Published online Oct 21, 2017. doi: 10.3748/wjg.v23.i39.7150
Peer-review started: August 19, 2017
First decision: August 30, 2017
Revised: September 21, 2017
Accepted: September 28, 2017
Article in press: September 28, 2017
Published online: October 21, 2017
Processing time: 64 Days and 7.5 Hours
To determine whether the presence of columnar-lined esophagus (CLE) is associated with the presence of esophageal varices (EVs) in male Japanese alcoholics.
The subjects were 1614 Japanese alcohol-dependent men (≥ 40 years of age) who had undergone upper gastrointestinal endoscopic screening. Digitalized records of high-quality endoscopic images that included the squamocolumnar junction and esophagogastric junction were retrospectively jointly reviewed by four expert endoscopists for the purpose of diagnosing CLE. The authors investigated whether and to what extent there were associations between the presence of CLE and the presence of EVs, especially in the group with liver cirrhosis (LC).
CLE ≥ 5 mm in length was found in 355 subjects (≥ 30 mm in 6 of them), LC without EVs in 152 subjects, LC with EVs in 174 subjects, and EVs without LC in 6 subjects. Advanced EVs, i.e., nodular, large or coiled forms, red color sign, or post-treatment, were found in 88 subjects. The incidence of CLE ≥ 5 mm decreased in the following order (P < 0.0001): 23.3% in the group without EVs, 17.4% in the group with small and straight EVs, and 5.7% in the group with advanced EVs. The multivariate ORs (95%CI) for EVs and advanced EVs in the group with LC were lower when CLE ≥ 5mm was present [0.46 (0.23-0.93) and 0.24 (0.08-0.74), respectively, vs 0-4 mm CLE].
The presence of CLE in male Japanese alcoholics was negatively associated with the presence of EVs.
Core tip: A positive association between excessive drinking and the presence of short-segmental columnar-lined esophagus (CLE) has been reported in Asians. Endoscopic screening of 1614 Japanese alcohol-dependent men revealed the presence of CLE ≥ 5 mm in length in 355 subjects and esophageal varices (EVs) in 180 subjects. The presence of CLE was negatively associated with the presence of EVs, and even more negatively associated with the presence of advanced forms of EVs. Since the first resistance vessels to EVs are the mucosal palisade vessels and submucosal veins at the lower end of the esophagus, the development of CLE may impede the development of EVs.
- Citation: Yokoyama A, Hirata K, Nakamura R, Omori T, Mizukami T, Aida J, Maruyama K, Yokoyama T. Presence of columnar-lined esophagus is negatively associated with the presence of esophageal varices in Japanese alcoholic men. World J Gastroenterol 2017; 23(39): 7150-7159
- URL: https://www.wjgnet.com/1007-9327/full/v23/i39/7150.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i39.7150
Esophageal varices (EVs) develop as a result of portal hypertension, which is mainly due to liver cirrhosis (LC) in alcoholics, and excessive alcohol drinking increases the risk of variceal bleeding and mortality[1-3]. Excessive alcohol consumption has been reported to be associated with the presence of gastroesophageal reflux disease (GERD) and a short-segmental columnar-lined esophagus (CLE) in East-Asian studies[4-8]. Long-segmental CLE is rare in Asians[8], and a large pooled analysis of the cases in Western studies did not show a positive association between heavy drinking and GERD or long-segmental CLE[9]. The differences between the results in East Asia and the West may be attributable to the differences between East Asia and Western countries in abdominal obesity, incidences of Helicobacter pylori infection, and gene polymorphisms of alcohol-metabolizing enzymes.
Our empirical impression based on the results of endoscopic screening examinations of Japanese alcoholic men is that EVs are less common among men with short-segmental CLE. The development of CLE has been shown to be accompanied by several histological changes around the palisade vessels, including the development of a double muscularis mucosae[10,11]. Since dilatation of the palisade vessels at the lower end of the esophagus has been suspected of being one of the major initial events in the development of EVs secondary to portal hypertension[12,13], some of the histological changes accompanying the development of CLE may protect against the development of EVs.
It is widely accepted that genetic polymorphisms of alcohol dehydrogenase-1B (ADH1B, rs1229984) and aldehyde dehydrogenase-2 (ALDH2, rs671) affect the susceptibility of East-Asians to alcoholism[14-16], and the presence of fast-metabolizing ADH1B encoded by the ADH1B*2 allele in Japanese alcoholics has been reported to be positively associated with the presence of advanced liver disease[17-19].
The aim of the present study was to determine whether and to what extent associations exist between the presence of EVs and the presence of CLE in Japanese alcoholic men based on the results of endoscopic screening examinations and their ADH1B/ALDH2 genotypes.
The reference population of this study consisted of 1902 Japanese alcoholic men 40 years of age and over who: (1) Came to the Kurihama Medical and Addiction Center for treatment of alcohol dependence for the first time between May 2004 and December 2011; (2) Were evaluated for the presence of physical comorbidities; (3) Underwent routine upper gastrointestinal endoscopic screening; (4) And underwent ADH1B and ALDH2 genotyping[19]. After excluding the 194 subjects with a history of either gastrectomy or treatment for esophageal cancer and the 94 subjects without a digitalized record of high-quality endoscopic images that included the squamocolumnar junction and esophagogastric junction, 1614 patients were selected as subjects.
All of the alcoholics who participated in this study met the DSM-IV criteria for alcohol dependence[20]. Just before the endoscopic screening examination we asked each participant when he was in a sober state about his drinking and smoking habits. Usual alcohol consumption during the preceding year was expressed in grams of ethanol per day calculated by using standard conversion factors for alcoholic beverages. Beer and low-malt beer were assumed to be 5% ethanol (v/v); wine, 12%; sake, 16%; shochu, 25%; and whiskey, 40%.
The clinical diagnoses of comorbidities were made after alcohol detoxification. Patients received a routine examination that included a physical examination, blood tests, chest X-ray and abdominal X-ray, upper gastrointestinal endoscopy, abdominal ultrasound examination, and abdominal computed tomography. The clinical diagnosis of LC was made on the basis of the results of the physical examination, blood tests, and imaging studies or detection of esophagogastric varices during the endoscopic examination. The severity of LC was graded according to the Child-Pugh scoring system based on the findings at the first visit[21]. Hepatitis B surface (HBs) antigen and second generation anti-hepatitis C (HCV) antibody were measured with Abbott enzyme immunosorbent assays (Abbott Japan Inc., Tokyo).
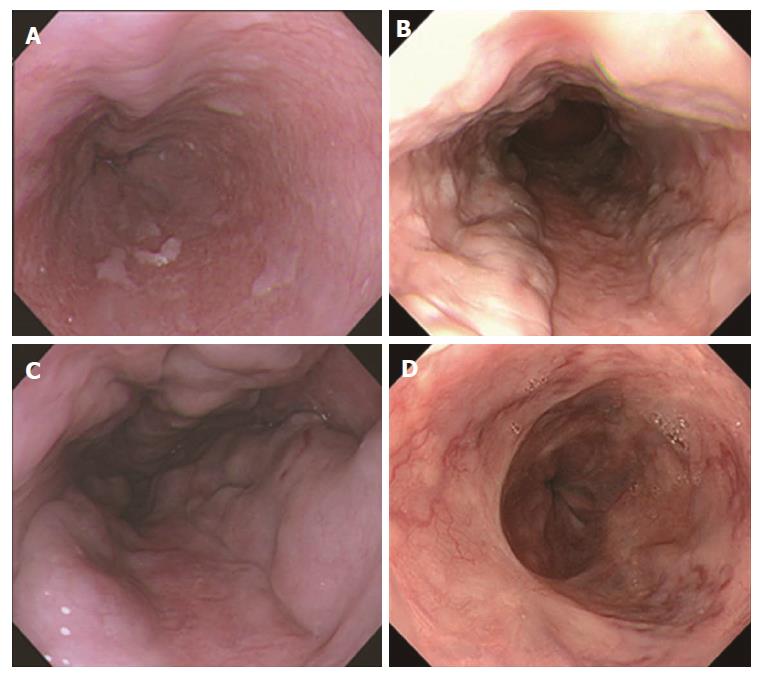
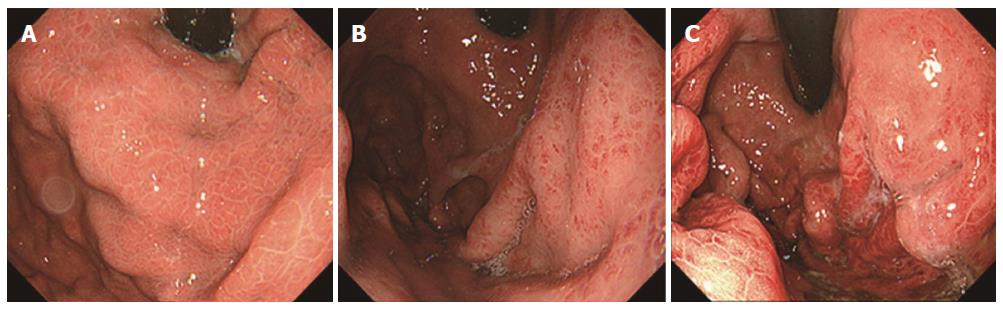
Endoscopy was performed with an Olympus XQ230, Q240, Q240Z, or Q260 panendoscope (Olympus Optical Co. Ltd., Tokyo, Japan). Esophagogastric varices were diagnosed according to the grading system for esophagogastric varices adopted by the Japanese Society for Portal Hypertension[22]; e.g., based on their form [small and straight (F1), nodular (F2), and large or coiled (F3)] and red color (RC) sign (Figure 1). The severity of portal hypertensive gastropathy (PHG) was evaluated according to Toyonaga’s grading system, which has been adopted by the Japanese Society for Portal Hypertension (Figure 2)[23]: All PHG lesions exhibit a snake-skin (mosaic) pattern in their background mucosa; Grade 1, erythematous flecks or maculae; Grade 2, red spots or diffuse redness; and Grade 3, intramucosal or luminal hemorrhage. Three patients could not be evaluated for PHG because of food residue in the stomach.
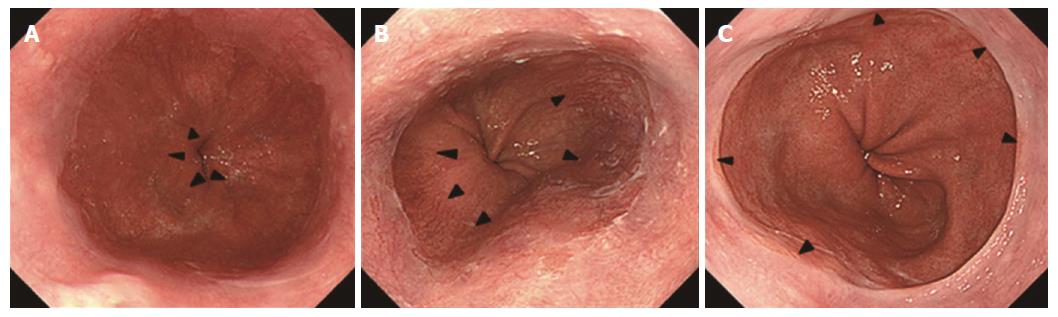
The digitalized images that included the squamocolumnar junction and esophagogastric junction acquired before advancing the endoscope into the stomach were stored by the medical imaging communication system. The endoscopic digitalized images were retrospectively assessed during a joint review by four expert endoscopists (Yokoyama A, Hirata K, Nakamura R, Omori T) to diagnose CLE and hiatal hernia according to the classification system adopted by the Japan Esophageal Society[24]. The endoscopic esophagogastric junction was defined as the lower limit of the palisade longitudinal vessels (Figure 3)[10,11]. It was defined as the upper limit of the gastric mucosal folds when the lower limit of the palisade vessels was unclear. The greatest axial lengths of CLE were classified into four categories: 0-4 mm, 5-9 mm, 10-29 mm, and ≥ 30 mm. The axial length of a hiatus hernia was defined as the distance between the esophagogastric junction and the hiatus represented by the diaphragmatic pinch. The images were examined for the presence or absence of a hiatal hernia whose axial length was ≥ 10 mm.
ADH1B and ALDH2 genotyping of every subject was performed by PCR-RFLP methods on a lymphocyte DNA sample[19].
The data have been summarized as means and standard errors or as percentage values. Student’s t-test was used to compare normally distributed continuous variables between groups; the Mann-Whitney U-test was used to compare non-normally distributed continuous variables between groups; and Fisher’s exact test or Cochran-Mantel-Haenszel test for trend was used to compare proportions between groups. Multiple logistic regression models were used to calculate adjusted odds ratios (ORs) and their 95% confidence intervals (CIs). A P value less than 0.05 was considered statistically significant. All analyses were performed by a biomedical statistician (Yokoyama T) using the SAS statistical package (version 9.4; SAS Institute, Cary, NC United States).
LC without EVs was diagnosed in 152 subjects, LC with EVs in 174 subjects, and EVs without LC in 6 subjects. The EVs were classified as F1 in 92 subjects, F2 or F3 in 41 subjects, RC-sign-positive in 21 subjects, and post-treatment in 26 subjects, 17 of whom were reported to have been treated for variceal rupture. All of the EVs in the subjects without LC were classified as F1. Table 1 shows the background factors of the 1614 subjects according to whether they had EVs. There were no significant differences between the group with EVs and the group without EVs in age, usual alcohol consumption, smoking, hepatitis C and B infection status, or ALDH2 and ADH1B genotypes. LC, gastric varices, and higher grade PHG were more common in the group with EVs than in the group without EVs (P < 0.0001). Most of the gastric varices in the group without EVs were found in the fornix alone, and gastric varices located in the cardia or cardia plus fornix predominanted in the group with EVs.
| Esophageal varices | |||
| Absent | Present | P value | |
| n | 1434 | 180 | |
| Age (yr) | |||
| mean ± SE | 55.8 ± 0.3 | 54.4 ± 0.7 | 0.079 |
| Alcohol consumption (g ethanol/d) | |||
| mean ± SE | 121 ± 2 | 133 ± 7 | 0.066 |
| Ever smoker | 1310 (91.4) | 159 (88.3) | 0.21 |
| Liver cirrhosis | |||
| Absent | 1282 (89.4) | 6 (3.3) | |
| Present | 152 (10.6) | 174 (96.7) | < 0.0001 |
| Gastric Varices | |||
| Absent | 1418 (98.9) | 147 (81.7) | |
| Cardia, cardia/fornix | 2 (0.1) | 23 (12.8) | |
| Fornix | 14 (1.0) | 10 (5.6) | < 0.0001 |
| Portal hypertensive gastropathy | 1432 | 179 | |
| Absence | 1172 (81.8) | 59 (33.0) | |
| Grade 1 | 191 (13.3) | 80 (44.7) | |
| Grade 2 | 53 (3.7) | 25 (14.0) | |
| Grade 3 | 16 (1.1) | 15 (8.4) | < 0.0001 |
| Anti-HCV antibody positive | 69 (4.8) | 13 (7.2) | 0.20 |
| HBs antigen positive | 17 (1.2) | 4 (2.2) | 0.28 |
| ALDH2 genotype | |||
| *1/*1 | 1216 (84.8) | 161 (89.4) | |
| *1/*2 | 218 (15.2) | 19 (10.6) | |
| *2/*2 | 0 (0.0) | 0 (0.0) | 0.12 |
| ADH1B genotype | |||
| *1/*1 | 406 (28.3) | 41 (22.8) | |
| *1/*2 | 470 (32.8) | 69 (38.3) | |
| *2/*2 | 558 (38.9) | 70 (38.9) | 0.20 |
CLE ≥ 5 mm was found in 355 (22.0%) of the subjects (5-9 mm, 13.6%; 10-29 mm, 8.1%; and ≥ 30 mm, 0.4%; Table 2). When advanced forms of EVs were defined as F2 - F3 varices, RC-sign-positive varices, and post-treatment varices, the proportion of subjects with CLE ≥ 5 mm decreased in the following order (P < 0.0001): 23.3% in the group without EVs, 17.4% in the group with F1 varices, and 5.7% in the group with advanced varices, and the findings were similar in the subgroup of subjects with LC (P = 0.0004). The incidence of CLE ≥ 5 mm in the group of subjects with advanced EVs did not differ significantly according to whether they had or had not received endoscopic treatment (7.7% and 4.8%, respectively, P = 0.63). The proportions with a hiatal hernia ≥ 10 mm (24.4%, 20.7%, and 13.6%, respectively) decreased in the same order as the order in which the proportions of subjects with CLE ≥ 5 mm decreased (P = 0.017), but the trend was not significant in the subgroup of subjects with LC.
| Esophageal varices | Absent | F1 varices | Advanced varices | P value |
| n | 1434 | 92 | 88 | |
| Columnar-lined esophagus | ||||
| 0-4 mm | 1100 (76.7) | 76 (82.6) | 83 (94.3) | |
| ≥ 5 mm | 334 (23.3) | 16 (17.4) | 5 (5.7) | < 0.0001 |
| 5-9 mm | 208 (14.5) | 8 (8.7) | 3 (3.4) | |
| 10-29 mm | 120 (8.4) | 8 (8.7) | 2 (2.3) | |
| ≥ 30 mm | 6 (0.4) | 0 (0.0) | 0 (0.0) | |
| Hiatal hernia ≥ 10 mm | ||||
| Absent | 1084 (75.6) | 73 (79.3) | 76 (86.4) | |
| Present | 350 (24.4) | 19 (20.7) | 12 (13.6) | 0.017 |
| Number of patients with liver cirrhosis | 152 | 86 | 88 | |
| Columnar-lined esophagus | ||||
| 0-4 mm | 117 (77.0) | 73 (84.9) | 83 (94.3) | |
| ≥ 5 mm | 35 (23.0) | 13 (15.1) | 5 (5.7) | 0.0004 |
| 5-9 mm | 22 (14.5) | 8 (9.3) | 3 (3.4) | |
| 10-29 mm | 12 (7.9) | 5 (5.8) | 2 (2.3) | |
| ≥ 30 mm | 1 (0.7) | 0 (0.0) | 0 (0.0) | |
| Hiatal hernia ≥ 10 mm | ||||
| Absent | 124 (81.6) | 71 (82.6) | 76 (86.4) | |
| Present | 28 (18.4) | 15 (17.4) | 12 (13.6) | 0.36 |
Table 3 shows the background factors of the 1614 subjects according to whether they had CLE ≥ 5 mm, and there were no significant differences between the two groups in age, usual alcohol consumption, smoking, or ALDH2 and ADH1B genotypes. Hiatal hernia was more common in the group with CLE ≥ 5 mm than in the other group (45.9% vs 17.3%, P < 0.0001). There was a significant difference between the group with CLE ≥ 5 mm and the group without CLE ≥ 5 mm in the proportions of ‘no LC and no EVs’ subjects, ‘LC and no EVs’ subjects, and the ‘EVs’ subjects they contained (P = 0.001). There were no significant difference between the group with CLE ≥ 5 mm and group without CLE ≥ 5 mm in the presence of gastric varices or the grade of PHG.
| Columnar-lined esophagus ≥ 5 mm | |||
| Absent | Present | P value | |
| n | 1259 | 355 | |
| Age (yr), mean ± SE | 55.6 ± 0.3 | 55.8 ± 0.5 | 0.75 |
| Usual alcohol consumption (g ethanol), mean ± SE | 123 ± 2 | 121 ± 4 | 0.69 |
| Ever smoker | 1151 (91.4) | 318 (89.6) | 0.29 |
| Hiatal hernia ≥ 10 mm | 218 (17.3) | 163 (45.9) | < 0.0001 |
| No liver cirrhosis and no EVs | 983 (78.1) | 299 (84.2) | |
| Liver cirrhosis and no EVs | 117 (9.3) | 35 (9.9) | |
| EVs | 159 (12.6) | 21 (5.9) | 0.001 |
| Gastric varices | |||
| Absent | 1215 (96.5) | 350 (98.6) | |
| Cardia, cardia and fornix | 23 (1.8) | 2 (0.6) | |
| Fornix | 21 (1.7) | 3 (0.8) | 0.13 |
| Portal hypertensive gastropathy | 1256 | 355 | |
| Absent | 947 (75.4) | 284 (80.0) | |
| Grade 1 | 218 (17.4) | 53 (14.9) | |
| Grade 2,3 | 91 (7.2) | 18 (5.1) | 0.17 |
| ALDH2 genotype, | |||
| *1/*1 | 1077 (85.5) | 300 (84.5) | |
| *1/*2 | 182 (14.5) | 55 (15.5) | |
| *2/*2 | 0 (0.0) | 0 (0.0) | 0.61 |
| ADH1B genotype, | |||
| *1/*1 | 348 (27.6) | 99 (27.9) | |
| *1/*2 | 407 (32.3) | 132 (37.2) | |
| *2/*2 | 504 (40.0) | 124 (34.9) | 0.15 |
A multiple logistic regression analysis to predict the presence of CLE ≥ 5 mm showed that the ORs (95%CI) for CLE ≥ 5 mm increased with age per +10 years [1.15 (1.00-1.32)] and with the presence of hiatal hernia ≥ 10 mm [4.33 (3.31-5.67)] and decreased with the presence of EVs [0.47 (0.26-0.87); Table 4]. The presence of LC, presence of gastric varices, and grade of PHG were not associated with the presence of CLE ≥ 5 mm.
| CLE, ≥ 5 mm vs 0-4 mm | |||
| Independent variables | OR | 95%CI | P value |
| Age, per +10 yr | 1.15 | (1.00-1.32) | 0.043 |
| Usual alcohol consumption, per +22 g ethanol | 0.99 | (0.96-1.03) | 0.75 |
| Ever smoker vs never smoker | 0.75 | (0.49-1.14) | 0.17 |
| Hiatal hernia ≥ 10 mm, presence vs absence | 4.33 | (3.31-5.67) | < 0.0001 |
| Liver cirrhosis, presence vs absence | 1.04 | (0.68-1.60) | 0.86 |
| Esophageal varices, presence vs absence | 0.47 | (0.26-0.87) | 0.015 |
| Gastric varices, presence vs absence | 0.59 | (0.21-1.64) | 0.31 |
| Portal hypertensive gastropathy | |||
| Absent | 1.00 | Referent | |
| Grade 1 | 1.23 | (0.86-1.78) | 0.26 |
| Grade 2,3 | 0.91 | (0.52-1.60) | 0.74 |
| Anti-HCV antibody, positive vs, negative | 0.93 | (0.53-1.66) | 0.81 |
| HBs antigen, positive vs, negative | 0.56 | (0.13-2.47) | 0.44 |
| ALDH2 genotype, *1/*1 vs *1/*2 | 0.94 | (0.67-1.33) | 0.73 |
| ADH1B genotype, *2 carrier vs *1/*1 | 0.97 | (0.73-1.28) | 0.81 |
Table 5 shows the background factors of the 326 subjects with LC according to whether they had EVs, and there were no significant differences between the two groups in age, usual alcohol consumption, smoking, hepatitis C and B infection status, or ALDH2 and ADH1B genotypes. The absence of CLE ≥ 5 mm (P = 0.003), presence of gastric varices (P < 0.0001), and higher grade PHG (P < 0.0001) were all more common in the LC group with EVs than in the LC group without EVs. Advanced Child-Pugh class (P = 0.011) was more common in the LC group with advanced EVs.
| Esophageal varices | |||||
| Absent | All forms | P value | Advanced forms | P1 value | |
| n | 152 | 174 | 88 | ||
| Age (yr) | |||||
| mean ± SE | 55.9 ± 0.7 | 54.5 ± 0.7 | 0.16 | 54.3 ± 1.0 | 0.21 |
| Alcohol consumption (g ethanol/d) | |||||
| mean ± SE | 124 ± 7 | 133 ± 7 | 0.37 | 142 ± 11 | 0.18 |
| Ever smoker | 132 (86.8) | 155 (89.1) | 0.61 | 83 (94.3) | 0.081 |
| Columnar-lined esophagus ≥ 5 mm | 35 (23.0) | 18 (10.3) | 0.003 | 5 (5.7) | 0.0005 |
| Hiatal hernia ≥ 10 mm | 28 (18.4) | 27 (15.5) | 0.55 | 12 (13.6) | 0.37 |
| Child-Pugh class | |||||
| A | 93 (61.2) | 88 (50.6) | 41 (46.6) | ||
| B | 50 (32.9) | 64 (36.8) | 32 (36.4) | ||
| C | 9 (5.9) | 22 (12.6) | 0.053 | 15 (17.0) | 0.011 |
| Gastric varices | |||||
| Absent | 137 (90.1) | 141 (81.0) | 65 (73.9) | ||
| Cardia, cardia and fornix | 2 (1.3) | 23 (13.2) | 17 (19.3) | ||
| Fornix | 13 (8.6) | 10 (5.7) | < 0.0001 | 6 (6.8%) | < 0.0001 |
| Portal hypertensive gastropathy | 151 | 173 | |||
| Absent | 88 (58.3) | 55 (31.8) | 27 (30.7) | ||
| Grade 1 | 50 (33.1) | 78 (45.1) | 39 (44.3) | ||
| Grade 2 | 10 (6.6) | 25 (14.5) | 12 (11.4) | ||
| Grade 3 | 3 (2.0) | 15 (8.7) | < 0.0001 | 15 (8.4) | < 0.0001 |
| Anti-HCV antibody positive | 8 (5.3) | 13 (7.5) | 0.50 | 8 (9.1) | 0.29 |
| HBs antigen positive | 4 (2.6) | 4 (2.3) | 1.00 | 1 (1.1) | 0.65 |
| ALDH2 genotype | |||||
| *1/*1 | 134 (88.2) | 156 (89.7) | 77 (87.5) | ||
| *1/*2 | 18 (11.8) | 18 (10.3) | 11 (12.5) | ||
| *2/*2 | 0 (0.0) | 0 (0.0) | 0.72 | 0 (0.0) | 1.00 |
| ADH1B genotype | |||||
| *1/*1 | 32 (21.1) | 36 (20.7) | 15 (17.0) | ||
| *1/*2 | 51 (33.6) | 68 (39.1) | 37 (42.0) | ||
| *2/*2 | 69 (45.4) | 70 (40.2) | 0.55 | 36 (40.9) | 0.41 |
A multiple logistic regression analysis showed that the ORs (95%CI) for EVs and advanced EVs in the subgroup of subjects with LC decreased with the presence of CLE ≥ 5 mm [0.46 (0.23-0.93) and 0.24 (0.08-0.74), respectively, vs CLE 0-4 mm; Table 6]. Child-Pugh class was not associated with EV status. The ORs for EVs and advanced EVs increased with progression of the grade of PHG, and the OR for advanced EVs increased with the presence of gastric varices.
| Cirrhosis with EVs vs cirrhosis without EVs | ||||
| All forms of EVs | Advanced forms of EVs | |||
| Independent variables | OR (95%CI) | P value | OR (95%CI) | P1 value |
| Age, per +10 yr | 0.88 (0.67-1.16) | 0.35 | 0.90 (0.64-1.26) | 0.55 |
| Usual alcohol consumption, per +22 g ethanol | 1.00 (0.94-1.06) | 0.97 | 1.02 (0.95-1.10) | 0.52 |
| Ever smoker vs never smoker | 1.04 (0.50-2.16) | 0.93 | 2.13 (0.69-6.57) | 0.19 |
| Columnar-lined esophagus, ≥ 5 mm vs 0-4 mm | 0.46 (0.23-0.93) | 0.030 | 0.24 (0.08-0.74) | 0.013 |
| Hiatal hernia ≥ 10 mm, presence vs absence | 1.10 (0.54-2.21) | 0.80 | 0.97 (0.38-2.49) | 0.95 |
| Child-Pugh class | ||||
| A | 1.00 Referent | 1.00 Referent | ||
| B | 1.18 (0.70-1.98) | 0.53 | 1.06 (0.55-2.05) | 0.87 |
| C | 1.56 (0.64-3.82) | 0.33 | 1.85 (0.67-5.16) | 0.24 |
| Gastric varices, presence vs absence | 1.94 (0.96-3.91) | 0.065 | 2.89 (1.29-6.47) | 0.010 |
| Portal hypertensive gastropathy | ||||
| Absent | 1.00 Referent | 1.00 Referent | ||
| Grade 1 | 2.24 (1.32-3.80) | 0.003 | 1.92 (0.97-3.77) | 0.060 |
| Grade 2,3 | 4.40 (2.11-9.15) | < 0.0001 | 5.29 (2.18-12.81) | 0.0002 |
| Anti-HCV antibody, positive vs negative | 1.09 (0.41-2.93) | 0.87 | 1.54 (0.49-4.88) | 0.46 |
| HBs antigen, positive vs, negative | 1.16 (0.26-5.16) | 0.85 | 0.61 (0.06-6.36) | 0.68 |
| ALDH2 genotype, *1/*1 vs *1/*2 | 1.02 (0.47-2.20) | 0.96 | 1.00 (0.38-2.60) | 1.00 |
| ADH1B genotype, *2 carrier vs *1/*1 | 1.10 (0.61-1.99) | 0.76 | 1.59 (0.72-3.51) | 0.25 |
The analysis of the results of endoscopic screening of 1614 Japanese alcoholic men in this study demonstrated that the presence of CLE was negatively associated with the presence of EVs, and even more negatively associated with the presence of advanced forms of EVs. The prevalence of CLE ≥ 30 mm has been reported to be very low (0.0%-0.2%) in Asians[8,25-27]. The prevalence of CLE ≥ 30 mm in the present study was 0.4%, and most of the CLE was short-segmental. The presence of a hiatal hernia was a strong determinant of the presence of CLE in this study, a finding that was consistent with the results of previous studies[5,7,8,26,28], suggesting that GERD in patients with a hiatal hernia contributes to the development of CLE. Asian studies have demonstrated positive associations between excessive alcohol consumption and both GERD and short-segmental CLE[4-8,26]. The prevalence of CLE ≥ 10 mm has been reported to be 1.8% in unselected Taiwanese, 4.0% in symptomatic Koreans[27], and 5.3% in a group of Japanese who underwent an endoscopic examination in a university hospital[8]. Thus, the high prevalence (8.1%) of CLE ≥ 10 mm in the population of alcoholics in our study was at least in part attributable to chronic heavy drinking.
As expected, the presence of EVs was positively associated with the presence of LC, gastric varices, and PHG. However, these factors were not associated with the presence of CLE, and adjustment for these factors revealed a negative association between the presence of CLE and the presence and severity of EVs. These findings taken together suggested a special relationship between CLE and EVs in the presence of LC and portal hypertension.
In the majority of LC patients the left gastric vein is the afferent vein to the EVs[29], and the first resistance vessels to the EVs are the mucosal palisade vessels and submucosal veins at the lower end of the esophagus, where there are many palisade vessels and few submucosal veins[12,13]. The palisade vessels penetrate the muscularis mucosae and connect to the submucosal veins 3-5 cm above the esophagogastric junction, the critical area for EV rupture[12,30,31]. Greatly enlarged palisade vessels disrupt the muscularis mucosae and form EVs across the proper mucosae and the submucosa[13], and they cause advanced EVs by connecting with submucosal veins or sub- and intra-epithelial channels accompanied by ‘varices on varices’ and positive RC signs[12,13,32].
Although there is no difference between the maximal size of the palisade vessels in the CLE and normal lower esophagus[11], it is conceivable that the development of CLE may change the microenvironment of the interstitium around the palisade vessels, e.g., increased interstitial fibrosis and the formation of the shallow muscularis mucosae of a double muscularis mucosae. A double muscularis mucosae was seen in 71% of the specimens obtained by endoscopic resection of a CLE[11]. The double muscularis mucosae in CLE divides the proper mucosae into two restricted compartments, and may increase the resistance to enlargement of palisade vessels and prevent the vessels from communicating with the submucosal vessels or sub- and intra-epithelial channels[13,32], thereby inhibiting the development of enlarged EVs and RC signs. Scars secondary to endoscopic ligation or sclerotherapy were present in some of the LC subjects with post-treatment varices and their presence may have influenced the development of CLE. However, the incidence of CLE in the LC group did not differ significantly according to whether there was a history of endoscopic treatment.
The presence of the ADH1B*2 allele in Japanese alcoholics has been demonstrated to be positively associated with the presence of advanced liver disease and the progression of Child-Pugh class[17-19], but the results of the present study showed no significant associations between the genetic polymorphism and the presence of EVs. The effect of the ADH1B*2 allele on EVs was probably eclipsed, because 46.6% of the LC subjects lacked EVs.
Our study had several potential limitations. The first potential limitation was that it was a cross-sectional study based on the results of the endoscopic screening, and the progression of CLE and EVs was not evaluated directly. Identification of a causal relationship between these endoscopic findings would require longitudinal follow-up examinations. The second potential limitation was that we evaluated the degree of CLE by retrospectively reviewing the endoscopic images, however, the review was performed jointly by four expert endoscopists. Pathological studies of autopsied cirrhotic subjects are warranted to clarify the pathological background underlying the negative association between the presence of CEL and the presence of EVs. We did not observe any effects of alcohol consumption during the preceding year on the presence of CLE or EVs, but that may have been because of the homogeneity of the study population in terms of their extremely high alcohol consumption. Generalization of the results obtained in our study based on investigations of alcoholic men treated in the Center requires confirmation in various drinking populations, including in a population with mild alcoholism.
Helicobacter pylori infection and chronic atrophic gastritis protect against the development of GERD and CLE[5,6,33]. If the observed associations between CLE and EVs and advanced EVs reflect causal relationships, the current trend toward lower Helicobacter pylori infection rates in Japan may result in lower EV rates and advanced EV rates in the future, and examination for CLE may benefit alcoholics with advanced liver disease by identifying their risk for the development or progression of EVs.
In conclusion, this cross-sectional observational study revealed a negative association between the presence of CLE and the presence of EVs in Japanese alcoholic men. Further studies should be conducted prospectively in a longitudinal fashion to confirm this finding.
Esophageal varices (EVs) develop as a result of portal hypertension, which is mainly due to liver cirrhosis (LC) in alcoholics. Excessive alcohol consumption has been reported to be associated with the presence of a short-segmental columnar-lined esophagus (CLE) in East-Asian studies. It has not been evaluated whether associations exist between the presence of EVs and the presence of CLE in alcoholics.
Our empirical impression based on the results of endoscopic screening examinations of Japanese alcoholic men is that EVs are less common among men with short-segmental CLE. The development of CLE is accompanied by several histological changes around the palisade vessels at the lower end of the esophagus. Some of the histological changes accompanying the development of CLE may protect against the development of EVs.
To determine whether and to what extent associations exist between the presence of EVs and the presence of CLE in Japanese alcoholic men based on the results of endoscopic screening examinations.
The subjects were 1614 Japanese alcohol-dependent men (≥ 40 years of age) who had undergone upper gastrointestinal endoscopic screening. Digitalized records of high-quality endoscopic images that included the squamocolumnar junction and esophagogastric junction were retrospectively jointly reviewed by four expert endoscopists for the purpose of diagnosing CLE. The authors investigated whether and to what extent there were associations between the presence of CLE and the presence of EVs, especially in the group with LC.
CLE ≥ 5 mm in length was found in 355 subjects, LC without EVs in 152 subjects, LC with EVs in 174 subjects, and EVs without LC in 6 subjects. Advanced EVs, i.e., nodular, large or coiled forms, red color sign, or post-treatment, were found in 88 subjects. The incidence of CLE ≥ 5 mm decreased in the following order (P < 0.0001): 23.3% in the group without EVs, 17.4% in the group with small and straight EVs, and 5.7% in the group with advanced EVs. The multivariate ORs (95%CI) for EVs and advanced EVs in the group with LC were lower when CLE ≥ 5 mm was present [0.46 (0.23-0.93) and 0.24 (0.08-0.74), respectively, vs 0-4 mm CLE]. To clarify the pathological backgrounds of the negative association between the presence of CEL and the presence of EVs, the further pathological studies of autopsied cirrhotic subjects may be warranted.
The presence of CLE was negatively associated with the presence of EVs, and even more negatively associated with the presence of advanced forms of EVs. Since the first resistance vessels to EVs are the mucosal palisade vessels and submucosal veins at the lower end of the esophagus, the development of CLE may impede the development of EVs. Helicobacter pylori infection and chronic atrophic gastritis protect against the development of GERD and CLE. If the observed associations between CLE and EVs and advanced EVs reflected causal relationships, the current trend toward to lower Helicobacter pylori infection rates in Japan may influence EV rates and advanced EV rates in the future, and examination for CLE may benefit alcoholics with advanced liver disease by identifying their risk for the development or progression of EVs. The further studies should be conducted prospectively in the longitudinal fashion to test this finding.
| 1. | Sutton R, Shields R. Alcohol and oesophageal varices. Alcohol Alcohol. 1995;30:581-589. [PubMed] |
| 2. | Stokkeland K, Ebrahim F, Ekbom A. Increased risk of esophageal varices, liver cancer, and death in patients with alcoholic liver disease. Alcohol Clin Exp Res. 2010;34:1993-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Liao WC, Hou MC, Chang CJ, Lee FY, Lin HC, Lee SD. Potential precipitating factors of esophageal variceal bleeding: a case-control study. Am J Gastroenterol. 2011;106:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 4. | Akiyama T, Inamori M, Iida H, Mawatari H, Endo H, Hosono K, Yoneda K, Fujita K, Yoneda M, Takahashi H. Alcohol consumption is associated with an increased risk of erosive esophagitis and Barrett’s epithelium in Japanese men. BMC Gastroenterol. 2008;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Gunji T, Sato H, Iijima K, Fujibayashi K, Okumura M, Sasabe N, Urabe A, Matsuhashi N. Risk factors for erosive esophagitis: a cross-sectional study of a large number of Japanese males. J Gastroenterol. 2011;46:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Minatsuki C, Yamamichi N, Shimamoto T, Kakimoto H, Takahashi Y, Fujishiro M, Sakaguchi Y, Nakayama C, Konno-Shimizu M, Matsuda R. Background factors of reflux esophagitis and non-erosive reflux disease: a cross-sectional study of 10,837 subjects in Japan. PLoS One. 2013;8:e69891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Lee HS, Jeon SW. Barrett esophagus in Asia: same disease with different pattern. Clin Endosc. 2014;47:15-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Matsuzaki J, Suzuki H, Kobayakawa M, Inadomi JM, Takayama M, Makino K, Iwao Y, Sugino Y, Kanai T. Association of Visceral Fat Area, Smoking, and Alcohol Consumption with Reflux Esophagitis and Barrett’s Esophagus in Japan. PLoS One. 2015;10:e0133865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Thrift AP, Cook MB, Vaughan TL, Anderson LA, Murray LJ, Whiteman DC, Shaheen NJ, Corley DA. Alcohol and the risk of Barrett’s esophagus: a pooled analysis from the International BEACON Consortium. Am J Gastroenterol. 2014;109:1586-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Takubo K, Vieth M, Aida J, Sawabe M, Kumagai Y, Hoshihara Y, Arai T. Differences in the definitions used for esophageal and gastric diseases in different countries: endoscopic definition of the esophagogastric junction, the precursor of Barrett’s adenocarcinoma, the definition of Barrett’s esophagus, and histologic criteria for mucosal adenocarcinoma or high-grade dysplasia. Digestion. 2009;80:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Aida J, Vieth M, Ell C, May A, Pech O, Hoshihara Y, Kumagai Y, Kawada K, Hishima T, Tateishi Y. Palisade vessels as a new histologic marker of esophageal origin in ER specimens from columnar-lined esophagus. Am J Surg Pathol. 2011;35:1140-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Noda T. Angioarchitectural study of esophageal varices. With special reference to variceal rupture. Virchows Arch A Pathol Anat Histopathol. 1984;404:381-392. [PubMed] |
| 13. | Arakawa M, Masuzaki T, Okuda K. Pathomorphology of esophageal and gastric varices. Semin Liver Dis. 2002;22:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Chen CC, Lu RB, Chen YC, Wang MF, Chang YC, Li TK, Yin SJ. Interaction between the functional polymorphisms of the alcohol-metabolism genes in protection against alcoholism. Am J Hum Genet. 1999;65:795-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 326] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Higuchi S, Matsushita S, Murayama M, Takagi S, Hayashida M. Alcohol and aldehyde dehydrogenase polymorphisms and the risk for alcoholism. Am J Psychiatry. 1995;152:1219-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 207] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Kim DJ, Choi IG, Park BL, Lee BC, Ham BJ, Yoon S, Bae JS, Cheong HS, Shin HD. Major genetic components underlying alcoholism in Korean population. Hum Mol Genet. 2008;17:854-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Yamauchi M, Maezawa Y, Mizuhara Y, Ohata M, Hirakawa J, Nakajima H, Toda G. Polymorphisms in alcohol metabolizing enzyme genes and alcoholic cirrhosis in Japanese patients: a multivariate analysis. Hepatology. 1995;22:1136-1142. [PubMed] |
| 18. | Whitfield JB. Meta-analysis of the effects of alcohol dehydrogenase genotype on alcohol dependence and alcoholic liver disease. Alcohol Alcohol. 1997;32:613-619. [PubMed] |
| 19. | Yokoyama A, Mizukami T, Matsui T, Yokoyama T, Kimura M, Matsushita S, Higuchi S, Maruyama K. Genetic polymorphisms of alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 and liver cirrhosis, chronic calcific pancreatitis, diabetes mellitus, and hypertension among Japanese alcoholic men. Alcohol Clin Exp Res. 2013;37:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed, American Psychiatric Association, Washington DC. 1994;. |
| 21. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 22. | Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (1991). Japanese Society for Portal Hypertension. World J Surg. 1995;19:420-422; discussion 423. [PubMed] |
| 23. | The Japanese Society for Portal Hypertension. The general rules for study of portal hypertension. The 2nd edition, Tokyo: Kanehara 2004; 12 (in Japanese). |
| 24. | The Japan Esophageal Society. Japanese Classification of Esophageal Cancer. Tokyo: Kanehara 2015; 56-57 (in Japanese). |
| 25. | Okita K, Amano Y, Takahashi Y, Mishima Y, Moriyama N, Ishimura N, Ishihara S, Kinoshita Y. Barrett’s esophagus in Japanese patients: its prevalence, form, and elongation. J Gastroenterol. 2008;43:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Peng S, Cui Y, Xiao YL, Xiong LS, Hu PJ, Li CJ, Chen MH. Prevalence of erosive esophagitis and Barrett’s esophagus in the adult Chinese population. Endoscopy. 2009;41:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Lee IS, Choi SC, Shim KN, Jee SR, Huh KC, Lee JH, Lee KJ, Park HS, Lee YC, Jung HY. Prevalence of Barrett’s esophagus remains low in the Korean population: nationwide cross-sectional prospective multicenter study. Dig Dis Sci. 2010;55:1932-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Cameron AJ. Barrett’s esophagus: prevalence and size of hiatal hernia. Am J Gastroenterol. 1999;94:2054-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Sharma M, Rameshbabu CS. Collateral pathways in portal hypertension. J Clin Exp Hepatol. 2012;2:338-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Vianna A, Hayes PC, Moscoso G, Driver M, Portmann B, Westaby D, Williams R. Normal venous circulation of the gastroesophageal junction. A route to understanding varices. Gastroenterology. 1987;93:876-889. [PubMed] |
| 31. | Yoshida H, Mamada Y, Taniai N, Yoshioka M, Hirakata A, Kawano Y, Mizuguchi Y, Shimizu T, Ueda J, Uchida E. Risk factors for bleeding esophagogastric varices. J Nippon Med Sch. 2013;80:252-259. [PubMed] |
| 32. | Spence RA, Sloan JM, Johnston GW, Greenfield A. Oesophageal mucosal changes in patients with varices. Gut. 1983;24:1024-1029. [PubMed] |
| 33. | Abe Y, Iijima K, Koike T, Asanuma K, Imatani A, Ohara S, Shimosegawa T. Barrett’s esophagus is characterized by the absence of Helicobacter pylori infection and high levels of serum pepsinogen I concentration in Japan. J Gastroenterol Hepatol. 2009;24:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chiu KW, Grgurevic I, Herbella F, Qi XS S- Editor: Wei LJ L- Editor: A E- Editor: Huang Y