Published online Oct 14, 2017. doi: 10.3748/wjg.v23.i38.6952
Peer-review started: August 13, 2017
First decision: August 30, 2017
Revised: September 11, 2017
Accepted: September 20, 2017
Article in press: September 19, 2017
Published online: October 14, 2017
Processing time: 64 Days and 16.8 Hours
Recurrent acute pancreatitis (RAP) is defined based on the occurrence of two or more episodes of acute pancreatitis. The initial evaluation fails to detect the cause of RAP in 10%-30% of patients, whose condition is classified as idiopathic RAP (IRAP). Idiopathic acute pancreatitis (IAP) is a diagnostic challenge for gastroenterologists. In view of associated morbidity and mortality, it is important to determine the aetiology of pancreatitis to provide early treatment and prevent recurrence. Endoscopic ultrasound (EUS) is an investigation of choice for imaging of pancreas and biliary tract. In view of high diagnostic accuracy and safety of EUS, a EUS based management strategy appears to be a reasonable approach for evaluation of patients with a single/recurrent idiopathic pancreatitis. The most common diagnoses by EUS in IAP is biliary tract disease. The present review aims to discuss the role of EUS in the clinical management and diagnosis of patients with IAP. It elaborates the diagnostic approach to IAP in relation to EUS and other different modalities. Controversial issues in IAP like when to perform EUS, whether to perform after first episode or recurrent episodes, comparison among different investigations and the latest evidence significance are detailed.
Core tip: The initial evaluation fails to detect the cause of recurrent acute pancreatitis (RAP) in 10%-30% of patients, whose condition is classified as idiopathic RAP. Idiopathic acute pancreatitis (IAP) is a diagnostic challenge for gastroenterologists. In view of high diagnostic accuracy and safety of endoscopic ultrasound (EUS), a EUS based management strategy appears to be a reasonable approach for evaluation of patients with a single/recurrent idiopathic pancreatitis. The most common diagnoses by EUS in IAP is biliary tract disease. This review aims to discuss the role of EUS in the clinical management and diagnosis of patients with IAP.
- Citation: Somani P, Sunkara T, Sharma M. Role of endoscopic ultrasound in idiopathic pancreatitis. World J Gastroenterol 2017; 23(38): 6952-6961
- URL: https://www.wjgnet.com/1007-9327/full/v23/i38/6952.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i38.6952
Acute pancreatitis (AP) is an inflammatory process of the pancreas affecting peripancreatic tissues and distant sites. In most patients after an attack of AP, aetiology can be found with gallstones and alcohol use being most often implicated. Approximately 20% of patients will develop subsequent attacks, defined as recurrent acute pancreatitis (RAP). Most aetiologies of AP can lead to recurrent attacks, if the underlying etiology persists[1,2,3]. RAP is a challenging condition, because it leads to significant patient morbidity, can progress to chronic pancreatitis (CP), and has limited treatment options[3]. In up to 10% of patients with a single episode of AP and in up to 30% of patients with RAP, the aetiology is not identified after the initial evaluation. This condition is known as idiopathic pancreatitis[4]. Evaluation and treatment is important as 50% of untreated patients with IRAP experience current episodes that may lead to CP[2]. Endoscopic ultrasound (EUS) is minimally invasive, highly accurate imaging modality for studying the pancreas and the biliary tree[5]. The goal of this review is to discuss the role of EUS in idiopathic pancreatitis, focusing on the methodology, findings, and limitations of the available literature.
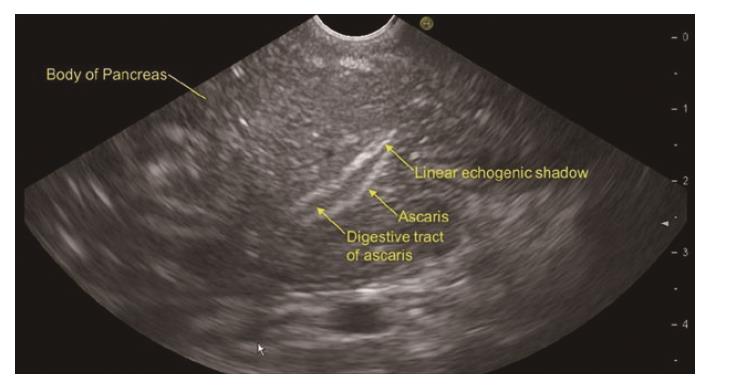
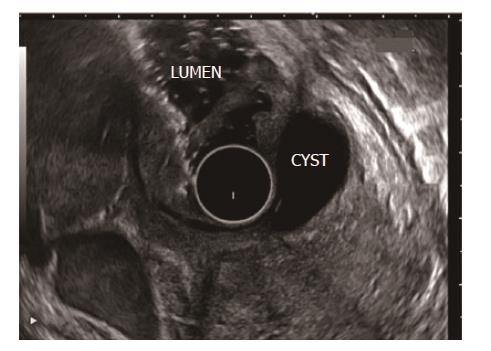
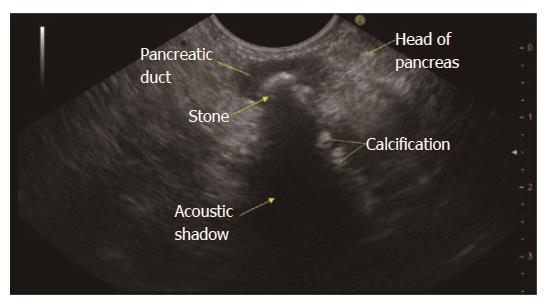
RAP has various definitions. RAP is defined as the occurrence of two or more episodes of AP without concurrent clinical or imaging evidence suggestive of CP[3]. Another definition is two or more episodes of AP with or without complete or near complete resolution of symptoms between episodes[6]. AP or RAP can be from an identifiable etiology or idiopathic (unidentifiable etiology). Initial evaluation of a patient with AP/RAP can identify the aetiology in 70%-90% of cases. When the etiology could not be identified on initial evaluation as it happens in 10%-30% of the patients, AP/RAP can be defined as IAP (idiopathic acute pancreatitis)/IRAP[2,3,7]. The initial evaluation includes a thorough history (including medication review and family history of pancreatitis), physical examination, laboratory studies (serum liver tests, calcium and triglyceride levels), transabdominal right upper quadrant ultrasound, contrast-enhanced abdominal computerized tomography (CT), or magnetic resonance cholangiopancreatography (MRCP). Various explanations for non-detection of aetiology of AP includes microlithiasis as the cause being difficult to diagnose using standard imaging analysis; pancreatic inflammation/necrosis, preventing visualization of pancreatic solid or cystic tumours; and biological abnormalities occurring during the initial days of AP, making difficult to diagnose lipid- or calcium metabolism abnormalities[8]. There are limited studies regarding the natural history following a single attack of IAP. These studies suggest relapse rates varying from 14% to 24%[9-11]. According to the latest prospective study by Wilcox et al[12], the relapse rate is 24. Various diagnostic studies to investigate the remaining 10%-30% cases includes microscopic bile examination (MBE), pancreatic function testing, genetic testing for mutations associated with pancreatitis, secretin-enhanced MRCP (MRCP-S), EUS, EUS elastography, and endoscopic retrograde cholangiopancreatography (ERCP) with sphincter of oddi manometry (SOM). This additional workup usually leads to the diagnosis of microlithiasis or biliary sludge, sphincter of oddi dysfunction (SOD), pancreas divisum (PD), hereditary pancreatitis, cystic fibrosis, a choledochocele, annular pancreas, an anomalous pancreatobiliary junction, pancreatobiliary tumours, duodenal duplication cyst, periampullary diverticulum or pancreatic-biliary ascariasis (Figures 1 and 2)[2,4,13-16]. After a complete additional advanced work-up, the aetiology remains unknown in no more than 10% of RAP, which can then be defined as true IRAP[2,3]. However, which test(s) to perform is not standardized. EUS is emerging as a diagnostic tool of choice for the patients with idiopathic pancreatitis[12].
Since the early 1980s, EUS has been in use and is a safe, minimally invasive diagnostic procedure in patients with RAP. Various advantages includes close proximity of EUS probe to the pancreas and availability of high frequency EUS probes leading to high resolution images, and non-interference of the intestinal gases with image acquisition. EUS has few complications[17,18]. The diagnostic yield of EUS in various studies varies from 29%-80%. In a recent systemic review of 13 studies evaluating the role of EUS in IAP, the most frequent aetiology was biliary tract disease (biliary stones, microlithiasis and sludge). In the above mentioned 13 studies, different diagnostic modalities (1 or combination of 2 or more) were mentioned for diagnostic accuracy. They were ultrasonography, CT, MRCP, ERCP, SOM. Reference standard varied depending on the study. EUS identified pancreatic disease like CP, PD, periampullary tumours or pancreatic parenchymal change and/or pancreatic ductal change in 22.1% ± 26.6% of patients with IAP. Overall, EUS identified additional diagnostic information in 61% of patients with IAP, with 41% having biliary tract disease[19]. Various studies on the sensitivity of EUS to detect biliary tract disease suggests that EUS has superior sensitivity to other commonly used tests like ultrasonography (USG), CT, MRCP or MBE[12,17,18-22]. EUS is a reliable diagnostic method to detect PD[23,24]. EUS is quite useful to diagnose biliary and pancreatic tumours with a diagnostic accuracy higher than CT particularly in tumours smaller than 2.5 cm in diameter[25]. EUS is highly specific in the diagnosis of pancreatic cancer with a negative predictive value of 100%[26]. EUS can detect pancreatic solid tumours and cystic tumours at an early stage before they are identified by USG or CT. Detecting them earlier can lead to early operative management and better prognosis[27]. EUS is a useful diagnostic technique to detect the presence of CP in patients initially diagnosed with IRAP[28]. EUS is the least expensive initial investigation for the diagnostic evaluation of patients with IAP with gallbladder in situ[29].
Before the advent of EUS, ERCP was a primary diagnostic and therapeutic tool with a diagnostic yield of about 80%; however the contribution of EUS and MRCP has changed the diagnostic algorithm of IAP[3]. Established (microlithiasis, neoplasms) and controversial (PD, SOD) aetiologies of IAP may now be identified with EUS, limiting the role of ERCP to its therapeutic arm[3]. EUS has a diagnostic yield of about 80% which is about the same as the diagnostic yield of ERCP but with much less complication rates compared to ERCP which is 10%-15%[4].
Along with the lesser complication rates compared to ERCP, the added advantage of EUS is, EUS can diagnose biliary and pancreatic tumors especially tumors communicating with the pancreatic duct which can cause IAP[4]. In the past, ERCP was advised only after the second episode of IAP or after the first in severe IAP[30,31]. ERCP and EUS are considered the “gold standards” in clinical practice; however, MRCP has been proposed as a non-invasive alternative imaging technique to ERCP[32].
MRCP is a non-invasive investigation revealing detailed images of the hepatobiliary and pancreatic systems[32]. MRCP is indicated to diagnose PD, choledochocele, anomalous pancreatobiliary junction, or annular pancreas in patients with IARP[2,33]. Advantages includes no administration of intravenous contrast or ionizing radiation, can be used in all patients including infants or those with allergies to iodine-based contrast materials[33,34]. It is less operator-dependent than USG or ERCP[35]. Disadvantages includes no therapeutic manoeuvres possible as it is primarily diagnostic investigation. MRCP is less sensitive than EUS for microlithiasis, small ampullary lesions, and ductal strictures[36-38].There are various studies showing better diagnostic yield of MRCP after secretin stimulation(MRCP-S) in CP,PD, SOD and pancreaticobiliary malformation as compared to simple MRCP[32,39-43]. In view of the available literature, MRCP-S may be more beneficial in IAP instead of MRCP if available.
There are three studies which has directly compared EUS and MRCP in IAP[18,43,44]. All have found higher diagnostic yield of EUS particularly to exclude biliary causes. In a prospective study of 49 patients with IAP, diagnoses were compared between EUS and MRCP. The diagnostic yield of EUS was more than that of MRCP (51% vs 20%, P = 0.001). It was concluded that EUS should be performed for establishing a possible biliary aetiology in patients with intact gall bladder (GB)[44]. In a study of 40 patients with MRCP negative IAP by Rana et al[18], the diagnostic yield of EUS was 55%. The most common aetiology was occult biliary tract disease in 50 percent[18].
PD is controversial aetiology of IAP[3]. Various studies have assessed the role of EUS as a reliable substitute for ERCP in patients of PD. Some believe that the sensitivity and specificity of EUS need further evaluation[5,45]. However, various studies considers EUS as an accurate, minimally invasive diagnostic method for PD[23,24,46-48]. In a study by Rana et al[46], the sensitivity, specificity, positive predictive value, negative predictive value of EUS for diagnosis of PD was 100%, 96%, 80%, 100% and 96%, respectively. In a recent retrospective cohort study of 45 consecutive patients diagnosed with PD on ERCP, diagnostic accuracy of EUS was compared to CT and MRCP. The sensitivity of EUS was 86.7%, significantly higher than CT (15.5%) or MRCP (60%).It was concluded that EUS is a sensitive test for diagnosing PD and is superior to MDCT and MRCP[48]. As per recent systemic review comparing diagnostic accuracy of MRCP with MRCP-S, MRCP-S has significant higher diagnostic accuracy and should be preferred for diagnosis of PD[42]. However, MRCP-S is not easily available. We believe that EUS is excellent diagnostic investigation for PD instead of MRCP[24].
USG (abdomen) has high accuracy in detecting gallstones, with reported sensitivities ranging from 92% to 96%[49]. However, when stones are less than 3 mm in diameter or are located in the GB infundibulum, the sensitivity is only 65%[50]. EUS is superior than USG for GB imaging due to its high-image resolution and close proximity to the biliary system. In a prospective study of 100 consecutive patients with AP, EUS was more sensitive than USG in detecting gallstones (100% vs 84%). The sensitivities of ERCP and EUS for choledocholithiasis were both 97%. It was concluded that EUS can be useful in selecting patients with AP who require therapeutic ERCP, thereby avoiding complications associated with diagnostic ERCP[49].
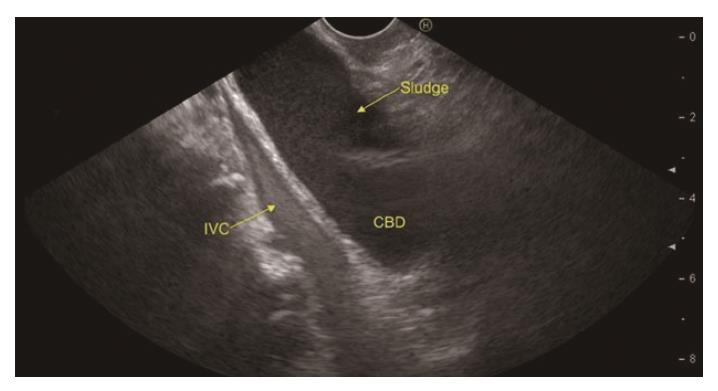
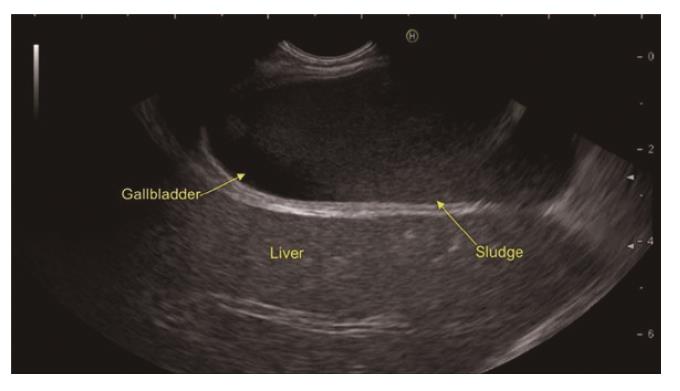
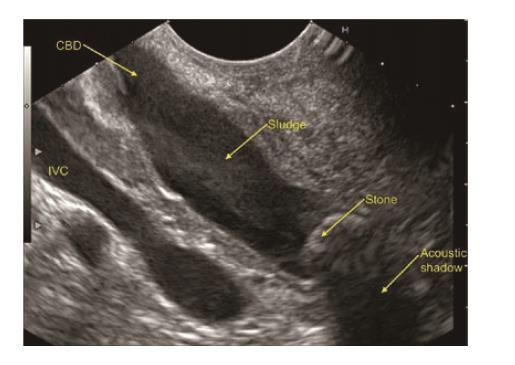
In a recent systemic review of 13 studies evaluating the role of EUS in IAP, the most frequent aetiology was biliary tract disease (41%)[19]. Various studies have found biliary microlithiasis/sludge (Figure 3, Figure 4 , Figure 5) as a cause of IAP in up to 75% of these patients[7,51,52]. However, there are few studies where biliary aetiology is not the most common cause of IAP[27,53].
In patients with intact GB, the most common cause of IAP is biliary microlithiasis/sludge which is detected in up to 80% of patients[7,51]. In the past, MBE was considered the gold standard for microlithiasis[54]. However, the disadvantages include high false negative rate, time consuming and high failure rate[4]. EUS has now replaced ERCP and bile sampling to diagnose microlithiasis in IRAP[8]. There are various reasons due to which EUS is highly accurate in detecting biliary microlithiasis. These includes GB being closely related to the stomach/duodenal wall; the median distance between the EUS transducer and the gallbladder is small (0.5 mm); the whole gallbladder is examined, even under acute pancreatitis; and use of high frequency probe[18].
The prevalence of the various aetiologies of RAP depends on whether the gallbladder is present or absent. If GB is present insitu, then the prevalence of microlithiasis would be about 50%, SOD-20%, CP-15%, PD-10% and choledocholithiasis 5%[29]. In patients who underwent cholecystectomy the prevalence of microlithiasis falls to 10%-15% whereas the prevalence of SOD, PD and CP rises[45].
RAP is a diagnostic and therapeutic challenge in clinical practice. It is sometimes impossible to differentiate clearly between recurrent attacks of AP and the early stages of CP[55]. Recurrent attacks of AP may complicate the course of chronic subclinical pancreatitis[13] (Figure 6). Although one of the limitations of EUS is being operator dependent, EUS has high diagnostic accuracy for the pancreatic parenchyma. EUS can detect early signs of CP, although the diagnostic significance of some changes is still debated[43]. In a study by Yusoff et al[27], EUS findings consistent with CP were reported as the most common finding in patients with IAP.
There is controversy in the literature about the timing of EUS in IAP whether to perform after first episode or recurrent episodes[21,27,56]. In a study by Yusoff et al[27], the diagnostic yield of EUS was not significantly different after a single attack or recurrent attacks of IAP. Therefore, the authors concluded that it is reasonable to perform EUS after the first episode, especially in older patients. Many authors have supported this opinion[12,18,21,57]. On the other hand there are few studies where the diagnostic yield is more in recurrent episodes than a single episode[51,58].
IAP is a common condition but the natural history is not well studied and the best diagnostic approach to both single and multiple attacks remains undefined. The suggested diagnostic approach in various studies and guidelines to IAP is highly variable[12]. As per the American Gastroenterological Association, EUS is not recommended after first episode of IAP in patients less than 40 years of age. It recommends EUS as investigation of choice in IRAP and after first episode in patients with more than 40 years of age[59]. As per American College of Gastroenterology, extensive evaluation using EUS or MRCP is recommended following recurrent attacks of IAP[60]. As per the International Association of pancreatology, patients with IAP should undergo EUS as the investigation of choice irrespective of first or recurrent episodes and, if negative, then, a MRCP-S as the next step[61].
In a recent landmark prospective study of patients with IAP over a 10-year period, 201 patients were enrolled. 80 were with single attack and 121 with multiple attacks of IAP. After EUS, 54% of patients with a single attack were categorized as idiopathic, while for multiple attacks 14 % were idiopathic. Long-term follow-up documented recurrence of pancreatitis in 24% of the patients with a single attack and in 49% of the patients with multiple attacks. On multivariate analysis, number of previous attacks was found to significant predictor of recurrence. It was concluded that following a single idiopathic attack of pancreatitis and a negative EUS examination, relapse was infrequent. A negative EUS after a single attack had significant prognostic value as it identified low rate of recurrence. This study suggest that EUS may be an useful test following a single attack given its yield and potential prognostic value. This study had prospective EUS based approach to idiopathic pancreatitis[12]. In a recent systemic review of 13 studies by Smith et al[19], the diagnostic yield of EUS was not influenced by recurrent disease. Considering the available literature, we recommend EUS after first episode of idiopathic pancreatitis.
Intravenous secretin when used with MRCP (MRCP-S) or EUS (EUS-S) may help the visualization of the pancreatic ductal system and can recognise some undetected ductal abnormalities that are of diagnostic importance in IRAP patients. In a study by Mariani et al[43], the diagnostic yield of EUS-S in IRAP was 13.6% and 16.7% higher than MRCP-S and ERCP respectively. ERCP alone did not find a diagnosis in any case missed by the other two investigations. It was concluded that both MRCP-S and EUS-S should be used as complementary, first-line, imaging techniques, rather than ERCP in the diagnostic work-up of IAP[43]. However, there are limited studies on the EUS-S and further data are required regarding its efficacy in IAP as compared to EUS without IV secretin.
The timing of performing EUS examination after an episode of IAP remains controversial and unclear. Different studies have used different timings[4]. In a recent prospective study of patients with IAP, EUS was performed 1 mo or more after hospital discharge[12]. Rana et al[18] performed EUS at least 1 mo after the episode of AP when the patients were asymptomatic for abdominal pain. In a study by Norton et al[62], EUS was performed when patients resume food intake while Liu et al[20] perform EUS after resolution of AP during admission. Yusoff et al[27] performed EUS atleast 4 wk after episode of AP to make sure that acute pancreatic parenchymal changes have resolved when EUS is performed.
Thevenot et al[8] suggested to perform MRCP/EUS at a longer interval after the initial AP as inflammation and/or necrosis can prevent visualization of pancreatic lesions during the acute phase. In two recent studies investigating the role of EUS/MRCP in IAP, these examinations were performed1 mo after initial AP when patients were eating normally. The explanation for delayed examination is that fasting is usually advised as initial treatment of AP which can induce GB sludge and, consequently, can result in a false-positive diagnosis of biliary AP if the examination is made early although this is not proven by studies[43,44].
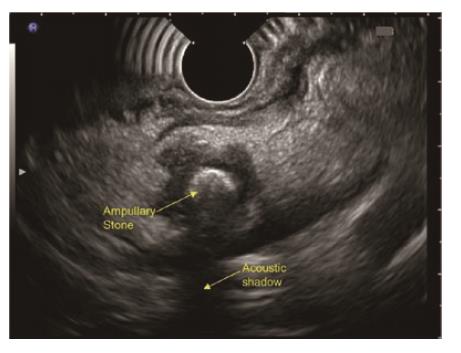
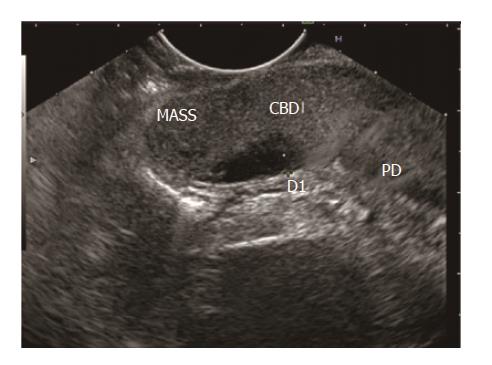
Disadvantages of late EUS after 4 wk of AP includes papillary/ampullary stones (Figure 7) not being diagnosed early with the risk of relapse of AP, early/small periampullary tumours (Figure 8) might be missed as patient may lost to follow up after hospital discharge[4] and possible low diagnostic accuracy as microlithiasis may be missed[21]. Further studies exploring the role of early EUS in IAP are required to come to final conclusion regarding the ideal timing.
There is slight controversy regarding the first investigation after an episode of IAP. Different studies/authors/guidelines have variable opinions on this topic. Although MRCP is less invasive, cheaper and widely available than EUS, and has recently benefited using IV secretin, performance of EUS remains higher than that of MRCP[8]. However, few studies[43,44] have demonstrated that MRCP can
detect few aetiologies undiagnosed by EUS. Further prospective studies are required regarding the cost benefit analysis for EUS/MRCP but at present they should be considered complimentary rather than competitive.
After reviewing the extensive literature on IAP, our opinion which is shared by many authors/guidelines [12,19,21,22,52,57,59,61] and not agreed by few authors/guidelines[32,59], is performing EUS as the first investigation after IAP with intact GB. There is some debate regarding the choice of investigation in post cholecystectomy patients where CP, SOD and PD are the most common diagnoses in which MRCP has demonstrated good diagnostic accuracy[21,52]. As per the latest systemic review in 2015 comparing the diagnostic accuracy of EUS and MRCP in diagnosis of choledocholithiasis, both tests were found to be similar and the choice of test will be decided by availability and contra-indications to each test[63]. However, EUS is superior to MRCP in detecting choledocholithiasis smaller than 5 mm[64]. Various authors and studies believe that the decision to perform EUS or MRCP as the first diagnostic investigation in post cholecystectomy patients must take into account various factors like local expertise, availability and patient details such as claustrophobia, gastric surgery etc. ERCP should always be considered a therapeutic intervention when required[61,63-65].
The role of PD and SOD as causes of IAP remains highly controversial. In a study by Coté et al[66] and Wilcox et al[12], inspite of endoscopic therapy, RAP occurred in almost 50% patients with SOD. In addition, despite repeat endoscopic interventions, further relapse was also very common. These studies questions the role of endoscopic therapy in PD and SOD. These studies shows that diagnosing SOD and PD may not alter the natural history/prognosis of these patients presenting with IAP. Hence, MRCP and ERCP with SOM may not be that useful in diagnostic workup of IRAP. This also supports EUS as first investigation of choice after IAP irrespective of gallbladder status and is agreed by many studies[12,19,21,22,54,59].
As per the recent prospective study done by Wilcox et al[12] on EUS based approach to the evaluation of Idiopathic Pancreatitis and after reviewing the literature, EUS as a first strategy towards the etiological evaluation of IAP appears to be useful not only as a diagnostic but as an important prognostic yield irrespective of gallbladder status. In view of high incidence of biliary tract disease as a cause of IAP in most of the studies and given the high diagnostic accuracy of EUS in identifying them, EUS should be considered as an initial diagnostic step for IAP after conventional radiography fails to identify the aetiology.
Considering all the prior studies, the diagnostic yield of EUS is not influenced by whether the episode is first or recurrent. Hence, EUS should be performed after the first episode of IAP if possible. MRCP preferably MRCP-S can be performed if EUS expertise is not available. MRCP can be complimentary to EUS in identifying controversial aetiologies like PD and SOD. ERCP can be then be performed to treat biliary stones and PD. However, in view of high risk of post ERCP pancreatitis, ERCP with SOM should be reserved in those patients in whom MRCP and EUS has found to be negative for diagnostic and therapeutic evaluation especially with post cholecystectomy cases. We recommend an EUS as the first-line examination in the evaluation of patients with idiopathic pancreatitis, because it is minimally invasive, low risk and accurately identifies most occult causes of pancreatitis.
Pran Prakash (Graphic designer) for his contribution.
| 1. | Steinberg W, Tenner S. Acute pancreatitis. N Engl J Med. 1994;330:1198-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 597] [Article Influence: 18.7] [Reference Citation Analysis (1)] |
| 2. | Levy MJ, Geenen JE. Idiopathic acute recurrent pancreatitis. Am J Gastroenterol. 2001;96:2540-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Md PS, Md UN. Role of ERCP in Patients With Idiopathic Recurrent Acute Pancreatitis. Curr Treat Options Gastroenterol. 2016;14:327-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Vila JJ. Endoscopic ultrasonography and idiopathic acute pancreatitis. World J Gastrointest Endosc. 2010;2:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Al-Haddad M, Wallace MB. Diagnostic approach to patients with acute idiopathic and recurrent pancreatitis, what should be done? World J Gastroenterol. 2008;14:1007-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 6. | Romagnuolo J, Guda N, Freeman M, Durkalski V. Preferred designs, outcomes, and analysis strategies for treatment trials in idiopathic recurrent acute pancreatitis. Gastrointest Endosc. 2008;68:966-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Lee SP, Nicholls JF, Park HZ. Biliary sludge as a cause of acute pancreatitis. N Engl J Med. 1992;326:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 313] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Thevenot A, Bournet B, Otal P, Canevet G, Moreau J, Buscail L. Endoscopic ultrasound and magnetic resonance cholangiopancreatography in patients with idiopathic acute pancreatitis. Dig Dis Sci. 2013;58:2361-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Yadav D, O’Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am J Gastroenterol. 2012;107:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (3)] |
| 10. | Lankisch PG, Breuer N, Bruns A, Weber-Dany B, Lowenfels AB, Maisonneuve P. Natural history of acute pancreatitis: a long-term population-based study. Am J Gastroenterol. 2009;104:2797-805; quiz 2806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Cavestro GM, Leandro G, Di Leo M, Zuppardo RA, Morrow OB, Notaristefano C, Rossi G, Testoni SG, Mazzoleni G, Alessandri M. A single-centre prospective, cohort study of the natural history of acute pancreatitis. Dig Liver Dis. 2015;47:205-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Wilcox CM, Seay T, Kim H, Varadarajulu S. Prospective Endoscopic Ultrasound-Based Approach to the Evaluation of Idiopathic Pancreatitis: Causes, Response to Therapy, and Long-term Outcome. Am J Gastroenterol. 2016;111:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Testoni PA. Acute recurrent pancreatitis: Etiopathogenesis, diagnosis and treatment. World J Gastroenterol. 2014;20:16891-16901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Sharma M, Somani P. Endoscopic ultrasound of pancreatic duct ascariasis. Dig Endosc. 2016;28:483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Somani P, Sharma M, Pathak A, Patil A, Kumar A, Sreesh S. Endoscopic ultrasound imaging of pancreatic duct ascariasis. Endoscopy. 2016;48 Suppl 1 UCTN:E33-E34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Sharma M, Choudhary NS, Puri R. A child with unexplained etiology of acute pancreatitis diagnosed by endoscopic ultrasound. Endosc Ultrasound. 2014;3:135-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Frossard JL, Sosa-Valencia L, Amouyal G, Marty O, Hadengue A, Amouyal P. Usefulness of endoscopic ultrasonography in patients with “idiopathic” acute pancreatitis. Am J Med. 2000;109:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 18. | Rana SS, Bhasin DK, Rao C, Singh K. Role of endoscopic ultrasound in idiopathic acute pancreatitis with negative ultrasound, computed tomography, and magnetic resonance cholangiopancreatography. Ann Gastroenterol. 2012;25:133-137. [PubMed] |
| 19. | Smith I, Ramesh J, Kyanam Kabir Baig KR, Mönkemüller K, Wilcox CM. Emerging Role of Endoscopic Ultrasound in the Diagnostic Evaluation of Idiopathic Pancreatitis. Am J Med Sci. 2015;350:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Liu CL, Lo CM, Chan JK, Poon RT, Fan ST. EUS for detection of occult cholelithiasis in patients with idiopathic pancreatitis. Gastrointest Endosc. 2000;51:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Vila JJ, Vicuña M, Irisarri R, de la Higuera BG, Ruiz-Clavijo D, Rodríguez-Gutiérrez C, Urman JM, Bolado F, Jiménez FJ, Arín A. Diagnostic yield and reliability of endoscopic ultrasonography in patients with idiopathic acute pancreatitis. Scand J Gastroenterol. 2010;45:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Tandon M, Topazian M. Endoscopic ultrasound in idiopathic acute pancreatitis. Am J Gastroenterol. 2001;96:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Rana SS, Gonen C, Vilmann P. Endoscopic ultrasound and pancreas divisum. JOP. 2012;13:252-257. [PubMed] |
| 24. | Sharma M, Pathak A, Rameshbabu CS, Rai P, Kirnake V, Shoukat A. Imaging of pancreas divisum by linear-array endoscopic ultrasonography. Endosc Ultrasound. 2016;5:21-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D, Kopecky K. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 335] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 26. | Klapman JB, Chang KJ, Lee JG, Nguyen P. Negative predictive value of endoscopic ultrasound in a large series of patients with a clinical suspicion of pancreatic cancer. Am J Gastroenterol. 2005;100:2658-2661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Yusoff IF, Raymond G, Sahai AV. A prospective comparison of the yield of EUS in primary vs. recurrent idiopathic acute pancreatitis. Gastrointest Endosc. 2004;60:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Teshima CW, Sandha GS. Endoscopic ultrasound in the diagnosis and treatment of pancreatic disease. World J Gastroenterol. 2014;20:9976-9989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Wilcox CM, Kilgore M. Cost minimization analysis comparing diagnostic strategies in unexplained pancreatitis. Pancreas. 2009;38:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Bank S, Indaram A. Causes of acute and recurrent pancreatitis. Clinical considerations and clues to diagnosis. Gastroenterol Clin North Am. 1999;28:571-589, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (2)] |
| 31. | Gregor JC, Ponich TP, Detsky AS. Should ERCP be routine after an episode of “idiopathic” pancreatitis? A cost-utility analysis. Gastrointest Endosc. 1996;44:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Gn Y, Amin WG, Shaheen FA, Zargar S, Javid G. The efficacy of magnetic resonance cholangiopancreatography in assessing the etiology of acute idiopathic pancreatitis. Int J Hepatobiliary Pancreat Dis. 2014;4:32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Safari MT, Miri MB, Ebadi S, Shahrokh S, Mohammad Alizadeh AH. Comparing the Roles of EUS, ERCP and MRCP in Idiopathic Acute Recurrent Pancreatitis. Clin Med Insights Gastroenterol. 2016;9:35-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Hachiya J, Haradome H. [The utilities and outlooks of MR cholangiography (MRCP) as a non contrast and noninvasive technique]. Nihon Rinsho. 1998;56:2747-2754. [PubMed] |
| 35. | Makary MA, Duncan MD, Harmon JW, Freeswick PD, Bender JS, Bohlman M, Magnuson TH. The role of magnetic resonance cholangiography in the management of patients with gallstone pancreatitis. Ann Surg. 2005;241:119-124. [PubMed] |
| 36. | Gosset J, Deviere J, Matos C. Magnetic resonance imaging of acute pancreatitis: the pancreatogram. JOP. 2004;5:48-50. [PubMed] |
| 37. | Carroll JK, Herrick B, Gipson T, Lee SP. Acute pancreatitis: diagnosis, prognosis, and treatment. Am Fam Physician. 2007;75:1513-1520. [PubMed] |
| 38. | Barish MA, Yucel EK, Ferrucci JT. Magnetic resonance cholangiopancreatography. N Engl J Med. 1999;341:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 141] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Sugiyama M, Haradome H, Atomi Y. Magnetic resonance imaging for diagnosing chronic pancreatitis. J Gastroenterol. 2007;42 Suppl 17:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Heverhagen JT, Burbelko M, Schenck zu Schweinsberg T, Funke C, Wecker C, Walthers EM, Rominger M. [Secretin-enhanced magnetic resonance cholangiopancreaticography: value for the diagnosis of chronic pancreatitis]. Rofo. 2007;179:790-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 41. | Pereira SP, Gillams A, Sgouros SN, Webster GJ, Hatfield AR. Prospective comparison of secretin-stimulated magnetic resonance cholangiopancreatography with manometry in the diagnosis of sphincter of Oddi dysfunction types II and III. Gut. 2007;56:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Rustagi T, Njei B. Magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: a systematic review and meta-analysis. Pancreas. 2014;43:823-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Mariani A, Arcidiacono PG, Curioni S, Giussani A, Testoni PA. Diagnostic yield of ERCP and secretin-enhanced MRCP and EUS in patients with acute recurrent pancreatitis of unknown aetiology. Dig Liver Dis. 2009;41:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Ortega AR, Gómez-Rodríguez R, Romero M, Fernández-Zapardiel S, Céspedes Mdel M, Carrobles JM. Prospective comparison of endoscopic ultrasonography and magnetic resonance cholangiopancreatography in the etiological diagnosis of “idiopathic” acute pancreatitis. Pancreas. 2011;40:289-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Kapetanos D. Endoscopic management of acute recurrent pancreatitis. Ann Gastroenterol. 2010;23:31-37. |
| 46. | Rana SS, Bhasin DK, Sharma V, Rao C, Singh K. Role of endoscopic ultrasound in the diagnosis of pancreas divisum. Endosc Ultrasound. 2013;2:7-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 47. | Lai R, Freeman ML, Cass OW, Mallery S. Accurate diagnosis of pancreas divisum by linear-array endoscopic ultrasonography. Endoscopy. 2004;36:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Kushnir VM, Wani SB, Fowler K, Menias C, Varma R, Narra V, Hovis C, Murad FM, Mullady DK, Jonnalagadda SS. Sensitivity of endoscopic ultrasound, multidetector computed tomography, and magnetic resonance cholangiopancreatography in the diagnosis of pancreas divisum: a tertiary center experience. Pancreas. 2013;42:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Liu CL, Lo CM, Chan JK, Poon RT, Lam CM, Fan ST, Wong J. Detection of choledocholithiasis by EUS in acute pancreatitis: a prospective evaluation in 100 consecutive patients. Gastrointest Endosc. 2001;54:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 50. | Kurol M, Forsberg L. Ultrasonography in the diagnosis of acute cholecystitis. Acta Radiol Diagn (Stockh). 1984;25:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Ros E, Navarro S, Bru C, Garcia-Pugés A, Valderrama R. Occult microlithiasis in ‘idiopathic’ acute pancreatitis: prevention of relapses by cholecystectomy or ursodeoxycholic acid therapy. Gastroenterology. 1991;101:1701-1709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 236] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Wilcox CM, Varadarajulu S, Eloubeidi M. Role of endoscopic evaluation in idiopathic pancreatitis: a systematic review. Gastrointest Endosc. 2006;63:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Garg PK, Tandon RK, Madan K. Is biliary microlithiasis a significant cause of idiopathic recurrent acute pancreatitis? A long-term follow-up study. Clin Gastroenterol Hepatol. 2007;5:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Levy MJ. The hunt for microlithiasis in idiopathic acute recurrent pancreatitis: should we abandon the search or intensify our efforts? Gastrointest Endosc. 2002;55:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 55. | Petrone MC, Arcidiacono PG, Testoni PA. Endoscopic ultrasonography for evaluating patients with recurrent pancreatitis. World J Gastroenterol. 2008;14:1016-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Chen RY, Hawes RH. Idopathic acute pancreatitis: Is EUS worth doing? Am J Gastroenterol. 2002;97:1244-1246. [PubMed] |
| 57. | Papachristou GI, Topazian M. Idiopathic recurrent pancreatitis: an EUS-based management approach. Gastrointest Endosc. 2011;73:1155-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 58. | Sajith KG, Chacko A, Dutta AK. Recurrent acute pancreatitis: clinical profile and an approach to diagnosis. Dig Dis Sci. 2010;55:3610-3616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Forsmark CE, Baillie J; AGA Institute Clinical Practice and Economics Committee; AGA Institute Governing Board. AGA Institute technical review on acute pancreatitis. Gastroenterology. 2007;132:2022-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 510] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 60. | Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-1415; 1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1428] [Article Influence: 109.8] [Reference Citation Analysis (3)] |
| 61. | Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1094] [Article Influence: 84.2] [Reference Citation Analysis (10)] |
| 62. | Norton SA, Alderson D. Endoscopic ultrasonography in the evaluation of idiopathic acute pancreatitis. Br J Surg. 2000;87:1650-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Giljaca V, Gurusamy KS, Takwoingi Y, Higgie D, Poropat G, Štimac D, Davidson BR. Endoscopic ultrasound versus magnetic resonance cholangiopancreatography for common bile duct stones. Cochrane Database Syst Rev. 2015;CD011549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Kondo S, Isayama H, Akahane M, Toda N, Sasahira N, Nakai Y, Yamamoto N, Hirano K, Komatsu Y, Tada M. Detection of common bile duct stones: comparison between endoscopic ultrasonography, magnetic resonance cholangiography, and helical-computed-tomographic cholangiography. Eur J Radiol. 2005;54:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 140] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Verma D, Kapadia A, Eisen GM, Adler DG. EUS vs MRCP for detection of choledocholithiasis. Gastrointest Endosc. 2006;64:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 66. | Coté GA, Imperiale TF, Schmidt SE, Fogel E, Lehman G, McHenry L, Watkins J, Sherman S. Similar efficacies of biliary, with or without pancreatic, sphincterotomy in treatment of idiopathic recurrent acute pancreatitis. Gastroenterology. 2012;143:1502-1509.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Li JJ, Stoos-Veic T, Zhang ZH S- Editor: Chen K
L- Editor: A E- Editor: Huang Y