and pseudo capillarization formed by discontinuous basement membranes, leading to potential microcirculatory disturbance and a risk of aggravating IRI[20]. Thus, easier apoptosis and detachment of liver sinusoidal endothelial cells of aged livers further aggravate microcirculatory disturbance, seriously influence post-transplant effects, and even cause failure of some liver transplantation[21]. According to the results of this study, aged groups appeared to be more sensitive to the possible protective effect of metformin. Furthermore, the histological observations by light and electron microscopy also support this conclusion.
In order to prevent cold ischemia injury, auxiliary protective intervention by drugs with different mechanisms of action, including the MAPK agonist, is being explored[22]. Scientists from different academic institutions have published research articles in which they revealed the molecular mechanism by which metformin could activate AMPK and inhibit the mTORC1 signaling pathway in livers via the AXIN/LKB1-v-ATPase-Ragulator pathway[23,24], protect the integrity of epithelial cells in multiple stressed conditions (such as inflammation, infection, and anoxia), and even demonstrate an anticancer effect[25-27]. Before revealing this molecular mechanism, it was known that AMPK activation plays a critical role in reducing liver IRI. However, how AMPK and eNOS phosphorylation direct their effects on the endothelial function is still elusive[28], even if there is plenty of evidence to show that AMPK activation can enhance the activity of eNOS, resulting in generation of nitric oxide[15,29,30]. Extension of cold preservation by maintaining a supercooled state can make donor liver cells remain viable up to 96 h, and human livers showed improvement in endothelial function with 2 h of HMP[31]. In the current study, the AMPK activator metformin was added to ex vivo HMP models of rat livers in UW solution after HMP for 12 h. Our results showed that all the biochemical indexes and inflammatory factors in the UWP group were significantly higher than those in the control group, but all the indexes in both the young and aged groups of MUWP, respectively, were appreciably lower than those in the young and aged groups of UWP. Histological observation by light and electron microscopy showed that injury of the related microstructure was milder in the MUWP group than in the UWP group. To a certain extent, it can be deduced that metformin activated protective mechanisms. Detection of expression levels of eNOS and AMPK in the liver sinusoidal endothelial cells of young rats showed that phosphorylation of AMPK and eNOS was increased in the MUWP group at 12 h after HMP, and this can be inferred as the AMPK/eNOS pathway was activated ex vivo by metformin.
This study confirmed that the addition of metformin to organ preservation solution can activate the AMPK/eNOS pathway, and this can not only significantly decrease the inevitable injury to donor livers caused by long-term HMP, but can reduce the difference between aged and young livers after HMP, protecting livers of aged rats, which should probably improve the utilization of marginal liver donor tissues. However, whether metformin can sequentially improve hepatic injury during reperfusion-ischemia requires further investigation. But at least, we provided a novel idea, which is also a simple procedure for drug auxiliary intervention with HMP in ex vivo rat donor liver and deserves further research with a promising prospect.
In conclusion, this study confirmed that metformin can activate the AMPK/eNOS pathway and reduce injury to ex vivo rat liver during cold ischemia in MP. The combination of metformin with the organ preservation solution effectively enhanced the quality of donor livers, with significant protective effects on livers especially of aged rats, which could be used to improve the utilization of marginal livers.
Liver transplantation is the only effective therapy for end-stage liver disease. At present, due to the shortage of liver donation, marginal donation, which includes aged donation, adipo-hepatic donation, and donation after cardiac death, increases the risk for more severe ischemic reperfusion injury (IRI) because of suboptimal function and long-term warm and cold ischemia. Therefore, there is a current pressing need to explore and improve methods of organ preservation and minimize IRI of donor livers during transplantation.
According to the latest research, machine perfusion has been meaningful for the preservation and repair of marginal liver donation. Another important direction of research on donor liver cold preservation is the auxiliary protective intervention of donor livers against IRI factors of microcirculation and hepatocyte metabolism through drugs. Activation of adenosine 5’-monophosphate-activated protein kinase (AMPK) signaling pathways increases eNOS activity to generate nitric oxide (NO), which plays an important role in the protection of liver sinusoidal endothelial cells. Metformin is an agonist of AMPK. Hence, we assumed that liver sinusoidal endothelial cells can be protected from injury by activating AMPK signaling pathways with the addition of metformin perfused in vitro, which could ultimately cause an improvement of liver donor organ preservation.
In this study, we added metformin to University of Wisconsin (UW) solution, to compare the effect of UW solution with or without metformin, an AMPK activator, for preserving standard and marginal criteria liver grafts of young and aged rats ex vivo by hypothermic machine perfusion (HMP).
Eighteen young (4-mo-old) and 18 aged (17-mo-old) healthy male SD rats were selected and randomly divided into three groups: control group, UW solution perfusion group (UWP), and UW solution with metformin perfusion group (MUWP). Aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), interleukin-18 (IL-18), and tumor necrosis factor-alpha (TNF-α) in the perfused liquid were tested. The expression levels of AMPK and eNOS in liver sinusoidal endothelial cells were also examined. Additionally, microscopic evaluation of the harvested perfused liver tissue samples was done.
AST, ALT, LDH, IL-18 and TNF-α levels in the young and aged liver-perfused liquid in the MUWP group were, respectively, significantly lower than those in the UWP group (P < 0.05), but no significant differences between the young and aged MUWP groups were found. Metformin increased the expression of AMPK and eNOS protein levels, and promoted the extracellular release of nitric oxide through activation of the AMPK-eNOS mediated pathway. Histological examination revealed that in the MUWP group, the extent of liver cells and tissue damage was significantly reduced compared with the UWP group.
This experiment confirmed that the addition of metformin to organ preservation solution can activate AMPK/eNOS pathway, which can not only reduce injury to ex vivo rat livers during cold ischemia, but can reduce the difference between aged and young livers after HMP, with especially significant effects of protecting livers of aged rats, which should probably improve the utilization of marginal liver donor tissues. However, whether metformin can sequentially improve hepatic injury during reperfusion-ischemia requires further investigation. But at least, we provided a novel idea, which is also a simple procedure for drug auxiliary intervention with HMP in ex vivo rat donor livers and deserves further research with a promising prospect.
| 1. | Potosek J, Curry M, Buss M, Chittenden E. Integration of palliative care in end-stage liver disease and liver transplantation. J Palliat Med. 2014;17:1271-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Matsuno N, Uchida K, Furukawa H. Impact of machine perfusion preservation of liver grafts from donation after cardiac death. Transplant Proc. 2014;46:1099-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Guan Z, Lv Y. The research progress of improving the quality of the marginal donor liver in liver transplantation. Yixue Zongshu. 2015;21:101-104. |
| 4. | Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 5. | He T, Zheng Z, Wu Y. Advances in research on ischemia/reperfusion injury in liver transplantation. Yixue Zongshu. 2011;17:1640-1642. |
| 6. | Miyashita T, Nakanuma S, Ahmed AK, Makino I, Hayashi H, Oyama K, Nakagawara H, Tajima H, Takamura H, Ninomiya I. Ischemia reperfusion-facilitated sinusoidal endothelial cell injury in liver transplantation and the resulting impact of extravasated platelet aggregation. Eu Surg. 2016;48:92-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Lowalekar SK, Cao H, Lu XG, Treanor PR, Thatte HS. Sub-normothermic preservation of donor hearts for transplantation using a novel solution, Somah: a comparative pre-clinical study. J Heart Lung Transplant. 2014;33:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Chinese college of cransplant doctors, Division of branch of surgery, Chinese medical association china liver transplant registry scientific committ. Chinese expert consensus on the organ protection of transplantation (2016 edition). Zhonghua Xiaohua Waike Zazhi. 2016;15:645-654. |
| 9. | Dirkes MC, Post IC, Heger M, van Gulik TM. A novel oxygenated machine perfusion system for preservation of the liver. Artif Organs. 2013;37:719-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Dutkowski P, Schlegel A, de Oliveira M, Müllhaupt B, Neff F, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2014;60:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 11. | Ravikumar R, Jassem W, Mergental H, Heaton N, Mirza D, Perera MT, Quaglia A, Holroyd D, Vogel T, Coussios CC. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am J Transplant. 2016;16:1779-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 381] [Article Influence: 38.1] [Reference Citation Analysis (1)] |
| 12. | Hara Y, Akamatsu Y, Maida K, Kashiwadate T, Kobayashi Y, Ohuchi N, Satomi S. A new liver graft preparation method for uncontrolled non-heart-beating donors, combining short oxygenated warm perfusion and prostaglandin E1. J Surg Res. 2013;184:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Padrissa-Altés S, Zaouali MA, Boncompagni E, Bonaccorsi-Riani E, Carbonell T, Bardag-Gorce F, Oliva J, French SW, Bartrons R, Roselló-Catafau J. The use of a reversible proteasome inhibitor in a model of Reduced-Size Orthotopic Liver transplantation in rats. Exp Mol Pathol. 2012;93:99-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Yang YM, Han CY, Kim YJ, Kim SG. AMPK-associated signaling to bridge the gap between fuel metabolism and hepatocyte viability. World J Gastroenterol. 2010;16:3731-3742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Tabka D, Bejaoui M, Javellaud J, Roselló-Catafau J, Achard JM, Abdennebi HB. Effects of Institut Georges Lopez-1 and Celsior preservation solutions on liver graft injury. World J Gastroenterol. 2015;21:4159-4168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Dumitrescu R, Mehedintu C, Briceag I, Purcărea VL, Hudita D. Metformin-clinical pharmacology in PCOs. J Med Life. 2015;8:187-192. [PubMed] |
| 17. | Jiménez-Romero C, Caso Maestro O, Cambra Molero F, Justo Alonso I, Alegre Torrado C, Manrique Municio A, Calvo Pulido J, Loinaz Segurola C, Moreno González E. Using old liver grafts for liver transplantation: where are the limits? World J Gastroenterol. 2014;20:10691-10702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (2)] |
| 18. | Chedid MF, Rosen CB, Nyberg SL, Heimbach JK. Excellent long-term patient and graft survival are possible with appropriate use of livers from deceased septuagenarian and octogenarian donors. HPB (Oxford). 2014;16:852-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Jiménez-Romero C, Cambra F, Caso O, Manrique A, Calvo J, Marcacuzco A, Rioja P, Lora D, Justo I. Octogenarian liver grafts: Is their use for transplant currently justified? World J Gastroenterol. 2017;23:3099-3110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 20. | Pezzati D, Ghinolfi D, De Simone P, Balzano E, Filipponi F. Strategies to optimize the use of marginal donors in liver transplantation. World J Hepatol. 2015;7:2636-2647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Ghinolfi D, Marti J, De Simone P, Lai Q, Pezzati D, Coletti L, Tartaglia D, Catalano G, Tincani G, Carrai P. Use of octogenarian donors for liver transplantation: a survival analysis. Am J Transplant. 2014;14:2062-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Kosieradzki M, Pratschke J, Kupiec-Węgliński J, Rowiński W. Ischemia/Reperfusion injury, its mechanisms, and prevention. J Transplant. 2012;2012:610370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Zhang CS, Li M, Ma T, Zong Y, Cui J, Feng JW, Wu YQ, Lin SY, Lin SC. Metformin Activates AMPK through the Lysosomal Pathway. Cell Metab. 2016;24:521-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 24. | Zhang CS, Jiang B, Li M, Zhu M, Peng Y, Zhang YL, Wu YQ, Li TY, Liang Y, Lu Z. The lysosomal v-ATPase-Ragulator complex is a common activator for AMPK and mTORC1, acting as a switch between catabolism and anabolism. Cell Metab. 2014;20:526-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 448] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 25. | Lei Y, Yi Y, Liu Y, Liu X, Keller ET, Qian CN, Zhang J, Lu Y. Metformin targets multiple signaling pathways in cancer. Chin J Cancer. 2017;36:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Kang JI, Hong JY, Lee HJ, Bae SY, Jung C, Park HJ, Lee SK. Anti-Tumor Activity of Yuanhuacine by Regulating AMPK/mTOR Signaling Pathway and Actin Cytoskeleton Organization in Non-Small Cell Lung Cancer Cells. PLoS One. 2015;10:e0144368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 2017;45:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 605] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 28. | Anavi S, Madar Z, Tirosh O. Non-alcoholic fatty liver disease, to struggle with the strangle: Oxygen availability in fatty livers. Redox Biol. 2017;13:386-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Fu P, Li W. Nitric Oxide in Liver Ischemia–Reperfusion Injury. Liver Pathophysiology. 2017;125-127 p. [DOI] [Full Text] |
| 30. | Bektas S, Karakaya K, Can M, Bahadir B, Guven B, Erdogan N, Ozdamar SO. The effects of tadalafil and pentoxifylline on apoptosis and nitric oxide synthase in liver ischemia/reperfusion injury. Kaohsiung J Med Sci. 2016;32:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Burlage LC, Karimian N, Westerkamp AC, Visser N, Matton APM, van Rijn R, Adelmeijer J, Wiersema-Buist J, Gouw ASH, Lisman T. Oxygenated hypothermic machine perfusion after static cold storage improves endothelial function of extended criteria donor livers. HPB (Oxford). 2017;19:538-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gilbert MR, Jones G, Otto G S- Editor: Wei LJ
L- Editor: A E- Editor: Ma YJ