Published online Oct 21, 2017. doi: 10.3748/wjg.v23.i39.7098
Peer-review started: July 24, 2017
First decision: August 15, 2017
Revised: August 27, 2017
Accepted: September 13, 2017
Article in press: September 13, 2017
Published online: October 21, 2017
Processing time: 91 Days and 17.7 Hours
To identify the optimal oral dosing time of Da-Cheng-Qi decoction (DCQD) in rats with acute pancreatitis (AP) based on the pharmacokinetic and pharmacodynamic parameters.
First, 24 male Sprague-Dawley rats were divided into a sham-operated group [NG(a)] and three model groups [4hG(a), 12hG(a) and 24hG(a)]. The NG(a) and model groups were administered DCQD (10 g/kg.BW) intragastrically at 4 h, 4 h, 12 h and 24 h, respectively, after AP models induced by 3% sodium taurocholate. Plasma samples were collected from the tails at 10 min, 20 min, 40 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h after a single dosing with DCQD. Plasma and pancreatic tissue concentrations of the major components of DCQD were determined by high-performance liquid chromatography tandem mass spectroscopy. The pharmacokinetic parameters and serum amylase were detected and compared. Second, rats were divided into a sham-operated group [NG(b)] and three treatment groups [4hG(b), 12hG(b) and 24hG(b)] with three corresponding control groups [MG(b)s]. Blood and pancreatic tissues were collected 24 h after a single dosing with DCQD. Serum amylase, inflammatory cytokines and pathological scores of pancreatic tissues were detected and compared.
The concentrations of emodin, naringin, honokiol, naringenin, aloe-emodin, chrysophanol and rheochrysidin in the 12hG(a) group were higher than those in the 4hG(a) group in the pancreatic tissues (P < 0.05). The area under the plasma concentration-time curve from time 0 to the time of the last measurable concentration values (AUC0→t) for rhein, chrysophanol, magnolol and naringin in the 12hG(a) group were larger than those in the 4hG(a) or 24hG(a) groups. The 12hG(a) group had a higher Cmax than the other two model groups. The IL-10 levels in the 12hG(b) and 24hG(b) groups were higher than in the MG(b)s (96.55 ± 7.84 vs 77.46 ± 7.42, 251.22 ± 16.15 vs 99.72 ± 4.7 respectively, P < 0.05), while in the 24hG(b) group, the IL-10 level was higher than in the other two treatment groups (251.22 ± 16.15 vs 154.41 ± 12.09/96.55 ± 7.84, P < 0.05). The IL-6 levels displayed a decrease in the 4hG(b) and 12hG(b) groups compared to the MG(b)s (89.99 ± 4.61 vs 147.91 ± 4.36, 90.82 ± 5.34 vs 171.44 ± 13.43, P < 0.05).
Late-time dosing may have higher concentrations of the most major components of DCQD, with better pharmacokinetics and pharmacodynamics of anti-inflammation than early-time dosing, which showed the late time to be the optimal dosing time of DCQD for AP.
Core tip: Our study group had raised the hypothesis of tissue pharmacology of an herbal recipe, which assumed the effect of an herb formula is related to its target tissue distribution or concentration of effective components in target tissues. This study was then designed to screen the optimal oral dosing time of Da-Cheng-Qi decoction (DCQD) in rats with acute pancreatitis based on the pharmacokinetics of the main absorbed components and the pharmacodynamics of DCQD targeting of the pancreas.
- Citation: Zhang YM, Zhu L, Zhao XL, Chen H, Kang HX, Zhao JL, Wan MH, Li J, Zhu L, Tang WF. Optimal timing for the oral administration of Da-Cheng-Qi decoction based on the pharmacokinetic and pharmacodynamic targeting of the pancreas in rats with acute pancreatitis. World J Gastroenterol 2017; 23(39): 7098-7109
- URL: https://www.wjgnet.com/1007-9327/full/v23/i39/7098.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i39.7098
Da-Cheng-Qi decoction (DCQD) was first described in Shang-Han-Lun, a classical work of Traditional Chinese Medicine (TCM). DCQD consists of four Chinese herbs: dahuang (Rheum palmatum L.), mangxiao (Mirabilite, Na2SO4·10H2O), houpu (Magnolia officinalis Rehd. et Wils.), and zhishi (Citrus aurantium L.). It has been widely used as a purgative to treat diseases with constipation and to clear away internal heat[1], such as in acute pancreatitis (AP), which has a consensus in the relative treatment guidelines in China. The efficacy of DCQD, administered orally or by coloclyster to patients with AP, is obvious. According to clinical observation, it can reduce intra-abdominal hypertension[2] and decrease the risk of developing acute respiratory distress syndrome in severe AP (SAP) patients with systemic inflammatory response syndrome, and shorten their length of hospitalization[3]. In animal experiments, it can ameliorate acute pancreatic, intestinal, lung and liver injury complicated with SAP[4,5]. It can also reduce the generation of reactive oxygen species in AR42J cells and regulate the apoptosis/necrosis switch, to ameliorate the pancreatic inflammation and pathological damage[6]. Other therapeutic activities of DCQD for AP, such as its antioxidant, anti-inflammatory and anti-ulcer properties[7] are important. Its numerous roles indicate the wide applicability of DCQD in AP.
However, early oral dosing with DCQD is contradictory to the conventional therapy that considers fasting and water deprivation are necessary for pancreatic rest in the early stage of AP. Conventionally, any stimulation of the exocrine function of the pancreas by fluid or solid nutrients would promote the release of proteolytic enzymes and affect the disease course negatively[8]. Early oral dosing with DCQD may increase gastric contents, worsen stomachache and abdominal distension, and even aggravate the disease severity. However, it is not clear whether early oral administration of DCQD increases pancreatic secretion and worsens the disease severity. One clinical observation revealed that the best time for gastrointestinal unblocking by herbs was within 48 h after the onset of AP[9]. At the same time, releasing excessive turbidity should be done sooner rather than later to prevent intestinal function failure and disease deterioration[9]. Some studies have indicated that AP patients must pass feces within 24 h after the onset of AP, as one method to support intestinal function to control systemic inflammatory response syndrome and protect organ functions[9]. It is still unclear if the optimal oral dosing time of DCQD should be earlier or later. Therefore, it is important to find the optimal dosing time of DCQD at which DCQD will not worsen the disease severity.
The necrosis of pancreatic acinar cells would worsen the disease, and the induction of apoptosis would decrease the disease severity[6]. Thus, based on the effect of DCQD on regulation of the apoptosis/necrosis switch of pancreatic acinar cells to ameliorate pancreatic inflammation and pathological damage[6], the study aimed to identify the optimal oral dosing time of DCQD in rats with AP according to the pharmacokinetics of the absorbed components and the pharmacodynamics of DCQD targeting of the pancreas.
Sprague-Dawley male rats (n = 66) aged 90 ± 5 d, with body weights of 280-300 g, were purchased from Chengdu Dashuo Bio-Technique Co. Ltd. (Chengdu, China). The animals were maintained as previously described[10]. Before AP induction, rats were fasted for 12 h. This study was performed according to the Guide for the Care and Use of Laboratory Animals of Sichuan University (Chengdu, China) and the Animal Ethics Committee Guidelines of the Animal Facility of the West China Hospital (Chengdu, China).
Sodium taurocholate was supplied by Sigma (St. Louis, MO, United States). Spray dried particles of dahuang, mangxiao, houpu, and zhishi were purchased from Chengdu Green Herbal Pharmaceutical Co. Ltd. (Chengdu, China). Chloral hydrate (10%), paraformaldehyde (4%) and methanol were obtained from Tedia Co. Ltd. (Nos. 509221 and 609144; Fairfield, OH, United States,). Glacial acetic acid (No. 20030911) and ethyl acetate (No. 20070116) were purchased from Chengdu Kelon Chemical Reagent Factory (Chengdu, China). Reference standards of the 10 components of DCQD were purchased from the same companies. According to the proportion of crude drugs (dahuang 12 g, houpu 24 g, zhishi 12 g, and mangxiao 9 g), the granules of the four drugs were stirred with ultra-pure water by magnetic stirrers with a speed setting of grade 5 for 1 h in a water bath at a temperature of 37 °C for 30 min. According to the Method of Pharmacology, the least dosage of DCQD is 0.6 g/100 g.BW for rats. In this study, the dosage was 1 g/100 g.BW (10 g/kg.BW) with the concentration of 1 g/mL.
The magnetic stirrer (C-MAG, MS 7) was provided by the IKA Company (Breisgau, Germany). The analytical balance (BSA-224S-CW) was provided by the Sartorius Company (Goettingen, Germany). The micro-infusion pump was obtained from KD Scientific (Holliston, MA, United States). Conventional operation instruments, fixation-machines for rats, and the 1.5 mL and 10 mL centrifuge tubes were purchased from Shimadzu (Kyoto, Japan). The high-performance liquid chromatography tandem mass spectroscopy (HPLC-MS/MS) system consisted of a SIL-HTc autosampler (Shimadzu), a LC-10ADvp pump (Shimadzu), and an API3000 triple-quadrupole LC-MS system (Applied Biosystems, Foster City, CA, United States). This system was controlled by Analyst 1.4. Software (Chinese Pharmacological Society, Beijing, China). The chromatographic column was an Ultimate XB-C18 (5 μm, 50 mm × 4.6 mm). The mobile phase consisted of methanol-water (92:8, v/v) delivered at a flow rate of 0.3 mL/min. The column was maintained at ambient temperature, and the injection volume was 80 μL. The Anke centrifuge TGL-16B was supplied by Shanghai An-Ting Science Technology Instrument Factory (Shanghai, China). All aqueous solutions and buffers were prepared with ultra-pure water from a Millipore RiosTM-16 water purifier (Millipore, Billerica, MA, United States). Standard stock solutions were prepared by dissolving the reference standards (100 μg/mL for emodin, aloe-emodin, chrysophanol, naringin, naringenin, hesperidin, magnolol and honokiol; 20 μg/mL for rhein and rheochrysidin) and internal standard (40 μg/mL for ibuprofen) in methanol[1]. Stock solutions were stored at -20 °C. Working standard solutions were prepared freshly by diluting stock solutions in sodium hydroxide solution (0.1 mol/L). Internal standard working solution (200 ng/mL) was prepared by diluting the stock solution in methanol-water (1:1, v/v)[1].
First part: Rats were randomly allocated into the following four groups (n = 6 each): a sham-operated group [NG(a)] and three model groups [4hG(a), 12hG(a) and 24hG(a)]. AP models were induced by retrograde perfusion of 3% sodium taurocholate (1 mL/kg.BW) into the biliopancreatic duct[11] at a rate of 6 mL/h with a micro-infusion pump after anesthetization with 10% chloral hydrate (3 mL/kg.BW) injected into the abdominal cavity. The NG(a) group was established by the same procedure, but with use of saline instead of sodium taurocholate. The dosing time of DCQD for the NG(a) and model groups were 4 h, 4 h, 12 h and 24 h after operation.
Second part: Forty-two rats were divided into a sham-operated group [NG(b)], and 4h-, 12h- and 24h-dosing treatment groups [4hG(b), 12hG(b) and 24hG(b), respectively] with three corresponding control groups [MG(b)s]. Only the three treatment groups were administered the same dosage of DCQD at 4 h, 12 h and 24 h, respectively, after AP induction. The three corresponding control groups were given saline instead of DCQD at the same time. Rats were euthanized at 24 h after drug dosing.
First part: Plasma samples (0.5 mL) were collected from the tails at 10 min, 20 min, 40 min, 1 h, 2 h, 4 h, 8 h, 12 h and 24 h after a single dosing of DCQD. Serum samples for amylase (AMS) and pancreatic tissues for the tissue concentrations of the absorbed components of DCQD were collected 24 h after drug dosing. A total of 0.05 mL of internal standard working solution and 0.1 mL of hydrochloric acid buffer solution were added into 0.2 mL of the plasma or tissue homogenate samples, followed by 3.0 mL of ethyl acetate. Then, the mixtures were extracted by vortex mixing for 7 min and centrifuged at 3000 rpm for 7 min at a low temperature. After that, 2.4 mL supernatants were evaporated at 45 °C, followed by incubation with 0.1 mL of double-solvents (methanol-water: 92:8, v/v). Thereafter, 20 μL of supernatant was injected automatically into the HPLC-MS/MS system for analysis. The 10 major components of DCQD (aloe-emodin, rhein, emodin, chrysophanol, honokiol, rheochrysophanol, magnolol, hesperidin, naringenin and naringin) were detected. The mean contents of the components were detected three times, as in our previous study[12]. The detected DCQD samples were stored in the Public Experiment Platform at West China Hospital (Chengdu, China).
Second part: Serum for AMS and inflammatory cytokines measurement and pancreatic tissues for pathological scoring were collected 24 h after drug dosing. Pancreatic samples were fixed in 10% neutral formalin for paraffin sections and stained with hematoxylin and eosin (HE). All the histopathology specimens were scored in a blinded fashion by two independent pathologists using a scoring system for the extent and severity of tissue injury (0-4, edema, neutrophil infiltration, necrosis and hemorrhage, respectively), as previously described[4,13]. The IL-10 and IL-6 levels were detected by enzyme-linked immunosorbent assay kits. The following formula was used to calculate the value of AMS: AMS (U/dL) = (blankOD - measurementOD)/blankOD × 0.4 × 0.5/10 × 30 min/7.5 min × 100/0.1 × 10
The pharmacokinetic parameters were processed by pharmacokinetic statistical software DAS2.0.1 programmed by the Chinese Pharmacological Society. The data were obtained by statistical moment calculation. The following pharmacokinetic parameters were calculated: the maximum plasma concentration (Cmax), the time to reach maximum concentration (Tmax), the mean residence time (MRT0→t), the elimination half-life (T1/2) and the area under the plasma concentration-time curve from time 0 to the time of the last measurable concentration (AUC0→t). The data were processed with statistical software PEMS3.1. All values were expressed as the mean ± SD. A one-way repeated-measure ANOVA, followed by multiple pairwise comparisons using the Student-Newman-Keuls procedure, was used to detect differences among the groups. P < 0.05 was considered a statistically significant difference.
In this study, all 10 major components of DCQD in pancreatic tissues could be detected by HPLC-MS/MS. The concentrations of naringin, hesperidin, naringenin, aloe-emodin and chrysophanol in pancreatic tissues were relatively high. Compared to the NG(a) group, the concentrations of emodin, naringin, hesperidin, aloe-emodin, chrysophanol and rheochrysidin were lower in the 4hG(a) group (P < 0.05), while the rhein concentration was higher (P < 0.05). The concentrations of emodin, naringin, honokiol, naringenin, aloe-emodin, chrysophanol and rheochrysidin in the 12hG(a) group were higher than in the 4hG(a) group (P < 0.05). The concentrations of rhein, naringenin, chrysophanol and rheochrysidin in the 24hG(a) group were lower than in the 12hG(a) group (P < 0.05), while the concentrations of naringin, magnolol and hesperidin in the 24hG(a) group were higher (P < 0.05) (Table 1).
| NG(a) | 4hG(a) | 12hG(a) | 24hG(a) | |
| Rhein | 12.65 ± 3.7 | 179.15 ± 77.6a | 199.89 ± 34.8 | 50.08 ± 17.6e |
| Emodin | 18.68 ± 8.7 | 1.33 ± 0.8a | 34.46 ± 10.3c | 39.75 ± 12.4 |
| Naringin | 415.30 ± 17.8 | 19.47 ± 1.4a | 32.10 ± 2.7c | 269.16 ± 12.9e |
| Honokiol | 2.38 ± 1.2 | 1.98 ± 0.1 | 19.79 ± 2.2c | 4.26 ± 1.0 |
| Magnolol | 1.34 ± 0.9 | 0.97 ± 0.2 | 0.97 ± 0.2 | 7.51 ± 0.8e |
| Hesperidin | 162.01 ± 34.3 | 34.94 ± 12.6a | 24.31 ± 10.3 | 113.26 ± 13.8e |
| Naringenin | 847.98 ± 76.9 | 858.58 ± 19.6 | 1077.06 ± 26.8c | 262.30 ± 100.6e |
| Aloe-emodin | 439.05 ± 179.8 | 89.53 ± 14.8a | 502.74 ± 70.7c | 501.45 ± 143.4 |
| Chrysophanol | 60.99 ± 16.4 | 5.16 ± 2.1a | 197.29 ± 17.9c | 113.43 ± 23.7e |
| Rheochrysidin | 6.79 ± 1.2 | 0.55 ± 0.2a | 17.92 ± 1.9c | 11.67 ± 0.9e |
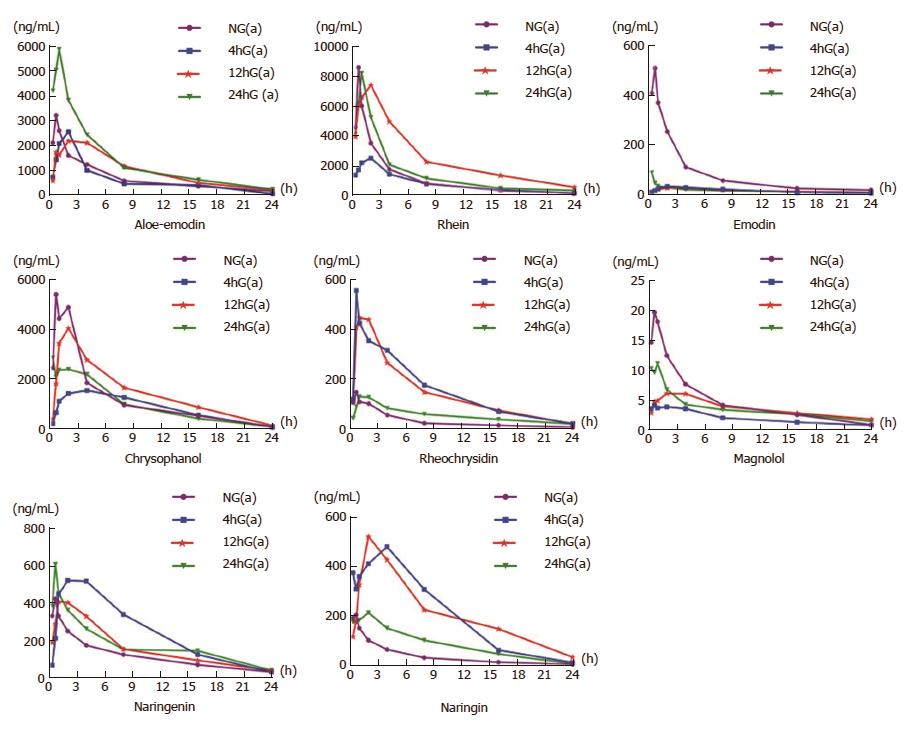
Only eight of the ten components were successfully fitted to the concentration-time curves according to the testing data (Figure 1). The Tmax values were all at 40 min (0.67 h) after a single dosage of DCQD in the NG(a) group. The Tmax of six components (aloe-emodin, rhein, emodin, chrysophanol, naringenin and naringin) in the 4hG(a) group, all components in the 12hG(a) group and that of five components (aloe-emodin, rhein, rheochrysidin, magnolol and naringin) in the 24hG(a) were delayed (Figure 1).
In the NG(a) group, the Cmax values of the components were as follows: aloe-emodin, 3218.33 ng/mL; rhein, 8638.42 ng/mL; emodin, 510.97 ng/mL; naringin, 204.56 ng/mL; chrysophanol, 5419.89 ng/mL; rheochrysidin, 146.75 ng/mL; naringenin, 419.94 ng/mL; and magnolol, 19.58 ng/mL. Compared to the NG(a) group, the Cmax values of aloe-emodin, rhein, emodin, chrysophanol and magnolol were reduced in the 4hG(a) group (Figure 1). Compared to the 4hG(a) or 12hG(a) groups, the Cmax values of aloe-emodin, rhein, emodin, naringenin and magnolol were higher in the 24hG(a) group, and they were as follows: 5885.13 ng/mL, 8245.18 ng/mL, 88.65 ng/mL, 606.41 ng/mL and 11.018 ng/mL, respectively. Among the three model groups, the Cmax values of chrysophanol and naringin were highest in the 12hG(a) group, and for rheochrysidin the Cmax was in the 4hG(a) group; the concentrations were 4054.73 ng/mL, 519.53 ng/mL and 557.06 ng/mL, respectively (Figure 1).
The pharmacokinetic parameters were analyzed by statistical moment calculation, and those in model groups were different from those in the NG(a) group (Table 2). Compared to the NG(a) group, the AUC0→t values of aloe-emodin, chrysophanol, emodin and magnolol were smaller in the 4hG(a) group (P < 0.05), while those of rheochrysidin, naringin and naringenin were larger (P < 0.05). The AUC0→t of aloe-emodin in the 24hG(a) group was larger than in the 4hG(a) or 12hG(a) groups, and those of rhein, chrysophanol, magnolol and naringin in the 12hG(a) group were larger than in the 4hG(a) or 24hG(a) groups. The T1/2 values of rhein, emodin, aloe-emodin, rheochrysidin, naringin and magnolol in the 24hG(a) group were longer than in the 4hG(a) or 12hG(a) groups.
| Parameter | NG(a) | 4hG(a) | 12hG(a) | 24hG(a) |
| Aloe-emodin | ||||
| T1/2 (h) | 7.5 ± 3.4 | 4.8 ± 1.9 | 7.3 ± 2.7 | 7.5 ± 4.3 |
| AUC0→t (μg/mL•h) | 16582.3 ± 523.7 | 15180.5 ± 245.3a | 23266.6 ± 2848.4b | 32517.8 ± 3109.6dc |
| MRT0→t (h) | 7.0 ± 1.8 | 6.5 ± 1.6 | 7.7 ± 0.9 | 6.2 ± 1.3 |
| Tmax (h) | 1.22 ± 1.38 | 1.45 ± 0.62 | 2.28 ± 1.44 | 0.67 ± 0.30dc |
| Cmax (μg/mL) | 4080.9 ± 2491.6 | 2890.5 ± 955.0 | 2948.2 ± 997.6 | 7706.6 ± 2366.7dc |
| Rhein | ||||
| T1/2 (h) | 4.7 ± 1.5 | 6.3 ± 1.8 | 6.2 ± 1.2 | 16.6 ± 2.1dc |
| AUC0→t (μg/mL•h) | 27164.9 ± 1686.9 | 19164.9 ± 1680.3 | 60705.9 ± 2870.4b | 35470.0 ± 1910.1dc |
| MRT0→t (h) | 4.7 ± 1.0 | 6.3 ± 1.5a | 7.4 ± 1.3 | 5.9 ± 3.2 |
| Tmax (h) | 0.7 ± 0.3 | 1.4 ± 0.9a | 1.3 ± 0.7 | 1.3 ± 0.4 |
| Cmax (μg/mL) | 11033.4 ± 3248.9 | 3037.9 ± 1040.5a | 9439.4 ± 3191.6b | 9938.6 ± 3349.6d |
| Chrysophanol | ||||
| T1/2 (h) | 5.2 ± 1.7 | 6.8 ± 3.6 | 4.5 ± 0.8 | 6.2 ± 3.8 |
| AUC0→t (μg/mL•h) | 28925.5 ± 7837.2 | 20214.5 ± 1460.6a | 34977.3 ± 1927.7b | 23102.4 ± 1614.8c |
| MRT0→t (h) | 5.7 ± 0.8 | 7.2 ± 1.7a | 7.3 ± 1.0 | 6.2 ± 1.2 |
| Tmax (h) | 1.2 ± 0.6 | 3.5 ± 2.5a | 1.7 ± 0.5 | 1.3 ± 0.5d |
| Cmax (μg/mL) | 7708.1 ± 2234.3 | 2296.1 ± 7643.8a | 4521.8 ± 1127.1b | 4772.6 ± 1078.4d |
| Emodin | ||||
| T1/2 (h) | 8.2 ± 6.6 | 14.7 ± 11.7 | 11.7 ± 6.0 | 20.8 ± 19.6 |
| AUC0→t (μg/mL•h) | 1891.5 ± 1692.8 | 395.3 ± 159.0a | 395.4 ± 82.4 | 391.2 ± 135.8 |
| MRT0→t (h) | 5.2 ± 1.9 | 8.4 ± 1.7a | 9.4 ± 0.8 | 8.6 ± 1.9 |
| Tmax (h) | 0.6 ± 0.3 | 3.8 ± 3.3a | 3.2 ± 2.6 | 0.7 ± 0.7dc |
| Cmax (μg/mL) | 546.2 ± 496.8 | 37.9 ± 18.4a | 34.1 ± 9.6 | 91.8 ± 37.4 |
| Rheochrysidin | ||||
| T1/2 (h) | 6.4 ± 0.9 | 4.6 ± 1.9 | 5.4 ± 2.1 | 8.3 ± 2.3dc |
| AUC0→t (μg/mL•h) | 740.2 ± 623.3 | 3680.6 ± 1903.0a | 3489.2 ± 1354.1 | 1301.8 ± 420.9dc |
| MRT0→t (h) | 5.7 ± 1.4 | 6.4 ± 0.9 | 6.3 ± 1.5 | 8.2 ± 1.1dc |
| Tmax (h) | 0.9 ± 0.6 | 2.1 ± 1.6 | 1.7 ± 1.3 | 1.5 ± 0.6 |
| Cmax (μg/mL) | 184.7 ± 92.4 | 713.8 ± 201.6a | 642.3 ± 211.5 | 155.5 ± 56.4dc |
| Magnolol | ||||
| T1/2 (h) | 8.5 ± 4.9 | 9.4 ± 2.8 | 12.2 ± 3.3 | 22.8 ± 7.6dc |
| AUC0→t (μg/mL•h) | 111.4 ± 37.2 | 44.4 ± 14.3a | 83.9 ± 27.2b | 81.5 ± 25.0d |
| MRT0→t (h) | 7.2 ± 0.9 | 7.9 ± 1.2 | 9.4 ± 1.1 | 8.9 ± 1.2 |
| Tmax (h) | 0.7 ± 0.3 | 1.3 ± 0.5∆ | 2.8 ± 1.4 | 0.6 ± 0.3dc |
| Cmax (μg/mL) | 24.2 ± 8.6 | 5.8 ± 2.3∆ | 7.7 ± 3.6 | 15.3 ± 13.1dc |
| Naringin | ||||
| T1/2 (h) | 5.8 ± 1.2 | 4.9 ± 1.8 | 5.3 ± 1.4 | 5.5 ± 1.4 |
| AUC0→t (μg/mL•h) | 918.1 ± 106.8 | 4920.6 ± 310.7a | 5054.4 ± 435.1 | 2044.1 ± 26.9dc |
| MRT0→t (h) | 5.9 ± 1.1 | 6.6 ± 0.8 | 7.9 ± 0.6 | 6.9 ± 1.0 |
| Tmax (h) | 0.7 ± 0.2 | 1.4 ± 1.4 | 2.5 ± 1.2 | 1.8 ± 1.3 |
| Cmax (μg/mL) | 229.3 ± 195.6 | 724.4 ± 584.7 | 595.1 ± 338.9 | 322.9 ± 148.4 |
| Naringenin | ||||
| T1/2 (h) | 7.2 ± 1.7 | 4.9 ± 1.1a | 9.3 ± 3.6b | 7.7 ± 1.3d |
| AUC0→t (μg/mL•h) | 2705.2 ± 164.6 | 5795.1 ± 291.4a | 3758.1 ± 250.1b | 4099.2 ± 148.8dc |
| MRT0→t (h) | 6.4 ± 1.7 | 7.3 ± 0.4 | 7.7 ± 1.2 | 7.9 ± 1.8 |
| Tmax (h) | 0.8 ± 0.6 | 2.3 ± 1.4 | 1.6 ± 1.3 | 0.6 ± 0.3d |
| Cmax (μg/mL) | 552.9 ± 226.7 | 720.4 ± 165.9 | 543.3 ± 110.4 | 624.9 ± 143.7 |
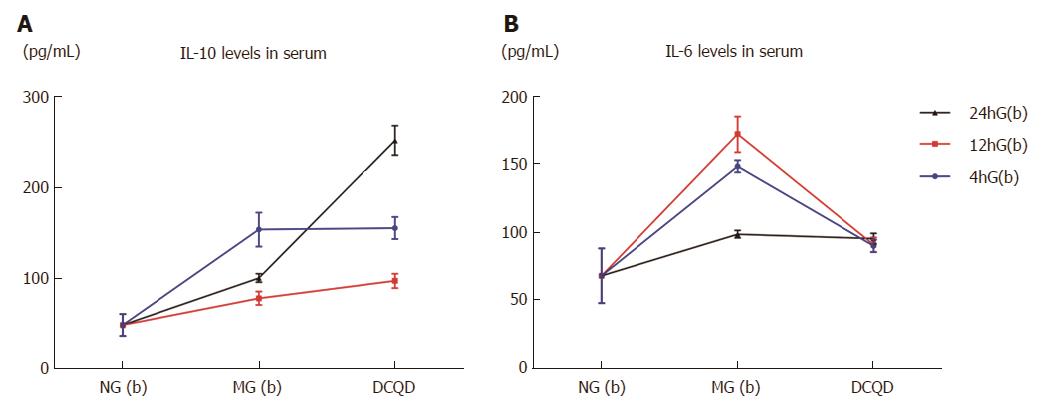
In serum, the IL-10 and IL-6 levels in the three corresponding control groups were all higher than in the NG(b) group (IL-10: 152.8 ± 18.58/77.46 ± 7.42/99.72 ± 4.7 vs 48 ± 12, P < 0.05; IL-6: 147.91 ± 4.36/171.44 ± 13.43/98.48 ± 2.7 vs 68 ± 20, P < 0.05). Compared to the corresponding control groups, the IL-10 levels in the 12hG(b) and 24hG(b) groups were increased (96.55 ± 7.84 vs 77.46 ± 7.42, 251.22 ± 16.15 vs 99.72 ± 4.7, P < 0.05), and the IL-10 level in the 24hG(b) group was higher than in the 4h(b) and 12hG(b) groups (251.22 ± 16.15 vs 154.41 ± 12.09/96.55 ± 7.84, P < 0.05) (Figure 2A). The IL-6 levels displayed a decrease in the 4hG(b) and 12hG(b) groups compared to their corresponding control groups (89.99 ± 4.61 vs 147.4.36, 90.82 ± 5.34 vs 171.44 ±13.43, P < 0.05) (Figure 2B).
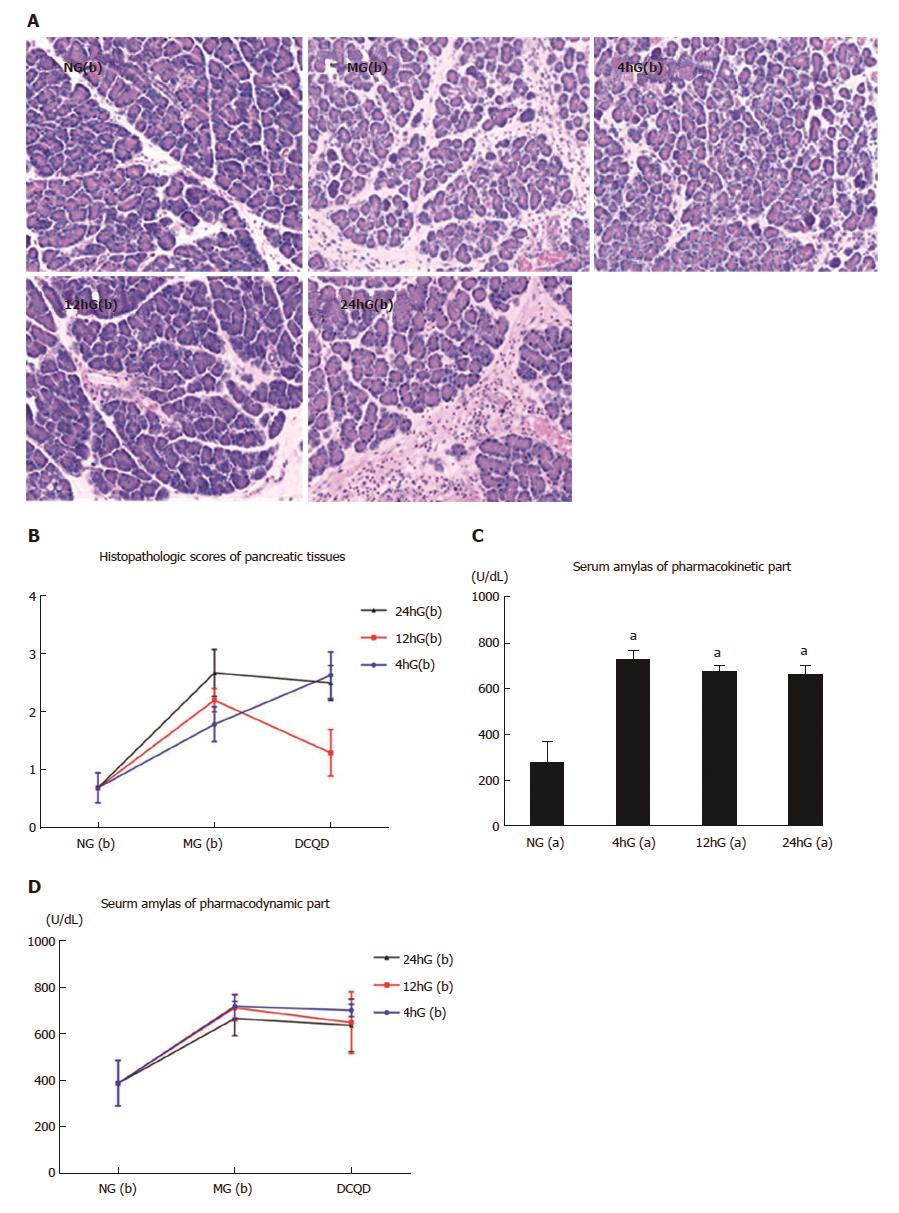
In the NG(b) group, the pancreatic tissues were edematous with a few neutrophils but without obvious hemorrhage and necrotic acinar tissues. In the MG(b) groups, edema, hemorrhage and neutrophils with some necrotic acinar tissues were obvious. In the 12hG(b) group, the pathological damages were improved, while those in the 4hG(b) or 24hG(b) groups had no obvious improvement (Figure 3A). Compared to the NG(b) group, the pathological scores in the MG(b) groups were increased (1.79 ± 0.3/2.2 ± 0.2/2.67 ± 0.67 vs 0.7 ± 0.26, P < 0.05). Those in the 12hG(b) group were evidently reduced compared to its control group (1.3 ± 0.4 vs 2.2 ± 0.2, P < 0.05) or the 4hG(b) and 24hG(b) groups (1.3 ± 0.4 vs 2.63 ± 0.4/2.5 ± 0.3, P < 0.05) (Figure 3B).
The AMS levels in the 4hG(a), 12hG(a) and 24hG(a) groups were all higher than in the NG(a) group (724.17 ± 42.8/673.67 ± 26.64/659.65 ± 41.38 vs 273.67 ± 93.23, P < 0.05) (Figure 3C). Compared to the NG(b) group, that in the MG(b) groups were higher (718.65 ± 51.04/711.68 ± 55.37/666.4 ± 73.4 vs 389 ± 98, P < 0.05). The AP model was successful. That in the 12hG(b) group was reduced compared to its control group (649 ± 131.69 vs 711.68 ± 55.37, P < 0.05) (Figure 3D).
According to the present study, AP reduced the concentrations of the major components of DCQD to the target pancreas, and the oral administration time also played an important role; their absorption may be better if the oral administration time of DCQD is approximately 12 h after the onset of AP (Table 1). AP delayed the Tmax and reduced the Cmax of the components of DCQD in the circulation of rats (Figure 1). The components of DCQD displayed a higher Cmax and a longer T1/2 when the oral administration time of DCQD was approximately 24 h after the onset of AP (Figure 1 and Table 2). The AUC0→t was larger when that time was approximately 12 h after the onset of AP (Table 2). DCQD increased the IL-10 levels and lowered the IL-6 levels (Figure 2A and B), and the later administration of the DCQD dose corresponded to higher IL-10 levels (Figure 2A). Therefore, administering the DCQD dose too early may not be appropriate for AP, and administration should at least be 12 h after the onset of AP.
Pathological circumstances may affect the absorption of the components of DCQD and affect the pharmacokinetics in AP. Pancreatic ischemia, reduced pancreatic blood flow and increased capillary permeability are usually common in AP[14]. The systemic hemodynamic disturbances lead to ischemia of the intestine[15]. In addition, one retrospective analysis (197 patients) showed that 65% of patients with AP had acute gastrointestinal mucosal lesions detected by upper gastrointestinal endoscopy[16]. As well, 59% of patients with AP showed gut barrier dysfunction with increased intestinal permeability in a meta-analysis of 18 studies[17]. Both propulsion and contractility were reduced in necrotizing pancreatitis of rats[18]. It was reported that permeability of the ileum was significantly increased at 6 h, the blood endotoxin level was elevated and bacterial translocation occurred 18 h after induction of SAP-induced by injection of 3% sodium deoxycholate[19].
As we know, oral medicines, including herbs, are generally absorbed by the gastrointestinal mucosa. The process of drug absorption into the blood circulation from the plasma membrane barrier proceeds as follows: drug molecule encounters the gastrointestinal mucous layer, brush border, epithelial cell membrane, intracellular fluid, basal lamina, lamina propria, externa of vessels, cytoplasm of vessels, intima of vessels and then the blood. Therefore, these aforementioned factors may play an important role in the delay of the Tmax of the components of DCQD in reaching the circulation and establishing their concentrations in pancreatic tissues.
Another factor may be the physicochemical properties of these components. Chrysophanol belongs to the Class II poorly water soluble drugs in the Biopharmaceutics Classification System (BCS), with low solubility but high permeability[20,21]. Magnolol, a small-molecule neolignan[22], has an extensive first-pass metabolism and low absorption[23]. Naringin is moderately soluble in water, and it is broken into its aglycon naringenin in the intestine by the gut microflora and then absorbed from the gut[24]. These different characteristics may influence the absorption of these components. Furthermore, the pharmacokinetics of phytochemicals have substantial variation[25] and circulating concentrations of phytochemicals, which could vary widely among individuals, even in the context of controlled feeding studies[26]. In brief, the internal environments of rats and the characteristics of the components of DCQD may be the major factors affecting the absorption of these components.
It is well known that the avoidance of gastric and intestinal secretion has been the cornerstone of management of patients with AP for nearly a century[27]. To espouse the “pancreatic rest” concept, fasting and water deprivation have become the fundamental treating rules. However, the benefits of oral dosing with DCQD in the early stage of AP have been demonstrated, especially in gastrointestinal internal environments. This approach could improve intestinal propulsive function, relieve abdominal distension and abdominal pressure[28], and protect the intestinal immune barrier, with amelioration of the levels of high mobility group box-1 protein (HMGB1) RNA and cyclooxygenase 2 (COX-2) RNA expression[29]. One meta-analysis showed that purgative therapy could shorten the time of first defecation and the hospitalization time[30]. Thus, the advantages of DCQD are obvious. Future studies should be done to determine whether the oral dose of DCQD could increase gastrointestinal and pancreatic secretion.
The finding of DCQD regulating the balance of pro-inflammation and anti-inflammation was consistent with our previous studies[4]. However, the current study showed that its oral dosing time might affect the inflammatory cytokines and pharmacokinetics of the effective components of DCQD targeting of pancreatic tissues and plasma. The chronomedicine of TCM, with thousands of years of history and in which the midnight-noon ebb-flow theory is typical, is similar to modern chronobiology. Theoretically, the function of Chinese medicine will be most effective at driving out pathogenic factors when the function of some meridian is at its peak[31]. For example, erythrocyte C3b receptor rosette and erythrocyte immune complex rosette can reach peak value when the kidney meridian has its most active function[32].
Along with the improvement of modern technology, the research on the relationship between the dosing time of Chinese medicine and plasma concentration or curative effect has been performed. Nishioka et al[33] demonstrated that the dosing time of Sho-Saiko-To could affect the plasma concentrations of the effective components (glycyrrhizin and baicalein). Additionally, the pharmacokinetic processes of emodin and aloe-emodin of DCQD presented a circadian rhythm phenomenon[34]. According to our study, the oral dosing time also affects the drug tissue concentrations. Therefore, the oral dosing time of DCQD is closely related to its pharmacokinetics and pharmacodynamics.
In this study, the pathological damages of pancreatic tissues had been improved only in the 12hG(b) group, and was not improved in the 4hG(b) or 24hG(b) groups. However, this finding was not consistent with those of a previous study showing that a similar dose of DCQD 2 h after AP induction confers some protection against pancreatic tissue damage. Although the weight of rats showed no difference, this finding may be related to the different experimenters, which may have resulted in large differences among the various groups. We should ensure the consistency of experimenters. In clinical practice, orally dosing or coloclysis of DCQD are performed immediately when patients with AP are admitted to hospital. Whether these approaches could increase secretion of the gastrointestinal tract remains unclear. Recently, little research on the optimal administration time of Chinese herbs has been reported, and there are no definite opinions on this topic in the Chinese guidelines, although the guidelines are generally used in clinical practice in China. Therefore, our conjecture needs further clinical studies to be confirmed.
In conclusion, AP and the oral administration time of DCQD could affect the pharmacokinetics of the absorbed components of DCQD in the pancreatic tissues and plasma of rats. Late-time dosing may result in higher concentrations of the major components of DCQD with better pharmacokinetics and pharmacodynamics of anti-inflammation than seen with early-time dosing, thereby showing the late time to be the optimal dosing time of DCQD for AP.
Oral administration with Da-Cheng-Qi decoction (DCQD) is the conventional therapy at the early phase of acute pancreatitis (AP) patients in China. But oral dosing with DCQD is contrary to the idea of pancreatic rest at the early stage of AP, which may inhibit the absorption of the components of DCQD, influence its pharmacokinetics or pharmacodynamics and even worsen the disease severity.
The necrosis of pancreatic acinar cells would worsen the disease and the induction of apoptosis would relieve the disease severity. In clinical practice, oral dosing or coloclysis of DCQD are performed immediately when patients with AP are admitted to hospital. Whether these approaches could increase secretion of gastrointestinal tract remain unclear. What’s more, little research on the optimal administration time of Chinese herbs has been reported, and there are no definite opinions on this topic in the Chinese guidelines. Thus, based on effect of DCQD regulating the apoptosis/necrosis switch of pancreatic acinar cells to ameliorate the pancreatic inflammation and pathological damage, the study aimed to screen the optional oral dosing time of DCQD in rats with AP according to the pharmacokinetics of the absorbed components and the pharmacodynamics of DCQD targeting of pancreas.
This objective was to screen the optional oral dosing time of DCQD in rats with AP based on the pharmacokinetic and pharmacodynamic parameters. We hoped to find an optimal dosing time of DCQD without increasing the severity of AP.
This animal experiment was divided into pharmacokinetic and pharmacodynamic parts. AP models were induced by 3% sodium taurocholate. Rats were dosed at three different times. Plasma samples were collected from the tails at nine different times. The main components’ concentrations of plasma and pancreatic tissues were detected by high-performance liquid chromatography tandem mass spectroscopy, confirmed as a specific, sensitive, accurate and reproducible method. The pharmacokinetic parameters [the maximum plasma concentration (Cmax), the time to reach maximum concentration (Tmax), the mean residence time (MRT0→t), the elimination half-life (T1/2) and the area under the plasma concentration-time curve from time 0 to the time of the last measurable concentration (AUC0→t)] were processed by pharmacokinetic statistical software DAS2.0.1 programmed by the Chinese Pharmacological Society. The IL-10, IL-6 and amylase concentrations in serum and pathological scores of pancreatic tissues were calculated.
According to the present study, AP reduced the concentrations of the major components of DCQD to the target pancreas, and the oral administration time also played an important role. AP delayed the Tmax and reduced the Cmax of the components of DCQD in the circulation of rats. The AUC0→t was larger when that time was approximately 12 h after the onset of AP. DCQD increased the IL-10 levels and lowered the IL-6 levels, and the later administration of the DCQD dose corresponded to higher IL-10 levels. Therefore, administering the DCQD dose too early may not be appropriate for AP. However, our results need further clinical studies to be confirmed.
Late-time dosing may result in higher concentrations of the major components of DCQD with better pharmacokinetics and pharmacodynamics of anti-inflammation than seen with early-time dosing, thereby showing the late time to be the optimal dosing time of DCQD for AP.
| 1. | Yu Q, Xiang J, Tang W, Liang M, Qin Y, Nan F. Simultaneous determination of the 10 major components of Da-Cheng-Qi decoction in dog plasma by liquid chromatography tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2025-2031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Wan MH, Li J, Huang W, Mukherjee R, Gong HL, Xia Q, Zhu L, Cheng GL, Tang WF. Modified Da-Cheng-Qi Decoction reduces intra-abdominal hypertension in severe acute pancreatitis: a pilot study. Chin Med J (Engl). 2012;125:1941-1944. [PubMed] |
| 3. | Wan MH, Li J, Gong HL, Xue P, Zhu L, Chen GY, Xia Q, Wen-Fu T. Clinical observation on the effect of dexamethasone and Chinese herbal decoction for purgation in severe acute pancreatitis patients. Chin J Integr Med. 2011;17:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 4. | Zhao X, Zhang Y, Li J, Wan M, Zhu S, Guo H, Xiang J, Thrower EC, Tang W. Tissue Pharmacology of Da-Cheng-Qi Decoction in Experimental Acute Pancreatitis in Rats. Evid Based Complement Alternat Med. 2015;2015:283175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Zhang YM, Ren HY, Zhao XL, Li J, Li JY, Wu FS, Su H, Tang WF. Pharmacokinetics and pharmacodynamics of Da-Cheng-Qi decoction in the liver of rats with severe acute pancreatitis. World J Gastroenterol. 2017;23:1367-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Wang J, Chen G, Gong H, Huang W, Long D, Tang W. Amelioration of experimental acute pancreatitis with Dachengqi Decoction via regulation of necrosis-apoptosis switch in the pancreatic acinar cell. PLoS One. 2012;7:e40160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Chen YT, Zheng RL, Jia ZJ, Ju Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic Biol Med. 1990;9:19-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 370] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Yousaf M, McCallion K, Diamond T. Management of severe acute pancreatitis. Br J Surg. 2003;90:407-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 9. | Tang XY, Cha JY, Xu K, Sheng HY, Ou Y. The timing of early intervention treatment in acute pancreatitis. Zhongyi Linchuang Zazhi. 2013;7:376-379. |
| 10. | Tang WF, Huang X, Yu Q, Qin F, Wan MH, Wang YG, Liang MZ. Determination and pharmacokinetic comparison of rhein in rats after oral dosed with Da-Cheng-Qi decoction and Xiao-Cheng-Qi decoction. Biomed Chromatogr. 2007;21:1186-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Hu YY, Zhou CH, Dou WH, Tang W, Hu CY, Hu DM, Feng H, Wang JZ, Qian MJ, Cheng GL. Improved autophagic flux is correlated with mTOR activation in the later recovery stage of experimental acute pancreatitis. Pancreatology. 2015;15:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Tang W, Wan M, Zhu Z, Chen G, Huang X. Simultaneous determination of eight major bioactive compounds in Dachengqi Tang (DT) by high-performance liquid chromatography. Chin Med. 2008;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Pitkäranta P, Kivisaari L, Nordling S, Nuutinen P, Schroder T. Vascular changes of pancreatic ducts and vessels in acute necrotizing, and in chronic pancreatitis in humans. Int J Pancreatol. 1991;8:13-22. [PubMed] |
| 14. | Rongione AJ, Kusske AM, Kwan K, Ashley SW, Reber HA, McFadden DW. Interleukin 10 reduces the severity of acute pancreatitis in rats. Gastroenterology. 1997;112:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 229] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Farrant GJ, Abu-Zidan FM, Liu X, Delahunt B, Zwi LJ, Windsor JA. The impact of intestinal ischaemia-reperfusion on caerulein-induced oedematous experimental pancreatitis. Eur Surg Res. 2003;35:395-400. [PubMed] |
| 16. | Chen TA, Lo GH, Lin CK, Lai KH, Wong HY, Yu HC, Hsu PI, Chen HH, Tsai WL, Chen WC. Acute pancreatitis-associated acute gastrointestinal mucosal lesions: incidence, characteristics, and clinical significance. J Clin Gastroenterol. 2007;41:630-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg. 2014;101:1644-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Rieger H, Runkel N, Spröder J, Buhr HJ. [Different mechanisms in intestinal paralysis in edematous and necrotizing pancreatitis of the rat]. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:409-412. [PubMed] |
| 19. | Yasuda T, Takeyama Y, Ueda T, Shinzeki M, Sawa H, Nakajima T, Kuroda Y. Breakdown of intestinal mucosa via accelerated apoptosis increases intestinal permeability in experimental severe acute pancreatitis. J Surg Res. 2006;135:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Singh D, Rawat MSM, Semalty A, Semalty M. Chrysophanol-phospholipid complex. J Thermal Analysis Calorimet. 2012;111:2069-2077. [DOI] [Full Text] |
| 21. | Wang S, Chen T, Chen R, Hu Y, Chen M, Wang Y. Emodin loaded solid lipid nanoparticles: preparation, characterization and antitumor activity studies. Int J Pharm. 2012;430:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Lin CF, Hwang TL, Al-Suwayeh SA, Huang YL, Hung YY, Fang JY. Maximizing dermal targeting and minimizing transdermal penetration by magnolol/honokiol methoxylation. Int J Pharm. 2013;445:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Tsai TH, Chou CJ, Lee TF, Wang LCH, Chen CF. Pharmacokinetic and Pharmacodynamic Studies of Magnolol after Oral Administration in Rats. Pharm Pharmacol Commun. 1996;2:191-193. |
| 24. | Choudhury R, Chowrimootoo G, Srai K, Debnam E, Rice-Evans CA. Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem Biophys Res Commun. 1999;265:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Mizunuma H, Khorram O, McCann SM. Blockade of stress-induced prolactin release in monosodium glutamate-treated rats. Brain Res Bull. 1983;10:23-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Kuijsten A, Arts IC, Vree TB, Hollman PC. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J Nutr. 2005;135:795-801. [PubMed] |
| 27. | Petrov MS. Gastric feeding and “gut rousing” in acute pancreatitis. Nutr Clin Pract. 2014;29:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Li DW, Wang CM. Effect of Da Cheng Qi decoction to the intestinal transtit of acute necrotizing pancreatitis (ANP) in rat. Dalian Yikedaxue Xuebao. 2012;34:455-457. |
| 29. | Shen Y, Jin W, Liao H, Zhang C, Zhang X, Wang Y. Protective Effect of Dachengqi Decoction on Intestinal Immune Barrier of Rat with Severe Acute Pancreatitis. Hubei Daxue Zhongyi Xuebao. 2015;63:438-463. |
| 30. | Miao B, Cui NQ, Li ZL, Ma T, Zhao G, Wang X. Systematic evaluation of the therapeutic efficacy of Tongli Gongxia herbs on severe acute pancreatitis. Shijie Huaren Xiaohua Zazhi. 2009;17:1042-1047. |
| 31. | Huang ZF, Wei JS, LI HZ, Zeng AP, Tan ZQ. Influence on Life Quality of Advanced Gastric Cancer Patients Treated with TCM Timing Therapy Combined with Chinese and Western Medicines. Zhongguo Zhongyao Zazhi. 2011;26:901-904. |
| 32. | Xie XQ. Research, developmen and application of new product of Chinese herbal medicine. Beijing: People’s Medical Publishing House 2000; 99-100. |
| 33. | Nishioka Y, Yang JZ. Effect of the dosing time of Sho-Saiko-To on the plasma concentrations of the effective components. Guowai Yixue. 1993;3:21-22. |
| 34. | Tang WF, Ren YY, Gong HL, Jiang L, Chen GY, Huang X. Circadian rhythm phenomenon of the effective components of Dachengqi decocotion in healthy rats. 2009;170. |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bourgoin SG, Yanev SG S- Editor: Qi Y L- Editor: Filipodia E- Editor: Huang Y