Published online Oct 21, 2017. doi: 10.3748/wjg.v23.i39.7160
Peer-review started: June 30, 2017
First decision: July 27, 2017
Revised: September 21, 2017
Accepted: September 29, 2017
Article in press: September 28, 2017
Published online: October 21, 2017
Processing time: 114 Days and 22.6 Hours
To identify the potential risk factors of cholangiocarcinoma, we determined the characteristics of cholangiocarcinoma patients among 5 different regions of Thailand.
All patients diagnosed with cholangiocarcinoma between 2008 and 2013 were identified using the Nationwide Hospital Admission Data registry (n = 39421). Baseline characteristics, comorbidities and survival were abstracted.
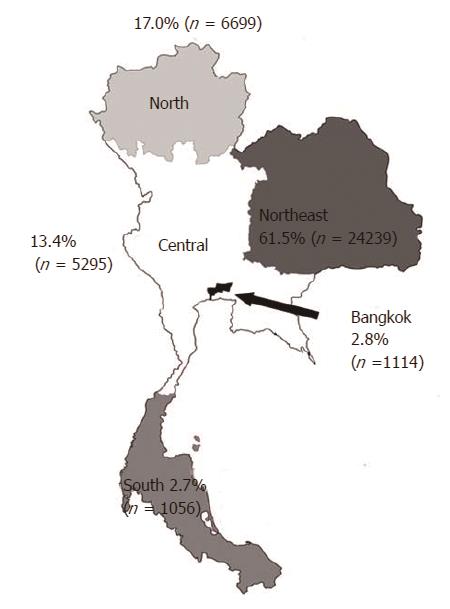
The annual incidence during the study period was stable in all regions. Most patients lived in the Northeast (62.8%), followed by the North (16.9%), Central (12.3%), Bangkok (5.4%), and South (n = 2.6%) regions (P < 0.0001). Significantly more cholangiocarcinoma patients had diabetes, cirrhosis, and chronic viral hepatitis B/C infection than non-cholangiocarcinoma participants (diabetes: 11.42% vs 5.28%; cirrhosis: 4.81% vs 0.92%; hepatitis B: 0.74% vs 0.12%; and hepatitis C: 0.50% vs 0.10%, P < 0.0001 for all, respectively). The overall 1-year mortality rate was 81.7%, with a stable trend over time.
Diabetes and chronic liver diseases may be associated with cholangiocarcinoma in the Thai population.
Core tip: Cholangiocarcinoma is highly prevalent in Thailand, particularly in the Northeast region. The high cholangiocarcinoma incidence in this region is known to be associated with a high prevalence of liver fluke infection. Cirrhosis, diabetes, and chronic viral hepatitis B and C infections have been recently identified as risk factors for cholangiocarcinoma in Western countries. In this study, we found that diabetes and chronic liver diseases may be associated with cholangiocarcinoma in the Thai population. Further study to determine the magnitude of the impact of these factors on cholangiocarcinoma development in the Thai population is necessary.
- Citation: Chaiteerakij R, Pan-ngum W, Poovorawan K, Soonthornworasiri N, Treeprasertsuk S, Phaosawasdi K. Characteristics and outcomes of cholangiocarcinoma by region in Thailand: A nationwide study. World J Gastroenterol 2017; 23(39): 7160-7167
- URL: https://www.wjgnet.com/1007-9327/full/v23/i39/7160.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i39.7160
Cholangiocarcinoma (CCA), a malignancy of the biliary tract epithelium, is of increasing importance due to its continually increasing incidence worldwide[1-7]. The incidence of CCA varies substantially from region to region, with a high incidence in Asia [e.g., 22.9, 7.5, and 5.6 cases per 100000 persons in Thailand[2], China[7] and South Korea[5], respectively] and a low incidence in the United States and Europe [e.g., 3.4, 1.6, 1.3, and 1.2 cases per 100000 persons in Italy[3], the United States[6], France[8], and the United Kingdom[4], respectively]. Variation in the global incidence of CCA is due to variations in risk factors, particularly host and environmental risk factors. Within Thailand, the incidence of CCA also varies geographically[9]. The Northeast region of Thailand has the most CCA cases, with an incidence rate of 85 cases per 100000 persons per year, whereas the South region has the lowest number of cases at 5.7 cases per 100000 persons per year. The high incidence of CCA in Northeast Thailand is related to the high prevalence of liver fluke or Opisthorchis viverrini (OV) infection[10].
In addition to liver fluke infection, primary sclerosing cholangitis (PSC) is another major risk factor for CCA[11]. PSC is a major cause of CCA in Western populations and is associated with an 80- to 160-fold increase in CCA risk[12,13]. Although PSC markedly increases CCA risk, PSC is present in only 20% of all CCA patients[14]. Most CCA patients do not have identifiable risk factors.
Cirrhosis, chronic viral hepatitis B and C infection, diabetes, obesity, and smoking have recently been shown to be associated with increased CCA risk[12-14]. These factors were believed to contribute to the increasing incidence of CCA in the United States[15]. The question regarding whether or not these preexisting conditions contribute to CCA development in the Thai population, particularly in people living in areas that are non-endemic for liver fluke infection, has not yet been studied. Accordingly, the objectives of this study were to compare the characteristics of CCA patients among the 5 different regions of Thailand. Secondarily, survival outcomes were determined. This population-based study was conducted using data from the National Health Security Office, Thailand.
The protocol of this study was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand (IRB No. 113/58).
The data were obtained from the Nationwide Hospital Admission Data (NHAD) registry. The data were already de-identified before the investigators accessed them. Briefly, the NHAD registry is a database that contains hospitalization data of Thai citizens who are covered by the national Medical Welfare Scheme (MWS). The MWS was established in Thailand in 2000 and is currently one of 3 major health insurance systems. During the study period, the MWS covered approximately 47 million of the 64 million total Thai people in all age groups across Thailand. Those who have health insurance coverage by the MWS can access health services in hospitals that are registered with the National Health Security Office. Patients have access to the hospitals that are assigned to each individual by the household registration system. At the time of data collection, there were 39421 hospitals in the registry, of which 14402 (36.5%) were primary or community hospitals that provide primary healthcare, 11277 (28.6%) were secondary hospitals that provide general healthcare, and 13742 (34.9%) were tertiary referral hospitals that provide specialized care for complex cases.
CCA cases: All patients diagnosed with CCA who were admitted to affiliated hospitals during the January 2008 to December 2013 study period were identified in the NHAD registry using the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) codes of C22.1 (intrahepatic cholangiocarcinoma), C24.0 (extrahepatic cholangiocarcinoma), C24.1 (cancer of the ampulla of Vater), C24.8 (cholangiocarcinoma whose subtype cannot be classified), and C24.9 (cholangiocarcinoma, unspecified subtype). Cancer of the ampulla of Vater was included in this study because it occurs within 2 cm of the distal end of the common bile duct. Demographic data, including age, gender, and residential area, were collected and recorded. Potential risk factors for CCA, including cirrhosis, diabetes, and chronic viral hepatitis B and C infection, were collected using the corresponding ICD-10 codes, as follows: HBV infection: B18.0 and B18.1; HCV infection: B18.2; cirrhosis: K74, K74.0, K74.1, K74.2, K74.6, K70.2, K70.3, and K70.9; and diabetes mellitus type 2: E11. Date of death was obtained from the death certificate.
Controls: There were 2 control cohorts in this study. First, to compare demographic differences between CCA patients and non-CCA individuals, all Thai citizens were used as controls (n = 64076033). Demographic data of the Thai citizens were obtained in the year 2011 (the midpoint of the study period) from the Department of Provincial Administration, Ministry of the Interior, Thailand (Table 1). Second, to explore the potential associations between cirrhosis, diabetes and chronic viral hepatitis B and C infections and CCA development in the Thai population, patients without CCA who were admitted to hospitals during the study period were used as controls (n = 18508448). The data of non-CCA controls were obtained from discharge summary notes in the same manner as CCA cases.
| Variable | CCA cases(n = 39421) | Thai population(n = 64076033) | P value |
| Gender | |||
| Male | 24120 (61.2) | 31529148 (49.2) | < 0.001 |
| Age (mean ± SD, yr) | 64.1 ± 11.7 | 34.91 | |
| Geographic region | < 0.0001 | ||
| Northeast | 24239 (61.5) | 21585883 (33.7) | < 0.001 |
| North | 6699 (17.0) | 11783311 (18.4) | < 0.001 |
| Central | 5295 (13.4) | 16060141 (25.1) | < 0.001 |
| Bangkok | 1114 (2.8) | 5674843 (8.8) | < 0.001 |
| South | 1056 (2.7) | 8971855 (14.0) | < 0.001 |
All data analyses were performed using PASW® Statistics version 18 (SPSS, Inc., Chicago, IL, United States) and STATA version 12.1 (StataCorp LP, College Station, TX, United States). The patients were divided into 1 of the following 5 geographic region groups: Northeast, North, Central, Bangkok, or South. Bangkok is the capital of Thailand and is located in the Central geographic region. However, we designated Bangkok as a separate region because it has several major university hospitals that may be able to provide more technologically advanced treatment options than most hospitals located in other parts of the country. The annual incidence rate of CCA was calculated by dividing the number of new patients who were admitted with a diagnosis of CCA annually by the general population in the same year. Underlying diseases and one-year mortality rates were compared among regions using Student’s t-test for continuous variables and chi-square test for categorical variables.
Demographic data of CCA patients and all Thai citizens were compared using chi-square and t-tests. The proportions of individuals with cirrhosis, diabetes and chronic viral hepatitis B and C infections between CCA and non-CCA patients who were admitted to the hospital were compared during the study period using a χ2 test. Data are presented as a number, number (%), or mean ± SD.
One-year mortality was calculated using the per-patient information in which the survival time was calculated from the subtraction between the date of death and the date of the first admission. One-year mortality was then defined as 1 if the survival time was less than a year and 0 otherwise. An analysis of the trend in CCA incidence and mortality was performed using “nptrend”, the nonparametric test for trend across ordered groups developed by Cuzick (1985), which is an extension of the Wilcoxon rank-sum test. A P-value of < 0.05 indicated statistical significance.
There were 39421 patients diagnosed with CCA who were admitted to the affiliated hospitals during the study period. The mean ± SD age was 64.0 ± 11.7 years, and most patients (61.2%) were male. When compared by geographic area, the number of CCA patients varied significantly. The Northeast region had the greatest number of CCA cases (24239; 61.5%), followed by the North (6699; 17.0%) and Central regions (5295; 13.4%) (P < 0.0001) (Figure 1). Bangkok had a relatively low number of CCA cases (1114; 2.8%), and the South region had the lowest number of CCA patients (1056; 2.7%) (Table 1). Differences in the demographic characteristics between CCA cases and the general population are shown in Table 1. During the study period, the annual incidence rate was stable over time, i.e., 15.8, 15.6, 16.3, 16.1, and 16.4 cases per 100000 persons-year in the years 2009, 2010, 2011, 2012, and 2013, respectively, Ptrend = 0.13. When classified by region, the annual incidence rate in each region remained stable over the course of the study period (Table 2).
| Region | Year | Ptrend value | ||||
| 2009 | 2010 | 2011 | 2012 | 2013 | ||
| All | 15.80 | 15.63 | 16.13 | 16.07 | 16.43 | 0.13 |
| Northeast | 28.71 | 28.33 | 28.96 | 29.30 | 28.83 | 0.25 |
| North | 19.59 | 18.80 | 19.46 | 18.80 | 20.84 | 0.70 |
| Central | 7.23 | 7.42 | 7.98 | 8.40 | 9.20 | 0.06 |
| Bangkok | 5.87 | 6.17 | 6.27 | 5.52 | 6.01 | 0.85 |
| South | 2.76 | 2.74 | 2.98 | 2.81 | 2.98 | 0.17 |
Among the comorbid diseases potentially associated with CCA that were studied, diabetes was the most frequently detected, affecting 11.42% (n = 4502) of the entire CCA cohort. Cirrhosis and chronic viral hepatitis B and C infection were present in 1896 (4.81%), 291 (0.74%), and 196 (0.50%) patients, respectively (Table 3). CCA patients with cirrhosis (n = 1896) were significantly more likely to have hepatitis B infection, hepatitis C infection and alcoholic liver disease than CCA patients without cirrhosis (n = 37525), i.e., 124 (6.5%) vs 132 (0.3%), 100 (5.3%) vs 84 (0.2%), and 197 (10.4%) vs 0 (0%) for hepatitis B infection, hepatitis C infection and alcoholic liver disease, respectively; P < 0.0001 for all.
| Conditions | CCA patients (n = 39421) | Non-CCA patients (n = 18508448) | P value |
| Cirrhosis | 1896 (4.81) | 170255 (0.92) | < 0.001 |
| Chronic viral hepatitis B infection | 291 (0.74) | 21797 (0.12) | < 0.001 |
| Chronic viral hepatitis C infection | 196 (0.50) | 18339 (0.10) | < 0.001 |
| Diabetes | 4502 (11.42) | 977973 (5.28)1 | < 0.001 |
In a comparison of the proportion of individuals with underlying diabetes and chronic liver diseases between CCA vs non-CCA patients, we found that a greater proportion of CCA patients had diabetes than non-CCA patients, i.e., 4502/39421 (11.42%) vs 977973/18508448 (5.28%), P < 0.001, respectively. Similarly, a significantly greater proportion of CCA patients had viral hepatitis B and C infection and cirrhosis, i.e., 291/39421 (0.74%) vs 21797/18508448 (0.12%), 196/39421 (0.50%) vs 18339/18508448 (0.10%), and 1896/39421 (4.81%) vs 170255/18508448 (0.92%) for viral hepatitis B, viral hepatitis C and cirrhosis, respectively; P < 0.001 for all comparisons (Table 3).
Compared to the Northeast region, all regions had a higher proportion of CCA cases with cirrhosis (5.39%, 6.84%, 7.46%, and 5.97% vs 3.97% for North, Central, Bangkok, and South vs Northeast, respectively; P < 0.0001 for all comparisons). Similarly, all regions except for Bangkok had a significantly greater proportion of CCA patients with chronic viral hepatitis B infection than the Northeast region (11.36%, 1.52%, and 1.11% in the North, South, and Central regions vs 0.44% in the Northeast, respectively; P < 0.001 for all comparisons) (Table 4). Bangkok had a borderline significantly greater proportion of individuals with chronic hepatitis B than the Northeast (P = 0.05) (Table 4). Taken together, these findings suggest that cirrhosis and chronic viral hepatitis B infection may be partly associated with the development of CCA in our population.
| Total(n = 39421) | Northeast(n = 24237) | North(n = 6696) | PNorthvsNortheast | Central(n = 5294) | PcentralvsNortheast | Bangkok(n = 1113) | PBangkokvsNortheast | South(n = 1055) | PSouthvsNortheast | Pfor all regions | |
| Diabetes | 4502 (11.42) | 2803 (11.56) | 572 (8.54) | < 0.0001 | 689 (13.01) | 0.11 | 389 (18.36) | < 0.0001 | 133 (12.60) | 0.25 | < 0.0001 |
| Cirrhosis | 1896 (4.81) | 963 (3.97) | 440 (5.39) | < 0.0001 | 362 (6.84) | < 0.0001 | 83 (7.46) | < 0.0001 | 63 (5.97) | < 0.0001 | < 0.0001 |
| Chronic hepatitis B | 291 (0.74) | 106 (0.44) | 91 (1.36) | < 0.0001 | 59 (1.11) | < 0.0001 | 18 (1.62) | 0.05 | 12 (1.52) | 0.002 | < 0.0001 |
| Chronic hepatitis C | 196 (0.50) | 92 (0.38) | 57 (0.85) | < 0.0001 | 20 (0.38) | 0.33 | 17 (1.53) | 0.12 | 3 (0.28) | < 0.001 | < 0.0001 |
At the end of the study period, 35190 (89.27%) patients had died, of whom 31366 had died within 1 year after CCA diagnosis, corresponding to a 1-year mortality rate of 81.68%. The study period mean one-year mortality rates were stable (80.6%, 82.6%, 81.8%, 82.0%, and 81.5% for the years 2009 to 2013; Ptrend = 0.85) (Table 5). A stable one-year mortality rate was also observed when evaluated by region (Table 5).
| Year | Region | Total | ||||
| Northeast | North | Central | Bangkok | South | ||
| 2009 | 3825 (79.6) | 1153 (85.5) | 754 (80.6) | 170 (78.7) | 149 (74.5) | 6051 (80.6) |
| 2010 | 3928 (82.4) | 1114 (86.1) | 782 (81.0) | 181 (80.4) | 148 (74.0) | 6153 (82.6) |
| 2011 | 3988 (82.1) | 1097 (83.0) | 830 (80.0) | 176 (78.2) | 172 (78.5) | 6263 (81.8) |
| 2012 | 4087 (82.5) | 1068 (82.3) | 893 (79.7) | 174 (81.3) | 176 (83.4) | 6398 (82.0) |
| 2013 | 3973 (81.9) | 1208 (84.2) | 986 (79.9) | 169 (72.5) | 165 (73.3) | 6501 (81.5) |
| Ptrend | 0.57 | 0.25 | 0.13 | 0.57 | 0.85 | 0.85 |
In this population-based study of 39421 Thai CCA patients, we observed substantial heterogeneity in the CCA incidence across various regions of Thailand. To a lesser degree, we also observed variation in mortality across the country. Our findings suggest that diabetes, liver cirrhosis, and chronic viral hepatitis B and C infection may contribute to CCA risk in the Thai population.
As expected, we found that approximately 62% of patients resided in Northeast Thailand, whereas only 3% of patients lived in the South region. The frequency of CCA cases in each region was correlated with the reported prevalence of OV infection in each area (as high as 67% in the Northeast and only 0.1% in the South)[16,17]. OV infection was shown to be associated with a 10-fold increased risk of CCA[10]. Accordingly, the eradication of OV infection could potentially reduce the incidence of CCA in Thailand. Indeed, a national program to control OV infection has been rigorously applied since 2000[17]. After the implementation of a comprehensive control program in a certain area in the Northeast in which the prevalence of OV infection was as high as 67%, the OV infection rate declined to 16%[17]. With the continuation of this control program, we may see a decline in CCA incidence in this particular study area over the next decade.
There is a body of evidence that suggests that chronic liver diseases and diabetes are potential risk factors for CCA[12-14,18,19]. Cirrhosis was shown to be associated with at least a 5-fold increase in CCA risk[12-14,20]. Previous studies have shown that CCA patients had a higher proportion of individuals with cirrhosis than controls, with proportions ranging from 3% to 10% depending on the enrolled population, study design, and subtype of CCA studied[12-14,20]. In this study, a significantly greater proportion of CCA patients had cirrhosis than non-CCA patients. Additionally, the proportions of CCA cases with cirrhosis in non-endemic areas of OV infection (Central, Bangkok, and South regions) ranged from 5.6% to 7.5%, which were comparable to the rate of 7.8% reported in a Korean study[20]. Interestingly, the proportions of CCA patients with cirrhosis in the non-endemic areas of OV infection were significantly higher than the proportion in the Northeast region. Taken together, these findings suggest that cirrhosis may be a potential factor that contributes to CCA in Thai individuals, particularly in those who are not infected with OV.
Chronic viral hepatitis B and C infections have recently been recognized as potential risk factors for CCA[12-14,20-22]. In line with previous reports, we found that significantly more Thai patients with CCA had chronic viral hepatitis B and C infections than non-CCA patients. Two recent meta-analyses reported that hepatitis B and C infection confer risks of CCA with odds ratios of 2.0 and 5.4, respectively[21,22]. The magnitude of the effect of hepatitis B infection on CCA risk was more pronounced in Asian than in non-Asian populations. For example, the odds ratios for hepatitis B infection were 4.1 and 8.9 in Korean and Chinese cohorts[18,23], but only 2.8 in an American cohort[13]. Data related to the effect of hepatitis B and C infection on CCA risk in the Thai population are scarce[24]. The only report we were able to identify in the literature did not identify a significant association between hepatitis B/C infection and CCA; however, the study was conducted in an area endemic to OV infection, and the effect of hepatitis B and C on CCA risk may have been masked by the higher effect magnitude of OV infection[24].
We observed that an approximately 2-fold greater proportion of CCA patients had diabetes than non-CCA patients (11.4% vs 5.3%). A recent large case-control study of 2395 CCA cases and 4769 controls conducted in the United States reported that the proportion of subjects with diabetes among CCA cases and controls was 18.2% and 10.0%, respectively[13]. Accordingly, diabetes increased the risk of developing CCA by 2.7-fold, and this magnitude of risk was consistent with a reported odds ratio of 2.6 for developing CCA among Korean individuals with diabetes[13,18].
Taken together, our findings suggest that all of the studied underlying comorbid diseases potentially contribute to the risk of developing CCA in a Thai population. Future studies to confirm these findings and to determine the magnitude of the effect of these factors in a Thai population are warranted.
The major strength of this study is that our data were obtained from the Nationwide Hospital Admission Data (NHAD) registry, which is a database that includes the hospitalization data of 73% of all Thai citizens. As such, these findings most likely reflect an overall characterization of this cancer in Thailand. This study also has some mentionable limitations. First, the annual incidence estimated in this study might be underestimated due to the unique features of this database. The database solely includes information during hospitalization. Because data of patients seen at outpatient departments were not available, this could raise a concern that some patients, particularly those with advanced stage CCA who were only seen at outpatient departments and had never been admitted to a hospital, might be missed. We admit that this is possible; however, based on the current practice in our country, most CCA patients require at least one hospitalization, either for performing an investigation for a definite diagnosis or for receiving treatment by surgery, biliary drainage with palliative chemotherapy or even the best supportive care. Thus, we believe that the number of CCA patients who did not have any hospitalization was not large, and this number did not significantly affect the overall findings observed in this study. Second, the diagnosis of CCA was made by ICD codes that were obtained from hospital discharge summary notes and without histopathological confirmation. Consequently, the actual number of CCA patients during the study period could be higher than the number presented here. However, the proportions of CCA patients by region were consistent with the rates reported in a previous study[2]. As a result, it is likely that the different incidence rates among the 5 different regions are a valid finding. Another concern was that the proportion of CCA patients in each region shown in this study might not reflect the actual region of residence of patients because some local people might migrate to other regions; in particular, a portion of Northeastern people migrate to work in Bangkok. Thai people are covered by the national MWS only if they visit their primary hospital, which is designated by household registration. Patients who require hospitalization are usually referred back to their primary hospital. If necessary, the primary hospital will further refer patients to the nearby secondary or tertiary hospitals within the same region. Thus, the effect of migration across regions had a minimal impact on the proportion of patients in each region. Data on hepatitis B/C infection, diabetes, smoking, alcohol drinking, obesity, and metabolic syndrome were not available in the discharge summary notes of all participants, thus precluding us from estimating the effect of these conditions on CCA risk. Similarly, the prevalence of OV infection and the relationship between OV infection and CCA incidence cannot be determined because the diagnosis of OV infection was not available in the medical records. The diagnosis of CCA was obtained from ICD codes, which group hilar and distal CCA together as extrahepatic CCA; thus, an analysis by CCA subtypes could not be performed. Choledocholiathiasis, hepatolithiasis, choledochal cysts, Caroli’s syndrome and bile duct adenoma have been shown to be associated with CCA[1,14]. These conditions were not included in the analysis because this study aimed to explore the conditions that have recently been proposed to be related to CCA and may in part contribute to the increasing incidence of CCA worldwide.
In summary, CCA-related incidence and mortality among Thai patients vary by geographical region. Our findings yield indirect evidence that supports current epidemiological data that suggests that diabetes, cirrhosis, and chronic viral hepatitis B and C infection are associated with an increased risk of developing CCA. These conditions may be important factors contributing to CCA in Thai individuals.
The incidence of cholangiocarcinoma (CCA) has been increasing for a few decades for unclear reasons. Primary sclerosing cholangitis, liver fluke infection, and conditions with chronic biliary tract inflammation are established predisposing factors to CCA development. Accumulating evidence suggests that cirrhosis, chronic viral hepatitis B/C infection and diabetes are associated with CCA.
The results of this study provide indirect evidence supporting the current epidemiological knowledge about risk factors of CCA.
This study used the Nationwide Hospital Admission Data (NHAD) registry to identify potential risk factors for CCA among 5 different regions of Thailand. The two key findings were that the incidence of CCA varied geographically in Thailand and that significantly more CCA patients had diabetes, cirrhosis, and chronic viral hepatitis B/C infection than non-CCA participants.
This study suggests that diabetes, cirrhosis, and chronic viral hepatitis B and C infection may contribute to CCA development in the Thai population. Further study to confirm these associations and determine the magnitude of the impact of these factors on cholangiocarcinoma development in the Thai population is warranted.
Cholangiocarcinoma is a cancer of the bile duct epithelium. CCA can be categorized into 3 subtypes: intrahepatic, perihilar and distal cholangiocarcinoma. Although the three subtypes share some common features, each has its own characteristics, including genetic alterations, presentation, treatment and prognosis. Obtaining a diagnosis of cholangiocarcinoma using ICD codes precludes the analysis by subtypes because the ICD codes group perihilar and distal cholangiocarcinoma together as extrahepatic cholangiocarcinomas.
This study addresses an important healthcare problem in Thailand. This is an informative article about the risk factors of cholangiocarcinoma in Thailand using a nationwide database. This study aimed to identify potential risk factors of cholangiocarcinoma among 5 different regions of Thailand. The fact that even in Thailand, diabetes, viral hepatitis B/C infection and cirrhosis are risk factors of cholangiocarcinoma in a region where fluke infection is not endemic, is interesting.
| 1. | Bragazzi MC, Cardinale V, Carpino G, Venere R, Semeraro R, Gentile R, Gaudio E, Alvaro D. Cholangiocarcinoma: Epidemiology and risk factors. Transl Gastrointest Cancer. 2012;1:21-32. [DOI] [Full Text] |
| 2. | Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 391] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 3. | Alvaro D, Crocetti E, Ferretti S, Bragazzi MC, Capocaccia R; AISF Cholangiocarcinoma committee. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis. 2010;42:490-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94:1751-1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 5. | Shin HR, Oh JK, Lim MK, Shin A, Kong HJ, Jung KW, Won YJ, Park S, Park SJ, Hong ST. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J Korean Med Sci. 2010;25:1011-1016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Altekruse SF, Petrick JL, Rolin AI, Cuccinelli JE, Zou Z, Tatalovich Z, McGlynn KA. Geographic variation of intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and hepatocellular carcinoma in the United States. PLoS One. 2015;10:e0120574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang Y, Wiangnon S, Sripa B, Hong ST. Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma--focus on East and South-Eastern Asia. Asian Pac J Cancer Prev. 2010;11:1159-1166. [PubMed] |
| 8. | Lepage C, Cottet V, Chauvenet M, Phelip JM, Bedenne L, Faivre J, Bouvier AM. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol. 2011;54:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 600] [Cited by in RCA: 605] [Article Influence: 31.8] [Reference Citation Analysis (2)] |
| 10. | Xia J, Jiang SC, Peng HJ. Association between Liver Fluke Infection and Hepatobiliary Pathological Changes: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0132673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1070] [Cited by in RCA: 1012] [Article Influence: 77.8] [Reference Citation Analysis (6)] |
| 12. | Chaiteerakij R, Yang JD, Harmsen WS, Slettedahl SW, Mettler TA, Fredericksen ZS, Kim WR, Gores GJ, Roberts RO, Olson JE. Risk factors for intrahepatic cholangiocarcinoma: association between metformin use and reduced cancer risk. Hepatology. 2013;57:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 13. | Choi J, Ghoz HM, Peeraphatdit T, Baichoo E, Addissie BD, Harmsen WS, Therneau TM, Olson JE, Chaiteerakij R, Roberts LR. Aspirin use and the risk of cholangiocarcinoma. Hepatology. 2016;64:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 722] [Cited by in RCA: 710] [Article Influence: 47.3] [Reference Citation Analysis (1)] |
| 15. | Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 518] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 16. | Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88:229-232. [PubMed] |
| 17. | Sripa B, Tangkawattana S, Laha T, Kaewkes S, Mallory FF, Smith JF, Wilcox BA. Toward integrated opisthorchiasis control in northeast Thailand: the Lawa project. Acta Trop. 2015;141:361-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Lee BS, Park EC, Park SW, Nam CM, Roh J. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a case-control study in Korea. World J Gastroenterol. 2015;21:502-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (5)] |
| 19. | Jing W, Jin G, Zhou X, Zhou Y, Zhang Y, Shao C, Liu R, Hu X. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev. 2012;21:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 20. | Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, Kwon S, Lee SK, Seo DW, Kim MH. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008;103:1716-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Li M, Li J, Li P, Li H, Su T, Zhu R, Gong J. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta-analysis and systematic review. J Gastroenterol Hepatol. 2012;27:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Li H, Hu B, Zhou ZQ, Guan J, Zhang ZY, Zhou GW. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: evidence from a systematic review and meta-analysis of 16 case-control studies. World J Surg Oncol. 2015;13:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Zhou YM, Yin ZF, Yang JM, Li B, Shao WY, Xu F, Wang YL, Li DQ. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol. 2008;14:632-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Srivatanakul P, Honjo S, Kittiwatanachot P, Jedpiyawongse A, Khuhaprema T, Miwa M. Hepatitis viruses and risk of cholangiocarcinoma in northeast Thailand. Asian Pac J Cancer Prev. 2010;11:985-988. [PubMed] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Aoki H, Chang JH, Cheon YK, Li FY, Popescu I S- Editor: Ma YJ L- Editor: A E- Editor: Huang Y